

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Genome polyprotein (Hepatitis C virus) | BDBM50135740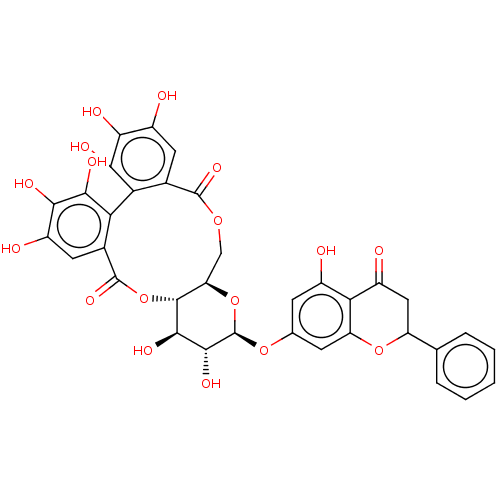 (2,3,4,5,6,7,14,15-octahydroxy-13-(5-hydroxy-4-oxo-...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 300 | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute Curated by ChEMBL | Assay Description Inhibitory activity of the compound evaluated in the HCV NS3 protease activity assay | Bioorg Med Chem Lett 13: 2925-8 (2003) BindingDB Entry DOI: 10.7270/Q2HX1C2D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus) | BDBM50135739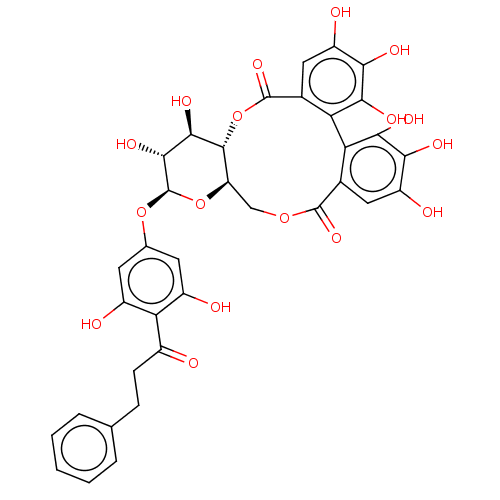 (13-[3,5-dihydroxy-4-(3-phenylpropanoyl)phenoxy]-2,...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 800 | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute Curated by ChEMBL | Assay Description Inhibitory activity of the compound evaluated in the HCV NS3 protease assay | Bioorg Med Chem Lett 13: 2925-8 (2003) BindingDB Entry DOI: 10.7270/Q2HX1C2D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Human rhinovirus B) | BDBM50135740 (2,3,4,5,6,7,14,15-octahydroxy-13-(5-hydroxy-4-oxo-...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 2.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute Curated by ChEMBL | Assay Description Inhibitory activity of the compound evaluated in the HCV protease binding assay | Bioorg Med Chem Lett 13: 2925-8 (2003) BindingDB Entry DOI: 10.7270/Q2HX1C2D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||