 Found 24 hits of Enzyme Inhibition Constant Data
Found 24 hits of Enzyme Inhibition Constant Data Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM50094392
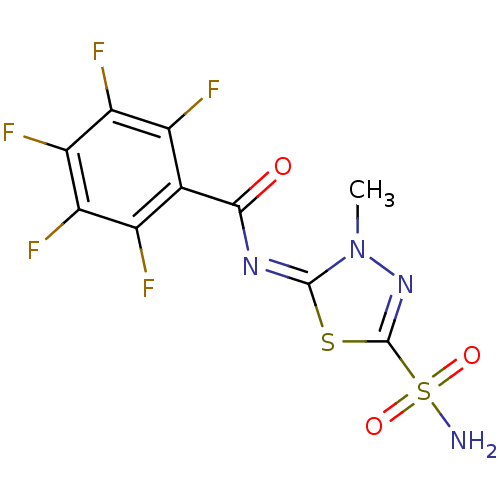
(2,3,4,5,6-Pentafluoro-N-(3-methyl-5-sulfamoyl-3H-[...)Show SMILES Cn1nc(s\c1=N\C(=O)c1c(F)c(F)c(F)c(F)c1F)S(N)(=O)=O Show InChI InChI=1S/C10H5F5N4O3S2/c1-19-9(23-10(18-19)24(16,21)22)17-8(20)2-3(11)5(13)7(15)6(14)4(2)12/h1H3,(H2,16,21,22)/b17-9+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 1.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Firenze
Curated by ChEMBL
| Assay Description
Compound was evaluated for inhibition against human carbonic anhydrase II |
Bioorg Med Chem Lett 14: 217-23 (2003)
BindingDB Entry DOI: 10.7270/Q2154HMF |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM11635

(4-sulfamoyl-N-[2-(4-sulfamoylphenyl)ethyl]benzamid...)Show SMILES NS(=O)(=O)c1ccc(CCNC(=O)c2ccc(cc2)S(N)(=O)=O)cc1 Show InChI InChI=1S/C15H17N3O5S2/c16-24(20,21)13-5-1-11(2-6-13)9-10-18-15(19)12-3-7-14(8-4-12)25(17,22)23/h1-8H,9-10H2,(H,18,19)(H2,16,20,21)(H2,17,22,23) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Firenze
Curated by ChEMBL
| Assay Description
Compound was evaluated for inhibition against human carbonic anhydrase II |
Bioorg Med Chem Lett 14: 217-23 (2003)
BindingDB Entry DOI: 10.7270/Q2154HMF |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM10887

(Sulfamate 7 | Topiramate (TPM) | US11535599, Examp...)Show SMILES CC1(C)O[C@@H]2CO[C@@]3(COS(N)(=O)=O)OC(C)(C)O[C@H]3[C@@H]2O1 |r| Show InChI InChI=1S/C12H21NO8S/c1-10(2)18-7-5-16-12(6-17-22(13,14)15)9(8(7)19-10)20-11(3,4)21-12/h7-9H,5-6H2,1-4H3,(H2,13,14,15)/t7-,8-,9+,12+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
PubMed
| 5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Firenze
Curated by ChEMBL
| Assay Description
Compound was evaluated for inhibition against human carbonic anhydrase II |
Bioorg Med Chem Lett 14: 217-23 (2003)
BindingDB Entry DOI: 10.7270/Q2154HMF |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Carbonic anhydrase 4
(Bos taurus (bovine)) | BDBM50094392

(2,3,4,5,6-Pentafluoro-N-(3-methyl-5-sulfamoyl-3H-[...)Show SMILES Cn1nc(s\c1=N\C(=O)c1c(F)c(F)c(F)c(F)c1F)S(N)(=O)=O Show InChI InChI=1S/C10H5F5N4O3S2/c1-19-9(23-10(18-19)24(16,21)22)17-8(20)2-3(11)5(13)7(15)6(14)4(2)12/h1H3,(H2,16,21,22)/b17-9+ | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Firenze
Curated by ChEMBL
| Assay Description
Compound was evaluated for inhibition against bovine carbonic anhydrase IV |
Bioorg Med Chem Lett 14: 217-23 (2003)
BindingDB Entry DOI: 10.7270/Q2154HMF |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM10880

(AZA | AZA2 | AZM acetazolamide | Acerazolamide, AA...)Show InChI InChI=1S/C4H6N4O3S2/c1-2(9)6-3-7-8-4(12-3)13(5,10)11/h1H3,(H2,5,10,11)(H,6,7,9) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
MMDB
PDB
PubMed
| 12 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Firenze
Curated by ChEMBL
| Assay Description
Compound was evaluated for inhibition against human carbonic anhydrase II |
Bioorg Med Chem Lett 14: 217-23 (2003)
BindingDB Entry DOI: 10.7270/Q2154HMF |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Carbonic anhydrase 4
(Bos taurus (bovine)) | BDBM11635

(4-sulfamoyl-N-[2-(4-sulfamoylphenyl)ethyl]benzamid...)Show SMILES NS(=O)(=O)c1ccc(CCNC(=O)c2ccc(cc2)S(N)(=O)=O)cc1 Show InChI InChI=1S/C15H17N3O5S2/c16-24(20,21)13-5-1-11(2-6-13)9-10-18-15(19)12-3-7-14(8-4-12)25(17,22)23/h1-8H,9-10H2,(H,18,19)(H2,16,20,21)(H2,17,22,23) | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 13 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Firenze
Curated by ChEMBL
| Assay Description
Compound was evaluated for inhibition against bovine carbonic anhydrase IV |
Bioorg Med Chem Lett 14: 217-23 (2003)
BindingDB Entry DOI: 10.7270/Q2154HMF |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM10881

(CHEMBL288100 | MZA3 | Methazolamide | Methazolamid...)Show InChI InChI=1S/C5H8N4O3S2/c1-3(10)7-4-9(2)8-5(13-4)14(6,11)12/h1-2H3,(H2,6,11,12) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
PubMed
| 14 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Firenze
Curated by ChEMBL
| Assay Description
Compound was evaluated for inhibition against human carbonic anhydrase II |
Bioorg Med Chem Lett 14: 217-23 (2003)
BindingDB Entry DOI: 10.7270/Q2154HMF |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM50137674
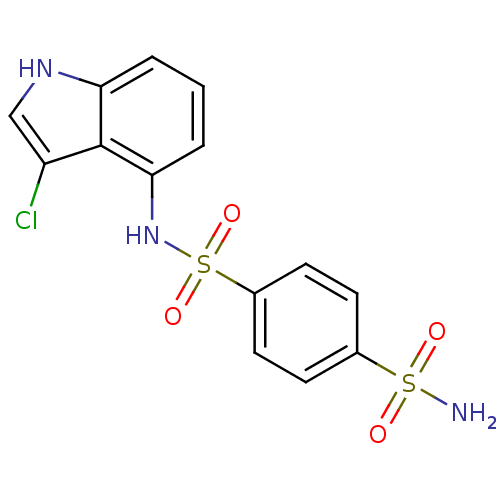
(Benzene-1,4-disulfonic acid 1-amide 4-[(3-chloro-1...)Show SMILES NS(=O)(=O)c1ccc(cc1)S(=O)(=O)Nc1cccc2[nH]cc(Cl)c12 Show InChI InChI=1S/C14H12ClN3O4S2/c15-11-8-17-12-2-1-3-13(14(11)12)18-24(21,22)10-6-4-9(5-7-10)23(16,19)20/h1-8,17-18H,(H2,16,19,20) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| PubMed
| 15 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Firenze
Curated by ChEMBL
| Assay Description
Compound was evaluated for inhibition against human carbonic anhydrase II |
Bioorg Med Chem Lett 14: 217-23 (2003)
BindingDB Entry DOI: 10.7270/Q2154HMF |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 9
(Homo sapiens (Human)) | BDBM11635

(4-sulfamoyl-N-[2-(4-sulfamoylphenyl)ethyl]benzamid...)Show SMILES NS(=O)(=O)c1ccc(CCNC(=O)c2ccc(cc2)S(N)(=O)=O)cc1 Show InChI InChI=1S/C15H17N3O5S2/c16-24(20,21)13-5-1-11(2-6-13)9-10-18-15(19)12-3-7-14(8-4-12)25(17,22)23/h1-8H,9-10H2,(H,18,19)(H2,16,20,21)(H2,17,22,23) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 18 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Firenze
Curated by ChEMBL
| Assay Description
Compound was evaluated for inhibition against human carbonic anhydrase IX |
Bioorg Med Chem Lett 14: 217-23 (2003)
BindingDB Entry DOI: 10.7270/Q2154HMF |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 9
(Homo sapiens (Human)) | BDBM50137674

(Benzene-1,4-disulfonic acid 1-amide 4-[(3-chloro-1...)Show SMILES NS(=O)(=O)c1ccc(cc1)S(=O)(=O)Nc1cccc2[nH]cc(Cl)c12 Show InChI InChI=1S/C14H12ClN3O4S2/c15-11-8-17-12-2-1-3-13(14(11)12)18-24(21,22)10-6-4-9(5-7-10)23(16,19)20/h1-8,17-18H,(H2,16,19,20) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| PubMed
| 24 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Firenze
Curated by ChEMBL
| Assay Description
Compound was evaluated for inhibition against human carbonic anhydrase IX |
Bioorg Med Chem Lett 14: 217-23 (2003)
BindingDB Entry DOI: 10.7270/Q2154HMF |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 9
(Homo sapiens (Human)) | BDBM10880

(AZA | AZA2 | AZM acetazolamide | Acerazolamide, AA...)Show InChI InChI=1S/C4H6N4O3S2/c1-2(9)6-3-7-8-4(12-3)13(5,10)11/h1H3,(H2,5,10,11)(H,6,7,9) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| 25 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Firenze
Curated by ChEMBL
| Assay Description
Compound was evaluated for inhibition against human carbonic anhydrase IX |
Bioorg Med Chem Lett 14: 217-23 (2003)
BindingDB Entry DOI: 10.7270/Q2154HMF |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 9
(Homo sapiens (Human)) | BDBM10881

(CHEMBL288100 | MZA3 | Methazolamide | Methazolamid...)Show InChI InChI=1S/C5H8N4O3S2/c1-3(10)7-4-9(2)8-5(13-4)14(6,11)12/h1-2H3,(H2,6,11,12) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| 27 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Firenze
Curated by ChEMBL
| Assay Description
Compound was evaluated for inhibition against human carbonic anhydrase IX |
Bioorg Med Chem Lett 14: 217-23 (2003)
BindingDB Entry DOI: 10.7270/Q2154HMF |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 9
(Homo sapiens (Human)) | BDBM50094392

(2,3,4,5,6-Pentafluoro-N-(3-methyl-5-sulfamoyl-3H-[...)Show SMILES Cn1nc(s\c1=N\C(=O)c1c(F)c(F)c(F)c(F)c1F)S(N)(=O)=O Show InChI InChI=1S/C10H5F5N4O3S2/c1-19-9(23-10(18-19)24(16,21)22)17-8(20)2-3(11)5(13)7(15)6(14)4(2)12/h1H3,(H2,16,21,22)/b17-9+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 29 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Firenze
Curated by ChEMBL
| Assay Description
Compound was evaluated for inhibition against human carbonic anhydrase IX |
Bioorg Med Chem Lett 14: 217-23 (2003)
BindingDB Entry DOI: 10.7270/Q2154HMF |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 1
(Homo sapiens (Human)) | BDBM50137674

(Benzene-1,4-disulfonic acid 1-amide 4-[(3-chloro-1...)Show SMILES NS(=O)(=O)c1ccc(cc1)S(=O)(=O)Nc1cccc2[nH]cc(Cl)c12 Show InChI InChI=1S/C14H12ClN3O4S2/c15-11-8-17-12-2-1-3-13(14(11)12)18-24(21,22)10-6-4-9(5-7-10)23(16,19)20/h1-8,17-18H,(H2,16,19,20) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| PubMed
| 31 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Firenze
Curated by ChEMBL
| Assay Description
Compound was evaluated for inhibition against human carbonic anhydrase I |
Bioorg Med Chem Lett 14: 217-23 (2003)
BindingDB Entry DOI: 10.7270/Q2154HMF |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 4
(Bos taurus (bovine)) | BDBM10881

(CHEMBL288100 | MZA3 | Methazolamide | Methazolamid...)Show InChI InChI=1S/C5H8N4O3S2/c1-3(10)7-4-9(2)8-5(13-4)14(6,11)12/h1-2H3,(H2,6,11,12) | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| 36 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Firenze
Curated by ChEMBL
| Assay Description
Compound was evaluated for inhibition against bovine carbonic anhydrase IV |
Bioorg Med Chem Lett 14: 217-23 (2003)
BindingDB Entry DOI: 10.7270/Q2154HMF |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 1
(Homo sapiens (Human)) | BDBM11635

(4-sulfamoyl-N-[2-(4-sulfamoylphenyl)ethyl]benzamid...)Show SMILES NS(=O)(=O)c1ccc(CCNC(=O)c2ccc(cc2)S(N)(=O)=O)cc1 Show InChI InChI=1S/C15H17N3O5S2/c16-24(20,21)13-5-1-11(2-6-13)9-10-18-15(19)12-3-7-14(8-4-12)25(17,22)23/h1-8H,9-10H2,(H,18,19)(H2,16,20,21)(H2,17,22,23) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Firenze
Curated by ChEMBL
| Assay Description
Compound was evaluated for inhibition against human carbonic anhydrase I |
Bioorg Med Chem Lett 14: 217-23 (2003)
BindingDB Entry DOI: 10.7270/Q2154HMF |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 1
(Homo sapiens (Human)) | BDBM10881

(CHEMBL288100 | MZA3 | Methazolamide | Methazolamid...)Show InChI InChI=1S/C5H8N4O3S2/c1-3(10)7-4-9(2)8-5(13-4)14(6,11)12/h1-2H3,(H2,6,11,12) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
PubMed
| 50 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Firenze
Curated by ChEMBL
| Assay Description
Compound was evaluated for inhibition against human carbonic anhydrase I |
Bioorg Med Chem Lett 14: 217-23 (2003)
BindingDB Entry DOI: 10.7270/Q2154HMF |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Carbonic anhydrase 4
(Bos taurus (bovine)) | BDBM10887

(Sulfamate 7 | Topiramate (TPM) | US11535599, Examp...)Show SMILES CC1(C)O[C@@H]2CO[C@@]3(COS(N)(=O)=O)OC(C)(C)O[C@H]3[C@@H]2O1 |r| Show InChI InChI=1S/C12H21NO8S/c1-10(2)18-7-5-16-12(6-17-22(13,14)15)9(8(7)19-10)20-11(3,4)21-12/h7-9H,5-6H2,1-4H3,(H2,13,14,15)/t7-,8-,9+,12+/m1/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| 54 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Firenze
Curated by ChEMBL
| Assay Description
Compound was evaluated for inhibition against bovine carbonic anhydrase IV |
Bioorg Med Chem Lett 14: 217-23 (2003)
BindingDB Entry DOI: 10.7270/Q2154HMF |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 9
(Homo sapiens (Human)) | BDBM10887

(Sulfamate 7 | Topiramate (TPM) | US11535599, Examp...)Show SMILES CC1(C)O[C@@H]2CO[C@@]3(COS(N)(=O)=O)OC(C)(C)O[C@H]3[C@@H]2O1 |r| Show InChI InChI=1S/C12H21NO8S/c1-10(2)18-7-5-16-12(6-17-22(13,14)15)9(8(7)19-10)20-11(3,4)21-12/h7-9H,5-6H2,1-4H3,(H2,13,14,15)/t7-,8-,9+,12+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| 58 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Firenze
Curated by ChEMBL
| Assay Description
Compound was evaluated for inhibition against human carbonic anhydrase IX |
Bioorg Med Chem Lett 14: 217-23 (2003)
BindingDB Entry DOI: 10.7270/Q2154HMF |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 4
(Bos taurus (bovine)) | BDBM50137674

(Benzene-1,4-disulfonic acid 1-amide 4-[(3-chloro-1...)Show SMILES NS(=O)(=O)c1ccc(cc1)S(=O)(=O)Nc1cccc2[nH]cc(Cl)c12 Show InChI InChI=1S/C14H12ClN3O4S2/c15-11-8-17-12-2-1-3-13(14(11)12)18-24(21,22)10-6-4-9(5-7-10)23(16,19)20/h1-8,17-18H,(H2,16,19,20) | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| PubMed
| 65 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Firenze
Curated by ChEMBL
| Assay Description
Compound was evaluated for inhibition against bovine carbonic anhydrase IV |
Bioorg Med Chem Lett 14: 217-23 (2003)
BindingDB Entry DOI: 10.7270/Q2154HMF |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 4
(Bos taurus (bovine)) | BDBM10880

(AZA | AZA2 | AZM acetazolamide | Acerazolamide, AA...)Show InChI InChI=1S/C4H6N4O3S2/c1-2(9)6-3-7-8-4(12-3)13(5,10)11/h1H3,(H2,5,10,11)(H,6,7,9) | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| 70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Firenze
Curated by ChEMBL
| Assay Description
Compound was evaluated for inhibition against bovine carbonic anhydrase IV |
Bioorg Med Chem Lett 14: 217-23 (2003)
BindingDB Entry DOI: 10.7270/Q2154HMF |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 1
(Homo sapiens (Human)) | BDBM10887

(Sulfamate 7 | Topiramate (TPM) | US11535599, Examp...)Show SMILES CC1(C)O[C@@H]2CO[C@@]3(COS(N)(=O)=O)OC(C)(C)O[C@H]3[C@@H]2O1 |r| Show InChI InChI=1S/C12H21NO8S/c1-10(2)18-7-5-16-12(6-17-22(13,14)15)9(8(7)19-10)20-11(3,4)21-12/h7-9H,5-6H2,1-4H3,(H2,13,14,15)/t7-,8-,9+,12+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| MMDB
PDB
PubMed
| 250 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Firenze
Curated by ChEMBL
| Assay Description
Compound was evaluated for inhibition against human carbonic anhydrase I |
Bioorg Med Chem Lett 14: 217-23 (2003)
BindingDB Entry DOI: 10.7270/Q2154HMF |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Carbonic anhydrase 1
(Homo sapiens (Human)) | BDBM10880

(AZA | AZA2 | AZM acetazolamide | Acerazolamide, AA...)Show InChI InChI=1S/C4H6N4O3S2/c1-2(9)6-3-7-8-4(12-3)13(5,10)11/h1H3,(H2,5,10,11)(H,6,7,9) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
PubMed
| 250 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Firenze
Curated by ChEMBL
| Assay Description
Compound was evaluated for inhibition against human carbonic anhydrase I |
Bioorg Med Chem Lett 14: 217-23 (2003)
BindingDB Entry DOI: 10.7270/Q2154HMF |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Carbonic anhydrase 1
(Homo sapiens (Human)) | BDBM50094392

(2,3,4,5,6-Pentafluoro-N-(3-methyl-5-sulfamoyl-3H-[...)Show SMILES Cn1nc(s\c1=N\C(=O)c1c(F)c(F)c(F)c(F)c1F)S(N)(=O)=O Show InChI InChI=1S/C10H5F5N4O3S2/c1-19-9(23-10(18-19)24(16,21)22)17-8(20)2-3(11)5(13)7(15)6(14)4(2)12/h1H3,(H2,16,21,22)/b17-9+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 270 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Firenze
Curated by ChEMBL
| Assay Description
Compound was evaluated for inhibition against human carbonic anhydrase I |
Bioorg Med Chem Lett 14: 217-23 (2003)
BindingDB Entry DOI: 10.7270/Q2154HMF |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data