

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM591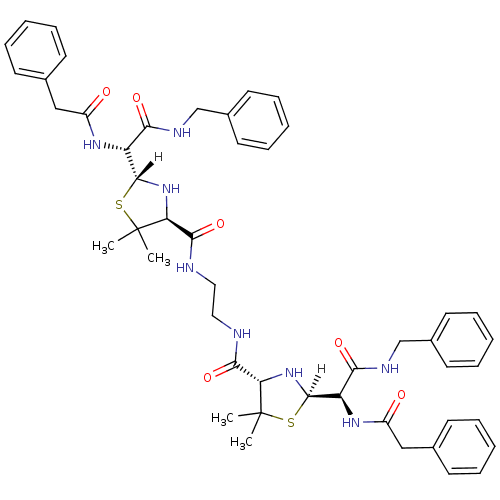 ((2R,4S)-2-[(R)-(benzylcarbamoyl)(1-phenylacetamido...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Group Research Limited Curated by ChEMBL | Assay Description Inhibitory activity towards HIV-1 protease | J Med Chem 35: 3080-1 (1992) BindingDB Entry DOI: 10.7270/Q27H1HJQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM589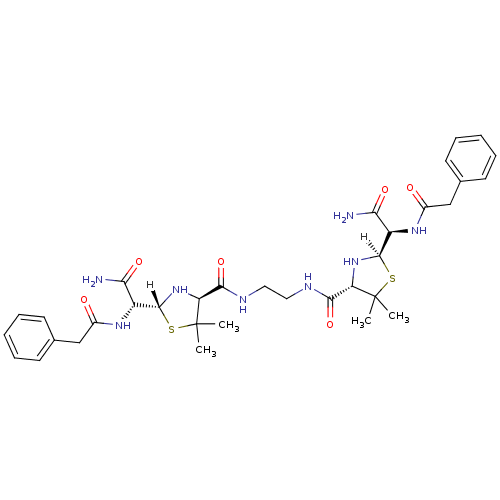 ((2R,4S)-2-[(R)-carbamoyl(1-phenylacetamido)methyl]...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Group Research Limited Curated by ChEMBL | Assay Description Inhibitory activity towards HIV-1 protease | J Med Chem 35: 3080-1 (1992) BindingDB Entry DOI: 10.7270/Q27H1HJQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM590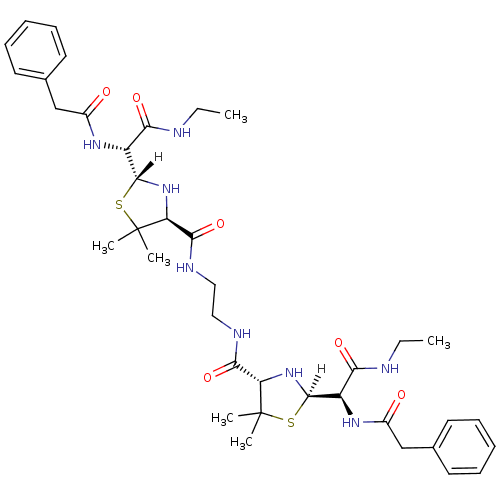 ((2R,4S)-2-[(R)-(ethylcarbamoyl)(1-phenylacetamido)...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 4.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Group Research Limited Curated by ChEMBL | Assay Description Inhibitory activity towards HIV-1 protease | J Med Chem 35: 3080-1 (1992) BindingDB Entry DOI: 10.7270/Q27H1HJQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM588 (CHEMBL104525 | [ 2R-[ 2a(R*),4B ] ]-4,4 -[ 1,2-Eth...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 60 | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Group Research Limited Curated by ChEMBL | Assay Description Inhibitory activity towards HIV-1 protease | J Med Chem 35: 3080-1 (1992) BindingDB Entry DOI: 10.7270/Q27H1HJQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50004601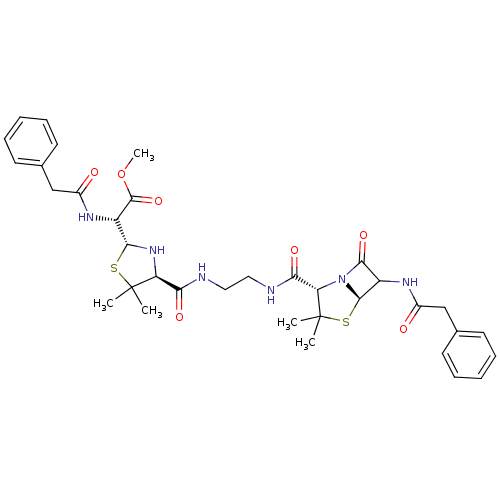 ((4-{2-[(3,3-Dimethyl-7-oxo-6-phenylacetylamino-4-t...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 500 | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Group Research Limited Curated by ChEMBL | Assay Description Inhibitory activity towards HIV-1 protease | J Med Chem 35: 3080-1 (1992) BindingDB Entry DOI: 10.7270/Q27H1HJQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50004600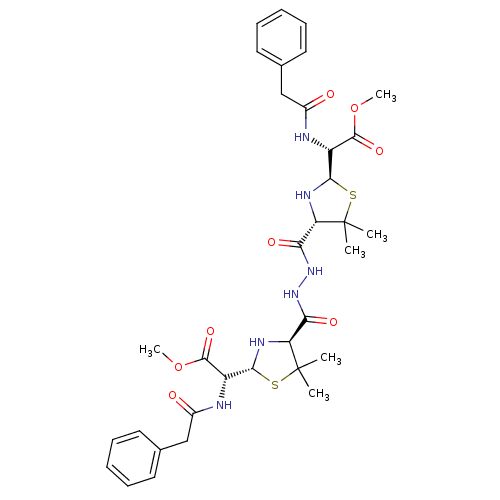 ((4-{N'-[2-(Methoxycarbonyl-phenylacetylamino-methy...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 6.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Group Research Limited Curated by ChEMBL | Assay Description Inhibitory activity towards HIV-1 protease | J Med Chem 35: 3080-1 (1992) BindingDB Entry DOI: 10.7270/Q27H1HJQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50004602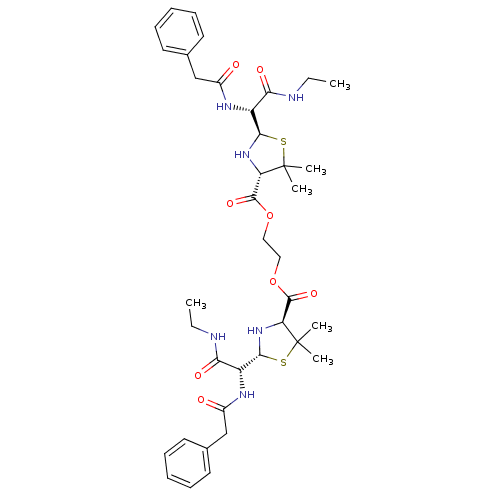 (2-[2-benzylcarboxamido(ethylcarbamoyl)methyl-5,5-d...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | >1.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Group Research Limited Curated by ChEMBL | Assay Description Inhibitory activity towards HIV-1 protease | J Med Chem 35: 3080-1 (1992) BindingDB Entry DOI: 10.7270/Q27H1HJQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50004598 (CHEMBL322454 | [4-(3-{[2-(Methoxycarbonyl-phenylac...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | >1.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Group Research Limited Curated by ChEMBL | Assay Description Inhibitory activity towards HIV-1 protease | J Med Chem 35: 3080-1 (1992) BindingDB Entry DOI: 10.7270/Q27H1HJQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50004603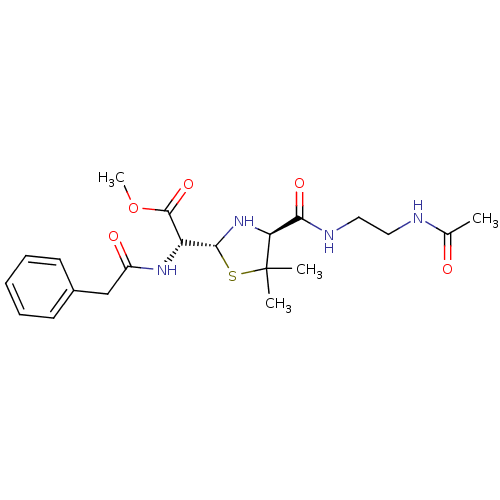 (CHEMBL105589 | [4-(2-Acetylamino-ethylcarbamoyl)-5...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | >5.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Group Research Limited Curated by ChEMBL | Assay Description Inhibitory activity towards HIV-1 protease | J Med Chem 35: 3080-1 (1992) BindingDB Entry DOI: 10.7270/Q27H1HJQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50004597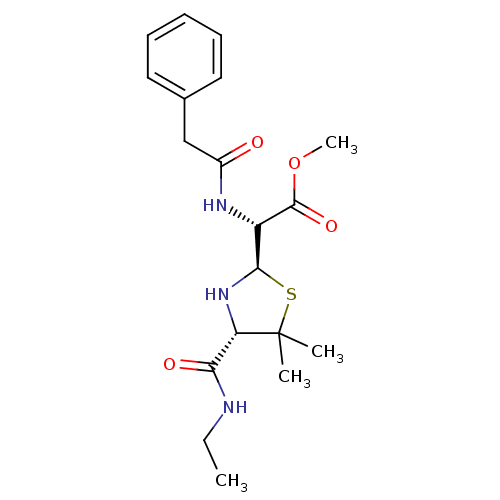 ((4-Ethylcarbamoyl-5,5-dimethyl-thiazolidin-2-yl)-p...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | >6.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Group Research Limited Curated by ChEMBL | Assay Description Inhibitory activity towards HIV-1 protease | J Med Chem 35: 3080-1 (1992) BindingDB Entry DOI: 10.7270/Q27H1HJQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50004599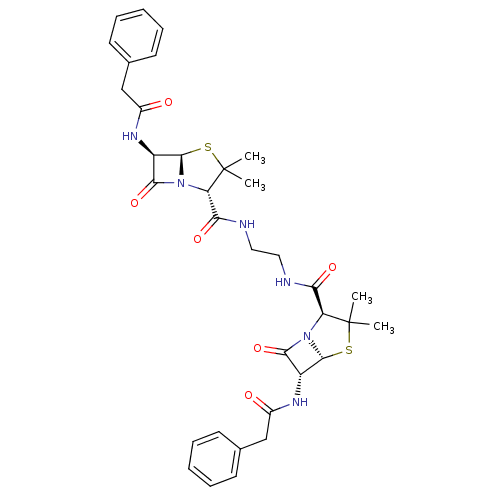 (1N-[3-{2-[6-benzylcarboxamido-2,2-dimethyl-5-oxo-(...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | >7.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Group Research Limited Curated by ChEMBL | Assay Description Inhibitory activity towards HIV-1 protease | J Med Chem 35: 3080-1 (1992) BindingDB Entry DOI: 10.7270/Q27H1HJQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||