 Found 22 hits of Enzyme Inhibition Constant Data
Found 22 hits of Enzyme Inhibition Constant Data Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Cathepsin K
(Homo sapiens (Human)) | BDBM50154180
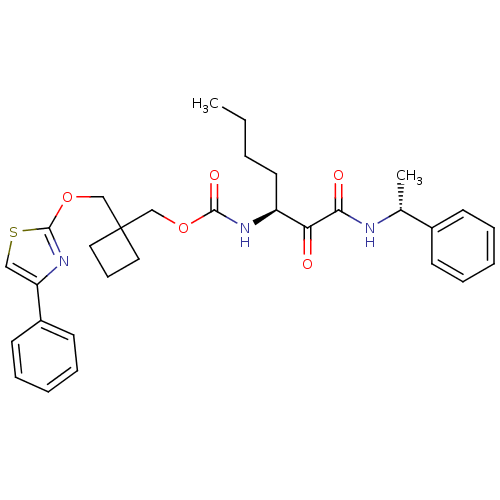
(CHEMBL187918 | [1-(1-Phenyl-ethylaminooxalyl)-pent...)Show SMILES CCCC[C@H](NC(=O)OCC1(COc2nc(cs2)-c2ccccc2)CCC1)C(=O)C(=O)N[C@H](C)c1ccccc1 Show InChI InChI=1S/C31H37N3O5S/c1-3-4-16-25(27(35)28(36)32-22(2)23-12-7-5-8-13-23)33-29(37)38-20-31(17-11-18-31)21-39-30-34-26(19-40-30)24-14-9-6-10-15-24/h5-10,12-15,19,22,25H,3-4,11,16-18,20-21H2,1-2H3,(H,32,36)(H,33,37)/t22-,25+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5.5 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibitory potency against human Cathepsin K |
J Med Chem 47: 5057-68 (2004)
Article DOI: 10.1021/jm040107n
BindingDB Entry DOI: 10.7270/Q23T9GP0 |
More data for this
Ligand-Target Pair | |
Cathepsin K
(Homo sapiens (Human)) | BDBM50154194
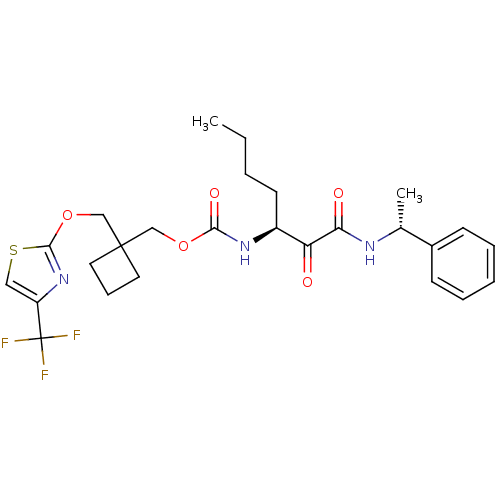
(CHEMBL361507 | [1-(1-Phenyl-ethylaminooxalyl)-pent...)Show SMILES CCCC[C@H](NC(=O)OCC1(COc2nc(cs2)C(F)(F)F)CCC1)C(=O)C(=O)N[C@H](C)c1ccccc1 Show InChI InChI=1S/C26H32F3N3O5S/c1-3-4-11-19(21(33)22(34)30-17(2)18-9-6-5-7-10-18)31-23(35)36-15-25(12-8-13-25)16-37-24-32-20(14-38-24)26(27,28)29/h5-7,9-10,14,17,19H,3-4,8,11-13,15-16H2,1-2H3,(H,30,34)(H,31,35)/t17-,19+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 7.90 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibitory potency against human Cathepsin K |
J Med Chem 47: 5057-68 (2004)
Article DOI: 10.1021/jm040107n
BindingDB Entry DOI: 10.7270/Q23T9GP0 |
More data for this
Ligand-Target Pair | |
Cathepsin K
(Homo sapiens (Human)) | BDBM50154187

(CHEMBL365081 | [1-(1-Phenyl-ethylaminooxalyl)-pent...)Show SMILES CCCC[C@H](NC(=O)OCC1(CSc2nccc(n2)-c2ccc(Cl)cc2)CCC1)C(=O)C(=O)N[C@H](C)c1ccccc1 Show InChI InChI=1S/C32H37ClN4O4S/c1-3-4-11-27(28(38)29(39)35-22(2)23-9-6-5-7-10-23)37-31(40)41-20-32(17-8-18-32)21-42-30-34-19-16-26(36-30)24-12-14-25(33)15-13-24/h5-7,9-10,12-16,19,22,27H,3-4,8,11,17-18,20-21H2,1-2H3,(H,35,39)(H,37,40)/t22-,27+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 9.5 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibitory potency against human Cathepsin K |
J Med Chem 47: 5057-68 (2004)
Article DOI: 10.1021/jm040107n
BindingDB Entry DOI: 10.7270/Q23T9GP0 |
More data for this
Ligand-Target Pair | |
Cathepsin K
(Homo sapiens (Human)) | BDBM50154197
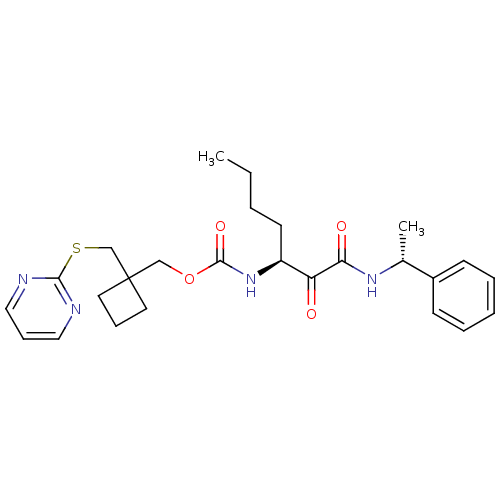
(CHEMBL186377 | [1-(1-Phenyl-ethylaminooxalyl)-pent...)Show SMILES CCCC[C@H](NC(=O)OCC1(CSc2ncccn2)CCC1)C(=O)C(=O)N[C@H](C)c1ccccc1 Show InChI InChI=1S/C26H34N4O4S/c1-3-4-12-21(22(31)23(32)29-19(2)20-10-6-5-7-11-20)30-25(33)34-17-26(13-8-14-26)18-35-24-27-15-9-16-28-24/h5-7,9-11,15-16,19,21H,3-4,8,12-14,17-18H2,1-2H3,(H,29,32)(H,30,33)/t19-,21+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibitory potency against human Cathepsin K |
J Med Chem 47: 5057-68 (2004)
Article DOI: 10.1021/jm040107n
BindingDB Entry DOI: 10.7270/Q23T9GP0 |
More data for this
Ligand-Target Pair | |
Cathepsin K
(Homo sapiens (Human)) | BDBM50154183
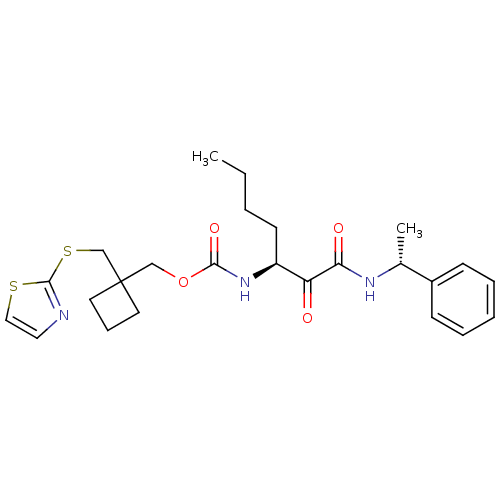
(CHEMBL359801 | [1-(1-Phenyl-ethylaminooxalyl)-pent...)Show SMILES CCCC[C@H](NC(=O)OCC1(CSc2nccs2)CCC1)C(=O)C(=O)N[C@H](C)c1ccccc1 Show InChI InChI=1S/C25H33N3O4S2/c1-3-4-11-20(21(29)22(30)27-18(2)19-9-6-5-7-10-19)28-23(31)32-16-25(12-8-13-25)17-34-24-26-14-15-33-24/h5-7,9-10,14-15,18,20H,3-4,8,11-13,16-17H2,1-2H3,(H,27,30)(H,28,31)/t18-,20+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 17 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibitory potency against human Cathepsin K |
J Med Chem 47: 5057-68 (2004)
Article DOI: 10.1021/jm040107n
BindingDB Entry DOI: 10.7270/Q23T9GP0 |
More data for this
Ligand-Target Pair | |
Cathepsin K
(Homo sapiens (Human)) | BDBM50154181
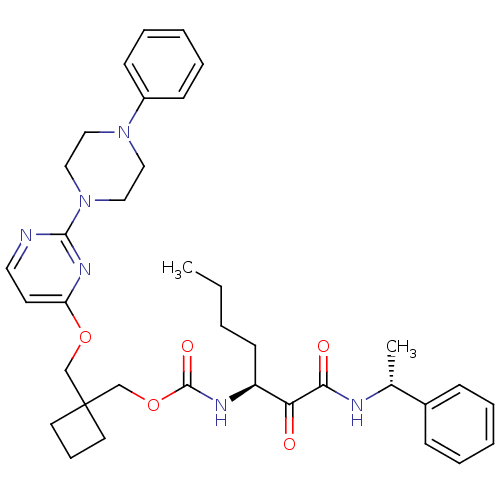
(CHEMBL187636 | [1-(1-Phenyl-ethylaminooxalyl)-pent...)Show SMILES CCCC[C@H](NC(=O)OCC1(COc2ccnc(n2)N2CCN(CC2)c2ccccc2)CCC1)C(=O)C(=O)N[C@H](C)c1ccccc1 Show InChI InChI=1S/C36H46N6O5/c1-3-4-16-30(32(43)33(44)38-27(2)28-12-7-5-8-13-28)39-35(45)47-26-36(18-11-19-36)25-46-31-17-20-37-34(40-31)42-23-21-41(22-24-42)29-14-9-6-10-15-29/h5-10,12-15,17,20,27,30H,3-4,11,16,18-19,21-26H2,1-2H3,(H,38,44)(H,39,45)/t27-,30+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 31 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibitory potency against human Cathepsin K |
J Med Chem 47: 5057-68 (2004)
Article DOI: 10.1021/jm040107n
BindingDB Entry DOI: 10.7270/Q23T9GP0 |
More data for this
Ligand-Target Pair | |
Cathepsin K
(Homo sapiens (Human)) | BDBM50154178
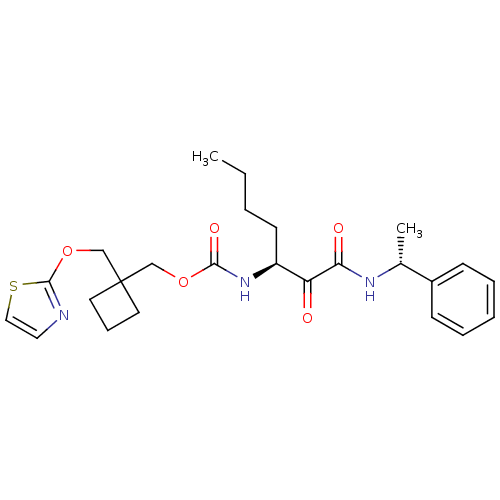
(CHEMBL188905 | [1-(1-Phenyl-ethylaminooxalyl)-pent...)Show SMILES CCCC[C@H](NC(=O)OCC1(COc2nccs2)CCC1)C(=O)C(=O)N[C@H](C)c1ccccc1 Show InChI InChI=1S/C25H33N3O5S/c1-3-4-11-20(21(29)22(30)27-18(2)19-9-6-5-7-10-19)28-23(31)32-16-25(12-8-13-25)17-33-24-26-14-15-34-24/h5-7,9-10,14-15,18,20H,3-4,8,11-13,16-17H2,1-2H3,(H,27,30)(H,28,31)/t18-,20+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 37 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibitory potency against human Cathepsin K |
J Med Chem 47: 5057-68 (2004)
Article DOI: 10.1021/jm040107n
BindingDB Entry DOI: 10.7270/Q23T9GP0 |
More data for this
Ligand-Target Pair | |
Cathepsin K
(Homo sapiens (Human)) | BDBM50154185
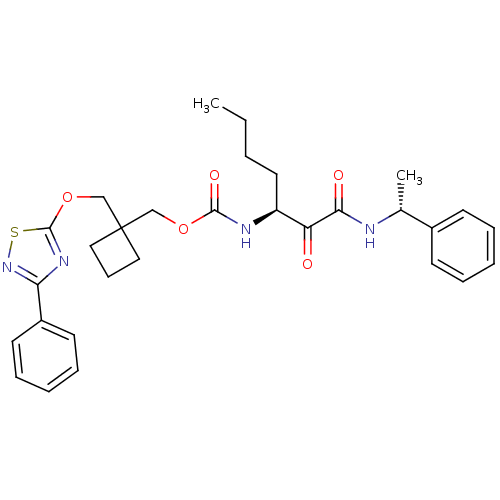
(CHEMBL189603 | [(S)-1-((R)-1-Phenyl-ethylaminooxal...)Show SMILES CCCC[C@H](NC(=O)OCC1(COc2nc(ns2)-c2ccccc2)CCC1)C(=O)C(=O)N[C@H](C)c1ccccc1 Show InChI InChI=1S/C30H36N4O5S/c1-3-4-16-24(25(35)27(36)31-21(2)22-12-7-5-8-13-22)32-28(37)38-19-30(17-11-18-30)20-39-29-33-26(34-40-29)23-14-9-6-10-15-23/h5-10,12-15,21,24H,3-4,11,16-20H2,1-2H3,(H,31,36)(H,32,37)/t21-,24+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibitory potency against human Cathepsin K |
J Med Chem 47: 5057-68 (2004)
Article DOI: 10.1021/jm040107n
BindingDB Entry DOI: 10.7270/Q23T9GP0 |
More data for this
Ligand-Target Pair | |
Cathepsin K
(Homo sapiens (Human)) | BDBM50154195
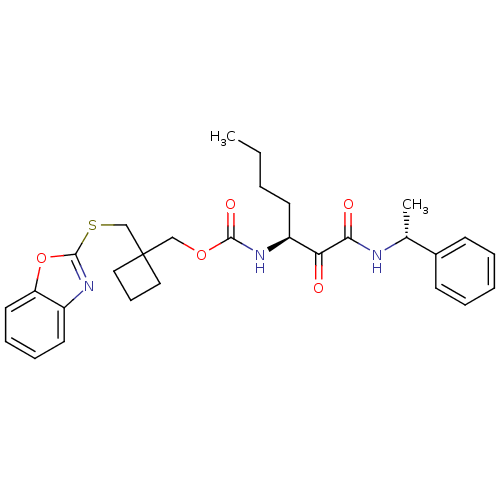
(CHEMBL360418 | [1-(1-Phenyl-ethylaminooxalyl)-pent...)Show SMILES CCCC[C@H](NC(=O)OCC1(CSc2nc3ccccc3o2)CCC1)C(=O)C(=O)N[C@H](C)c1ccccc1 Show InChI InChI=1S/C29H35N3O5S/c1-3-4-13-23(25(33)26(34)30-20(2)21-11-6-5-7-12-21)31-27(35)36-18-29(16-10-17-29)19-38-28-32-22-14-8-9-15-24(22)37-28/h5-9,11-12,14-15,20,23H,3-4,10,13,16-19H2,1-2H3,(H,30,34)(H,31,35)/t20-,23+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | 51 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibitory potency against human Cathepsin K |
J Med Chem 47: 5057-68 (2004)
Article DOI: 10.1021/jm040107n
BindingDB Entry DOI: 10.7270/Q23T9GP0 |
More data for this
Ligand-Target Pair | |
Cathepsin K
(Homo sapiens (Human)) | BDBM50154198
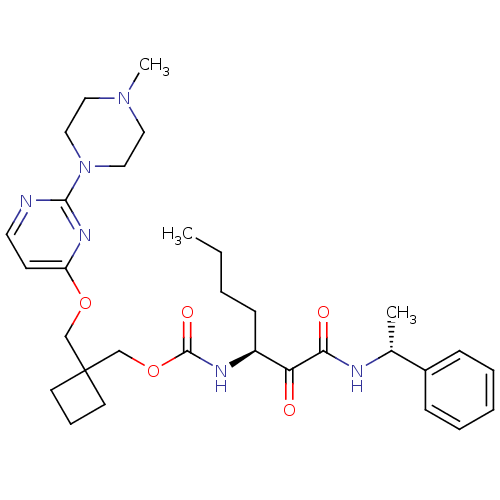
(CHEMBL364350 | [1-(1-Phenyl-ethylaminooxalyl)-pent...)Show SMILES CCCC[C@H](NC(=O)OCC1(COc2ccnc(n2)N2CCN(C)CC2)CCC1)C(=O)C(=O)N[C@H](C)c1ccccc1 Show InChI InChI=1S/C31H44N6O5/c1-4-5-12-25(27(38)28(39)33-23(2)24-10-7-6-8-11-24)34-30(40)42-22-31(14-9-15-31)21-41-26-13-16-32-29(35-26)37-19-17-36(3)18-20-37/h6-8,10-11,13,16,23,25H,4-5,9,12,14-15,17-22H2,1-3H3,(H,33,39)(H,34,40)/t23-,25+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 54 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibitory potency against human Cathepsin K |
J Med Chem 47: 5057-68 (2004)
Article DOI: 10.1021/jm040107n
BindingDB Entry DOI: 10.7270/Q23T9GP0 |
More data for this
Ligand-Target Pair | |
Cathepsin K
(Homo sapiens (Human)) | BDBM50154186
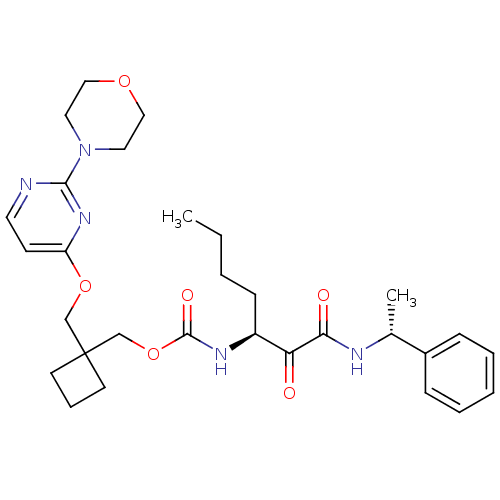
(CHEMBL188603 | [1-(1-Phenyl-ethylaminooxalyl)-pent...)Show SMILES CCCC[C@H](NC(=O)OCC1(COc2ccnc(n2)N2CCOCC2)CCC1)C(=O)C(=O)N[C@H](C)c1ccccc1 Show InChI InChI=1S/C30H41N5O6/c1-3-4-11-24(26(36)27(37)32-22(2)23-9-6-5-7-10-23)33-29(38)41-21-30(13-8-14-30)20-40-25-12-15-31-28(34-25)35-16-18-39-19-17-35/h5-7,9-10,12,15,22,24H,3-4,8,11,13-14,16-21H2,1-2H3,(H,32,37)(H,33,38)/t22-,24+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 54 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibitory potency against human Cathepsin K |
J Med Chem 47: 5057-68 (2004)
Article DOI: 10.1021/jm040107n
BindingDB Entry DOI: 10.7270/Q23T9GP0 |
More data for this
Ligand-Target Pair | |
Cathepsin K
(Homo sapiens (Human)) | BDBM50154193
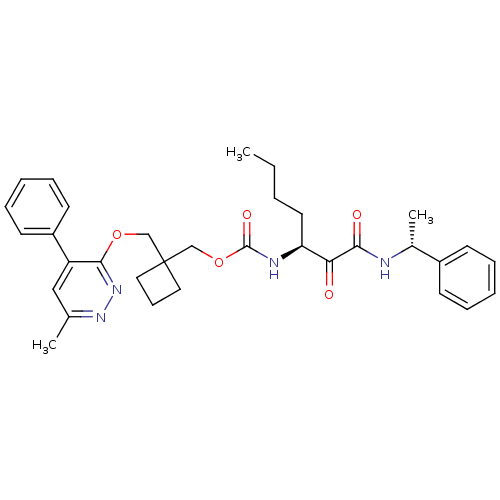
(CHEMBL186005 | [1-(1-Phenyl-ethylaminooxalyl)-pent...)Show SMILES CCCC[C@H](NC(=O)OCC1(COc2nnc(C)cc2-c2ccccc2)CCC1)C(=O)C(=O)N[C@H](C)c1ccccc1 Show InChI InChI=1S/C33H40N4O5/c1-4-5-17-28(29(38)30(39)34-24(3)25-13-8-6-9-14-25)35-32(40)42-22-33(18-12-19-33)21-41-31-27(20-23(2)36-37-31)26-15-10-7-11-16-26/h6-11,13-16,20,24,28H,4-5,12,17-19,21-22H2,1-3H3,(H,34,39)(H,35,40)/t24-,28+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | 57 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibitory potency against human Cathepsin K |
J Med Chem 47: 5057-68 (2004)
Article DOI: 10.1021/jm040107n
BindingDB Entry DOI: 10.7270/Q23T9GP0 |
More data for this
Ligand-Target Pair | |
Cathepsin K
(Homo sapiens (Human)) | BDBM50154177
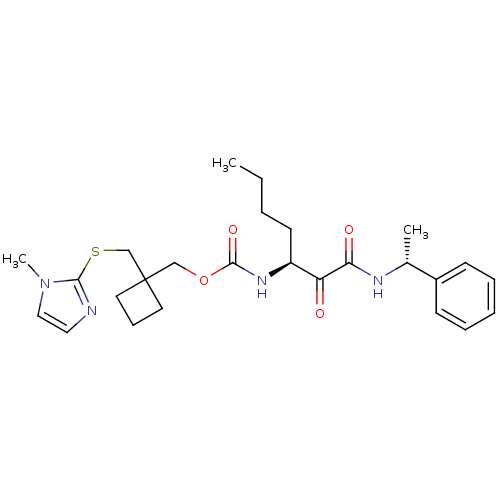
(CHEMBL184370 | [1-(1-Phenyl-ethylaminooxalyl)-pent...)Show SMILES CCCC[C@H](NC(=O)OCC1(CSc2nccn2C)CCC1)C(=O)C(=O)N[C@H](C)c1ccccc1 Show InChI InChI=1S/C26H36N4O4S/c1-4-5-12-21(22(31)23(32)28-19(2)20-10-7-6-8-11-20)29-25(33)34-17-26(13-9-14-26)18-35-24-27-15-16-30(24)3/h6-8,10-11,15-16,19,21H,4-5,9,12-14,17-18H2,1-3H3,(H,28,32)(H,29,33)/t19-,21+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 61 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibitory potency against human Cathepsin K |
J Med Chem 47: 5057-68 (2004)
Article DOI: 10.1021/jm040107n
BindingDB Entry DOI: 10.7270/Q23T9GP0 |
More data for this
Ligand-Target Pair | |
Cathepsin K
(Homo sapiens (Human)) | BDBM50154196
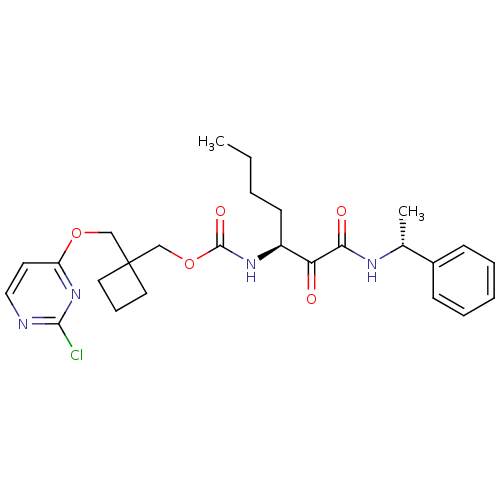
(CHEMBL188840 | [1-(1-Phenyl-ethylaminooxalyl)-pent...)Show SMILES CCCC[C@H](NC(=O)OCC1(COc2ccnc(Cl)n2)CCC1)C(=O)C(=O)N[C@H](C)c1ccccc1 Show InChI InChI=1S/C26H33ClN4O5/c1-3-4-11-20(22(32)23(33)29-18(2)19-9-6-5-7-10-19)30-25(34)36-17-26(13-8-14-26)16-35-21-12-15-28-24(27)31-21/h5-7,9-10,12,15,18,20H,3-4,8,11,13-14,16-17H2,1-2H3,(H,29,33)(H,30,34)/t18-,20+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 85 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibitory potency against human Cathepsin K |
J Med Chem 47: 5057-68 (2004)
Article DOI: 10.1021/jm040107n
BindingDB Entry DOI: 10.7270/Q23T9GP0 |
More data for this
Ligand-Target Pair | |
Cathepsin K
(Homo sapiens (Human)) | BDBM50154191
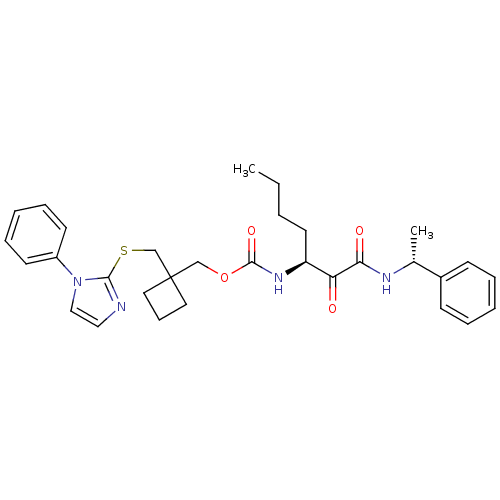
(CHEMBL364563 | [1-(1-Phenyl-ethylaminooxalyl)-pent...)Show SMILES CCCC[C@H](NC(=O)OCC1(CSc2nccn2-c2ccccc2)CCC1)C(=O)C(=O)N[C@H](C)c1ccccc1 Show InChI InChI=1S/C31H38N4O4S/c1-3-4-16-26(27(36)28(37)33-23(2)24-12-7-5-8-13-24)34-30(38)39-21-31(17-11-18-31)22-40-29-32-19-20-35(29)25-14-9-6-10-15-25/h5-10,12-15,19-20,23,26H,3-4,11,16-18,21-22H2,1-2H3,(H,33,37)(H,34,38)/t23-,26+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 87 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibitory potency against human Cathepsin K |
J Med Chem 47: 5057-68 (2004)
Article DOI: 10.1021/jm040107n
BindingDB Entry DOI: 10.7270/Q23T9GP0 |
More data for this
Ligand-Target Pair | |
Cathepsin K
(Homo sapiens (Human)) | BDBM50154190
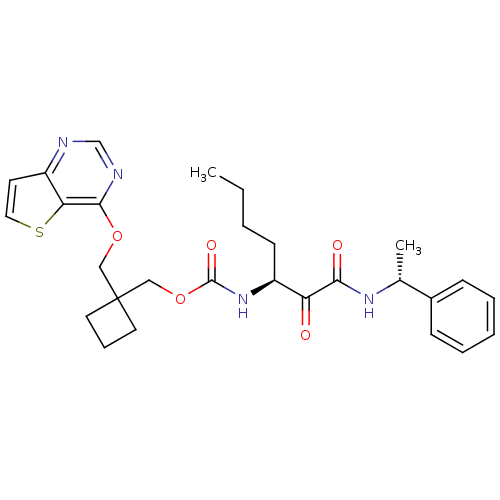
(CHEMBL186912 | [1-(1-Phenyl-ethylaminooxalyl)-pent...)Show SMILES CCCC[C@H](NC(=O)OCC1(COc2ncnc3ccsc23)CCC1)C(=O)C(=O)N[C@H](C)c1ccccc1 Show InChI InChI=1S/C28H34N4O5S/c1-3-4-11-21(23(33)25(34)31-19(2)20-9-6-5-7-10-20)32-27(35)37-17-28(13-8-14-28)16-36-26-24-22(12-15-38-24)29-18-30-26/h5-7,9-10,12,15,18-19,21H,3-4,8,11,13-14,16-17H2,1-2H3,(H,31,34)(H,32,35)/t19-,21+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | 98 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibitory potency against human Cathepsin K |
J Med Chem 47: 5057-68 (2004)
Article DOI: 10.1021/jm040107n
BindingDB Entry DOI: 10.7270/Q23T9GP0 |
More data for this
Ligand-Target Pair | |
Cathepsin K
(Homo sapiens (Human)) | BDBM50154179
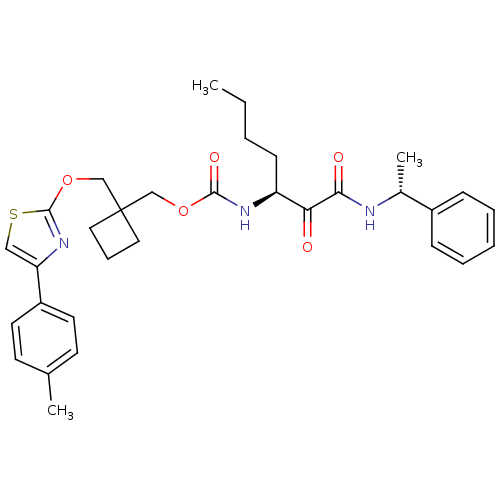
(CHEMBL362766 | [1-(1-Phenyl-ethylaminooxalyl)-pent...)Show SMILES CCCC[C@H](NC(=O)OCC1(COc2nc(cs2)-c2ccc(C)cc2)CCC1)C(=O)C(=O)N[C@H](C)c1ccccc1 Show InChI InChI=1S/C32H39N3O5S/c1-4-5-12-26(28(36)29(37)33-23(3)24-10-7-6-8-11-24)34-30(38)39-20-32(17-9-18-32)21-40-31-35-27(19-41-31)25-15-13-22(2)14-16-25/h6-8,10-11,13-16,19,23,26H,4-5,9,12,17-18,20-21H2,1-3H3,(H,33,37)(H,34,38)/t23-,26+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 150 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibitory potency against human Cathepsin K |
J Med Chem 47: 5057-68 (2004)
Article DOI: 10.1021/jm040107n
BindingDB Entry DOI: 10.7270/Q23T9GP0 |
More data for this
Ligand-Target Pair | |
Cathepsin K
(Homo sapiens (Human)) | BDBM50154192

(CHEMBL363538 | [1-(1-Phenyl-ethylaminooxalyl)-pent...)Show SMILES CCCC[C@H](NC(=O)OCC1(CSc2ncc(-c3ccc(Cl)cc3)n2C)CCC1)C(=O)C(=O)N[C@H](C)c1ccccc1 Show InChI InChI=1S/C32H39ClN4O4S/c1-4-5-12-26(28(38)29(39)35-22(2)23-10-7-6-8-11-23)36-31(40)41-20-32(17-9-18-32)21-42-30-34-19-27(37(30)3)24-13-15-25(33)16-14-24/h6-8,10-11,13-16,19,22,26H,4-5,9,12,17-18,20-21H2,1-3H3,(H,35,39)(H,36,40)/t22-,26+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 230 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibitory potency against human Cathepsin K |
J Med Chem 47: 5057-68 (2004)
Article DOI: 10.1021/jm040107n
BindingDB Entry DOI: 10.7270/Q23T9GP0 |
More data for this
Ligand-Target Pair | |
Cathepsin K
(Homo sapiens (Human)) | BDBM50154182
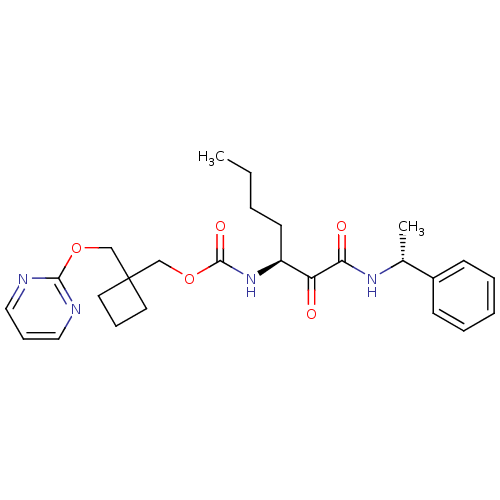
(CHEMBL188278 | [1-(1-Phenyl-ethylaminooxalyl)-pent...)Show SMILES CCCC[C@H](NC(=O)OCC1(COc2ncccn2)CCC1)C(=O)C(=O)N[C@H](C)c1ccccc1 Show InChI InChI=1S/C26H34N4O5/c1-3-4-12-21(22(31)23(32)29-19(2)20-10-6-5-7-11-20)30-25(33)35-18-26(13-8-14-26)17-34-24-27-15-9-16-28-24/h5-7,9-11,15-16,19,21H,3-4,8,12-14,17-18H2,1-2H3,(H,29,32)(H,30,33)/t19-,21+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | 260 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibitory potency against human Cathepsin K |
J Med Chem 47: 5057-68 (2004)
Article DOI: 10.1021/jm040107n
BindingDB Entry DOI: 10.7270/Q23T9GP0 |
More data for this
Ligand-Target Pair | |
Cathepsin K
(Homo sapiens (Human)) | BDBM50154184
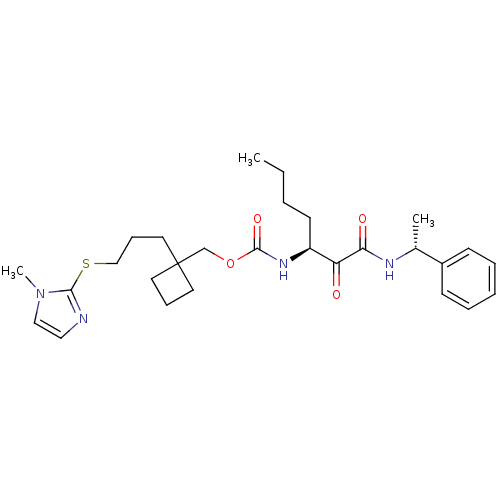
(CHEMBL188106 | [1-(1-Phenyl-ethylaminooxalyl)-pent...)Show SMILES CCCC[C@H](NC(=O)OCC1(CCCSc2nccn2C)CCC1)C(=O)C(=O)N[C@H](C)c1ccccc1 Show InChI InChI=1S/C28H40N4O4S/c1-4-5-13-23(24(33)25(34)30-21(2)22-11-7-6-8-12-22)31-27(35)36-20-28(14-9-15-28)16-10-19-37-26-29-17-18-32(26)3/h6-8,11-12,17-18,21,23H,4-5,9-10,13-16,19-20H2,1-3H3,(H,30,34)(H,31,35)/t21-,23+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibitory potency against human Cathepsin K |
J Med Chem 47: 5057-68 (2004)
Article DOI: 10.1021/jm040107n
BindingDB Entry DOI: 10.7270/Q23T9GP0 |
More data for this
Ligand-Target Pair | |
Cathepsin K
(Homo sapiens (Human)) | BDBM50154189
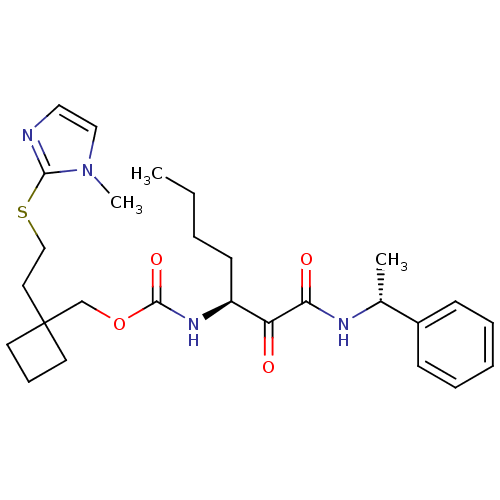
(CHEMBL184193 | [1-(1-Phenyl-ethylaminooxalyl)-pent...)Show SMILES CCCC[C@H](NC(=O)OCC1(CCSc2nccn2C)CCC1)C(=O)C(=O)N[C@H](C)c1ccccc1 Show InChI InChI=1S/C27H38N4O4S/c1-4-5-12-22(23(32)24(33)29-20(2)21-10-7-6-8-11-21)30-26(34)35-19-27(13-9-14-27)15-18-36-25-28-16-17-31(25)3/h6-8,10-11,16-17,20,22H,4-5,9,12-15,18-19H2,1-3H3,(H,29,33)(H,30,34)/t20-,22+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >1.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibitory potency against human Cathepsin K |
J Med Chem 47: 5057-68 (2004)
Article DOI: 10.1021/jm040107n
BindingDB Entry DOI: 10.7270/Q23T9GP0 |
More data for this
Ligand-Target Pair | |
Cathepsin K
(Homo sapiens (Human)) | BDBM50154188
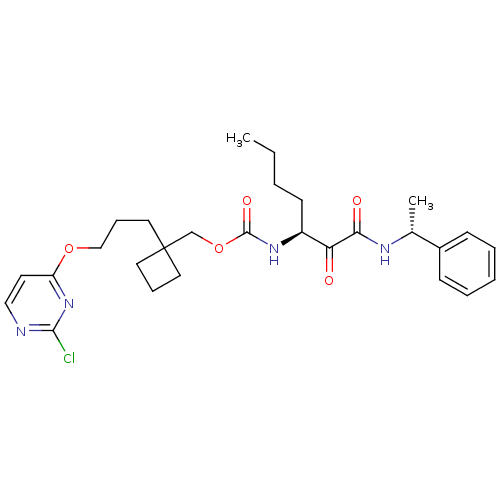
(CHEMBL188518 | [1-(1-Phenyl-ethylaminooxalyl)-pent...)Show SMILES CCCC[C@H](NC(=O)OCC1(CCCOc2ccnc(Cl)n2)CCC1)C(=O)C(=O)N[C@H](C)c1ccccc1 Show InChI InChI=1S/C28H37ClN4O5/c1-3-4-12-22(24(34)25(35)31-20(2)21-10-6-5-7-11-21)32-27(36)38-19-28(14-8-15-28)16-9-18-37-23-13-17-30-26(29)33-23/h5-7,10-11,13,17,20,22H,3-4,8-9,12,14-16,18-19H2,1-2H3,(H,31,35)(H,32,36)/t20-,22+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >1.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibitory potency against human Cathepsin K |
J Med Chem 47: 5057-68 (2004)
Article DOI: 10.1021/jm040107n
BindingDB Entry DOI: 10.7270/Q23T9GP0 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data