

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Tyrosine-protein phosphatase non-receptor type 1 (Homo sapiens (Human)) | BDBM84499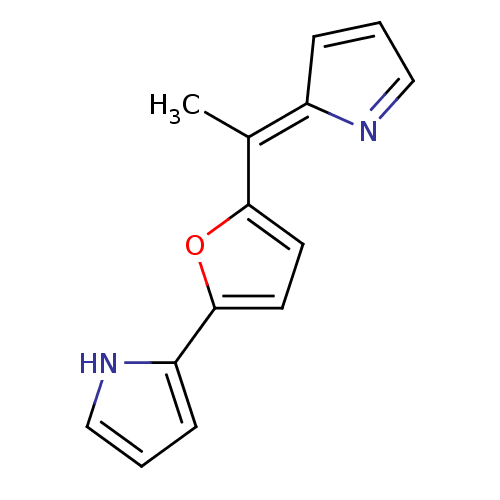 (2-(5-{1-[(2Z)-2H-pyrrol-2-ylidene]ethyl}furan-2-yl...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.30E+3 | n/a | n/a | n/a | n/a | 7.2 | 37 |
Max-Planck-Institut für Kohlenforschung | Assay Description Enzyme concentrations were chose to yield absorption changes of 0.2 OD at 405 nm within 80 min for 4-nitrophenyl phosphate (pNPP) in the absence of i... | Chembiochem 5: 1575-9 (2004) Article DOI: 10.1002/cbic.200400135 BindingDB Entry DOI: 10.7270/Q2QF8RCP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dual specificity protein phosphatase 3 (Homo sapiens (Human)) | BDBM84499 (2-(5-{1-[(2Z)-2H-pyrrol-2-ylidene]ethyl}furan-2-yl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.90E+3 | n/a | n/a | n/a | n/a | 6.5 | 37 |
Max-Planck-Institut für Kohlenforschung | Assay Description Enzyme concentrations were chose to yield absorption changes of 0.2 OD at 405 nm within 80 min for 4-nitrophenyl phosphate (pNPP) in the absence of i... | Chembiochem 5: 1575-9 (2004) Article DOI: 10.1002/cbic.200400135 BindingDB Entry DOI: 10.7270/Q2QF8RCP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 1 (Homo sapiens (Human)) | BDBM84498 (3-chloro-2-(5-{1-[(2Z)-2H-pyrrol-2-ylidene]ethyl}f...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6.00E+3 | n/a | n/a | n/a | n/a | 7.2 | 37 |
Max-Planck-Institut für Kohlenforschung | Assay Description Enzyme concentrations were chose to yield absorption changes of 0.2 OD at 405 nm within 80 min for 4-nitrophenyl phosphate (pNPP) in the absence of i... | Chembiochem 5: 1575-9 (2004) Article DOI: 10.1002/cbic.200400135 BindingDB Entry DOI: 10.7270/Q2QF8RCP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dual specificity protein phosphatase 3 (Homo sapiens (Human)) | BDBM84496 (Roseophilin, 2) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6.00E+3 | n/a | n/a | n/a | n/a | 6.5 | 37 |
Max-Planck-Institut für Kohlenforschung | Assay Description Enzyme concentrations were chose to yield absorption changes of 0.2 OD at 405 nm within 80 min for 4-nitrophenyl phosphate (pNPP) in the absence of i... | Chembiochem 5: 1575-9 (2004) Article DOI: 10.1002/cbic.200400135 BindingDB Entry DOI: 10.7270/Q2QF8RCP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 1 (Homo sapiens (Human)) | BDBM84496 (Roseophilin, 2) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6.00E+3 | n/a | n/a | n/a | n/a | 7.2 | 37 |
Max-Planck-Institut für Kohlenforschung | Assay Description Enzyme concentrations were chose to yield absorption changes of 0.2 OD at 405 nm within 80 min for 4-nitrophenyl phosphate (pNPP) in the absence of i... | Chembiochem 5: 1575-9 (2004) Article DOI: 10.1002/cbic.200400135 BindingDB Entry DOI: 10.7270/Q2QF8RCP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dual specificity protein phosphatase 3 (Homo sapiens (Human)) | BDBM84511 (Acyclic analogue, 16) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 8.00E+3 | n/a | n/a | n/a | n/a | 6.5 | 37 |
Max-Planck-Institut für Kohlenforschung | Assay Description Enzyme concentrations were chose to yield absorption changes of 0.2 OD at 405 nm within 80 min for 4-nitrophenyl phosphate (pNPP) in the absence of i... | Chembiochem 5: 1575-9 (2004) Article DOI: 10.1002/cbic.200400135 BindingDB Entry DOI: 10.7270/Q2QF8RCP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dual specificity protein phosphatase 3 (Homo sapiens (Human)) | BDBM84506 (Acyclic analogue, 12) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 9.00E+3 | n/a | n/a | n/a | n/a | 6.5 | 37 |
Max-Planck-Institut für Kohlenforschung | Assay Description Enzyme concentrations were chose to yield absorption changes of 0.2 OD at 405 nm within 80 min for 4-nitrophenyl phosphate (pNPP) in the absence of i... | Chembiochem 5: 1575-9 (2004) Article DOI: 10.1002/cbic.200400135 BindingDB Entry DOI: 10.7270/Q2QF8RCP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dual specificity protein phosphatase 3 (Homo sapiens (Human)) | BDBM84498 (3-chloro-2-(5-{1-[(2Z)-2H-pyrrol-2-ylidene]ethyl}f...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 9.00E+3 | n/a | n/a | n/a | n/a | 6.5 | 37 |
Max-Planck-Institut für Kohlenforschung | Assay Description Enzyme concentrations were chose to yield absorption changes of 0.2 OD at 405 nm within 80 min for 4-nitrophenyl phosphate (pNPP) in the absence of i... | Chembiochem 5: 1575-9 (2004) Article DOI: 10.1002/cbic.200400135 BindingDB Entry DOI: 10.7270/Q2QF8RCP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 1 (Homo sapiens (Human)) | BDBM84508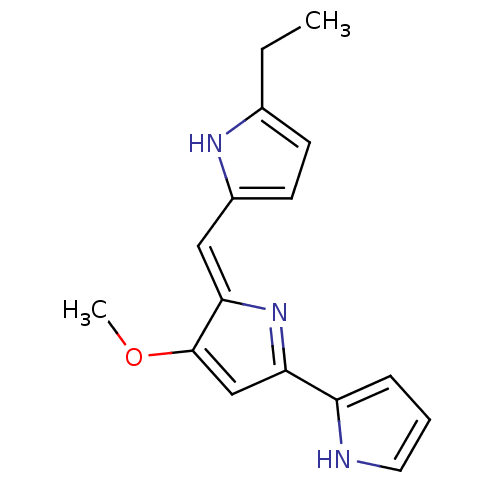 (Acyclic analogue, 14) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.00E+4 | n/a | n/a | n/a | n/a | 7.2 | 37 |
Max-Planck-Institut für Kohlenforschung | Assay Description Enzyme concentrations were chose to yield absorption changes of 0.2 OD at 405 nm within 80 min for 4-nitrophenyl phosphate (pNPP) in the absence of i... | Chembiochem 5: 1575-9 (2004) Article DOI: 10.1002/cbic.200400135 BindingDB Entry DOI: 10.7270/Q2QF8RCP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 1 (Homo sapiens (Human)) | BDBM84500 (Nonylprodigiosin, 6) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.00E+4 | n/a | n/a | n/a | n/a | 7.2 | 37 |
Max-Planck-Institut für Kohlenforschung | Assay Description Enzyme concentrations were chose to yield absorption changes of 0.2 OD at 405 nm within 80 min for 4-nitrophenyl phosphate (pNPP) in the absence of i... | Chembiochem 5: 1575-9 (2004) Article DOI: 10.1002/cbic.200400135 BindingDB Entry DOI: 10.7270/Q2QF8RCP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 1 (Homo sapiens (Human)) | BDBM84497 (Roseophilin analogue, 3) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.00E+4 | n/a | n/a | n/a | n/a | 7.2 | 37 |
Max-Planck-Institut für Kohlenforschung | Assay Description Enzyme concentrations were chose to yield absorption changes of 0.2 OD at 405 nm within 80 min for 4-nitrophenyl phosphate (pNPP) in the absence of i... | Chembiochem 5: 1575-9 (2004) Article DOI: 10.1002/cbic.200400135 BindingDB Entry DOI: 10.7270/Q2QF8RCP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 1 (Homo sapiens (Human)) | BDBM84507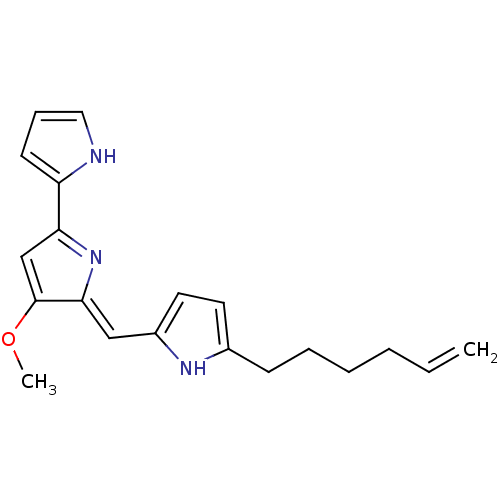 (Acyclic analogue, 13) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.10E+4 | n/a | n/a | n/a | n/a | 7.2 | 37 |
Max-Planck-Institut für Kohlenforschung | Assay Description Enzyme concentrations were chose to yield absorption changes of 0.2 OD at 405 nm within 80 min for 4-nitrophenyl phosphate (pNPP) in the absence of i... | Chembiochem 5: 1575-9 (2004) Article DOI: 10.1002/cbic.200400135 BindingDB Entry DOI: 10.7270/Q2QF8RCP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dual specificity protein phosphatase 3 (Homo sapiens (Human)) | BDBM84507 (Acyclic analogue, 13) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.10E+4 | n/a | n/a | n/a | n/a | 6.5 | 37 |
Max-Planck-Institut für Kohlenforschung | Assay Description Enzyme concentrations were chose to yield absorption changes of 0.2 OD at 405 nm within 80 min for 4-nitrophenyl phosphate (pNPP) in the absence of i... | Chembiochem 5: 1575-9 (2004) Article DOI: 10.1002/cbic.200400135 BindingDB Entry DOI: 10.7270/Q2QF8RCP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 1 (Homo sapiens (Human)) | BDBM84506 (Acyclic analogue, 12) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.20E+4 | n/a | n/a | n/a | n/a | 7.2 | 37 |
Max-Planck-Institut für Kohlenforschung | Assay Description Enzyme concentrations were chose to yield absorption changes of 0.2 OD at 405 nm within 80 min for 4-nitrophenyl phosphate (pNPP) in the absence of i... | Chembiochem 5: 1575-9 (2004) Article DOI: 10.1002/cbic.200400135 BindingDB Entry DOI: 10.7270/Q2QF8RCP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dual specificity protein phosphatase 3 (Homo sapiens (Human)) | BDBM84497 (Roseophilin analogue, 3) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.50E+4 | n/a | n/a | n/a | n/a | 6.5 | 37 |
Max-Planck-Institut für Kohlenforschung | Assay Description Enzyme concentrations were chose to yield absorption changes of 0.2 OD at 405 nm within 80 min for 4-nitrophenyl phosphate (pNPP) in the absence of i... | Chembiochem 5: 1575-9 (2004) Article DOI: 10.1002/cbic.200400135 BindingDB Entry DOI: 10.7270/Q2QF8RCP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 1 (Homo sapiens (Human)) | BDBM84512 (Acyclic analogue, 18) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.60E+4 | n/a | n/a | n/a | n/a | 7.2 | 37 |
Max-Planck-Institut für Kohlenforschung | Assay Description Enzyme concentrations were chose to yield absorption changes of 0.2 OD at 405 nm within 80 min for 4-nitrophenyl phosphate (pNPP) in the absence of i... | Chembiochem 5: 1575-9 (2004) Article DOI: 10.1002/cbic.200400135 BindingDB Entry DOI: 10.7270/Q2QF8RCP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| M-phase inducer phosphatase 1 (Homo sapiens (Human)) | BDBM84496 (Roseophilin, 2) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.10E+4 | n/a | n/a | n/a | n/a | 8.0 | 37 |
Max-Planck-Institut für Kohlenforschung | Assay Description Enzyme concentrations were chose to yield absorption changes of 0.2 OD at 405 nm within 80 min for 4-nitrophenyl phosphate (pNPP) in the absence of i... | Chembiochem 5: 1575-9 (2004) Article DOI: 10.1002/cbic.200400135 BindingDB Entry DOI: 10.7270/Q2QF8RCP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dual specificity protein phosphatase 3 (Homo sapiens (Human)) | BDBM84512 (Acyclic analogue, 18) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.10E+4 | n/a | n/a | n/a | n/a | 6.5 | 37 |
Max-Planck-Institut für Kohlenforschung | Assay Description Enzyme concentrations were chose to yield absorption changes of 0.2 OD at 405 nm within 80 min for 4-nitrophenyl phosphate (pNPP) in the absence of i... | Chembiochem 5: 1575-9 (2004) Article DOI: 10.1002/cbic.200400135 BindingDB Entry DOI: 10.7270/Q2QF8RCP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dual specificity protein phosphatase 3 (Homo sapiens (Human)) | BDBM84500 (Nonylprodigiosin, 6) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.10E+4 | n/a | n/a | n/a | n/a | 6.5 | 37 |
Max-Planck-Institut für Kohlenforschung | Assay Description Enzyme concentrations were chose to yield absorption changes of 0.2 OD at 405 nm within 80 min for 4-nitrophenyl phosphate (pNPP) in the absence of i... | Chembiochem 5: 1575-9 (2004) Article DOI: 10.1002/cbic.200400135 BindingDB Entry DOI: 10.7270/Q2QF8RCP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dual specificity protein phosphatase 3 (Homo sapiens (Human)) | BDBM84508 (Acyclic analogue, 14) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.10E+4 | n/a | n/a | n/a | n/a | 6.5 | 37 |
Max-Planck-Institut für Kohlenforschung | Assay Description Enzyme concentrations were chose to yield absorption changes of 0.2 OD at 405 nm within 80 min for 4-nitrophenyl phosphate (pNPP) in the absence of i... | Chembiochem 5: 1575-9 (2004) Article DOI: 10.1002/cbic.200400135 BindingDB Entry DOI: 10.7270/Q2QF8RCP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 1 (Homo sapiens (Human)) | BDBM84501 (Cyclic analogue, 7) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.30E+4 | n/a | n/a | n/a | n/a | 7.2 | 37 |
Max-Planck-Institut für Kohlenforschung | Assay Description Enzyme concentrations were chose to yield absorption changes of 0.2 OD at 405 nm within 80 min for 4-nitrophenyl phosphate (pNPP) in the absence of i... | Chembiochem 5: 1575-9 (2004) Article DOI: 10.1002/cbic.200400135 BindingDB Entry DOI: 10.7270/Q2QF8RCP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 1 (Homo sapiens (Human)) | BDBM84502 (Cyclic analogue, 8) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.40E+4 | n/a | n/a | n/a | n/a | 7.2 | 37 |
Max-Planck-Institut für Kohlenforschung | Assay Description Enzyme concentrations were chose to yield absorption changes of 0.2 OD at 405 nm within 80 min for 4-nitrophenyl phosphate (pNPP) in the absence of i... | Chembiochem 5: 1575-9 (2004) Article DOI: 10.1002/cbic.200400135 BindingDB Entry DOI: 10.7270/Q2QF8RCP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 1 (Homo sapiens (Human)) | BDBM84509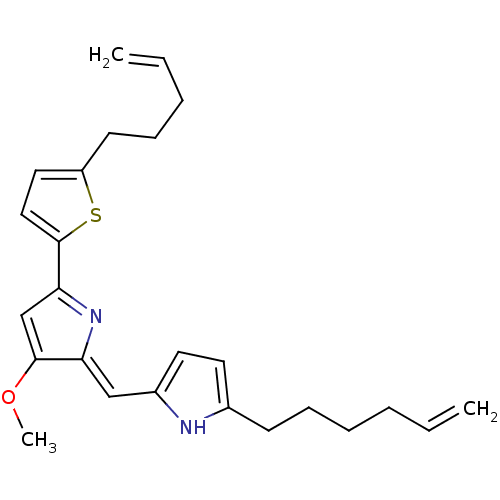 (Acyclic analogue, 15) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.80E+4 | n/a | n/a | n/a | n/a | 7.2 | 37 |
Max-Planck-Institut für Kohlenforschung | Assay Description Enzyme concentrations were chose to yield absorption changes of 0.2 OD at 405 nm within 80 min for 4-nitrophenyl phosphate (pNPP) in the absence of i... | Chembiochem 5: 1575-9 (2004) Article DOI: 10.1002/cbic.200400135 BindingDB Entry DOI: 10.7270/Q2QF8RCP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| M-phase inducer phosphatase 1 (Homo sapiens (Human)) | BDBM84495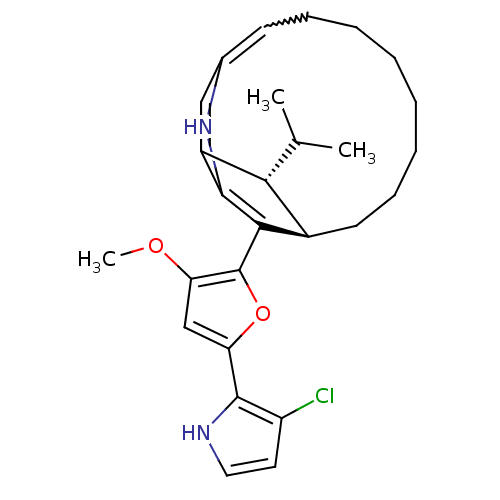 (CHEMBL1945189 | Roseophilin, 1) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.80E+4 | n/a | n/a | n/a | n/a | 8.0 | 37 |
Max-Planck-Institut für Kohlenforschung | Assay Description Enzyme concentrations were chose to yield absorption changes of 0.2 OD at 405 nm within 80 min for 4-nitrophenyl phosphate (pNPP) in the absence of i... | Chembiochem 5: 1575-9 (2004) Article DOI: 10.1002/cbic.200400135 BindingDB Entry DOI: 10.7270/Q2QF8RCP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dual specificity protein phosphatase 3 (Homo sapiens (Human)) | BDBM84501 (Cyclic analogue, 7) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.80E+4 | n/a | n/a | n/a | n/a | 6.5 | 37 |
Max-Planck-Institut für Kohlenforschung | Assay Description Enzyme concentrations were chose to yield absorption changes of 0.2 OD at 405 nm within 80 min for 4-nitrophenyl phosphate (pNPP) in the absence of i... | Chembiochem 5: 1575-9 (2004) Article DOI: 10.1002/cbic.200400135 BindingDB Entry DOI: 10.7270/Q2QF8RCP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dual specificity protein phosphatase 3 (Homo sapiens (Human)) | BDBM84503 (Cyclic analogue, 9) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.00E+4 | n/a | n/a | n/a | n/a | 6.5 | 37 |
Max-Planck-Institut für Kohlenforschung | Assay Description Enzyme concentrations were chose to yield absorption changes of 0.2 OD at 405 nm within 80 min for 4-nitrophenyl phosphate (pNPP) in the absence of i... | Chembiochem 5: 1575-9 (2004) Article DOI: 10.1002/cbic.200400135 BindingDB Entry DOI: 10.7270/Q2QF8RCP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dual specificity protein phosphatase 3 (Homo sapiens (Human)) | BDBM84495 (CHEMBL1945189 | Roseophilin, 1) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.20E+4 | n/a | n/a | n/a | n/a | 6.5 | 37 |
Max-Planck-Institut für Kohlenforschung | Assay Description Enzyme concentrations were chose to yield absorption changes of 0.2 OD at 405 nm within 80 min for 4-nitrophenyl phosphate (pNPP) in the absence of i... | Chembiochem 5: 1575-9 (2004) Article DOI: 10.1002/cbic.200400135 BindingDB Entry DOI: 10.7270/Q2QF8RCP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 1 (Homo sapiens (Human)) | BDBM84505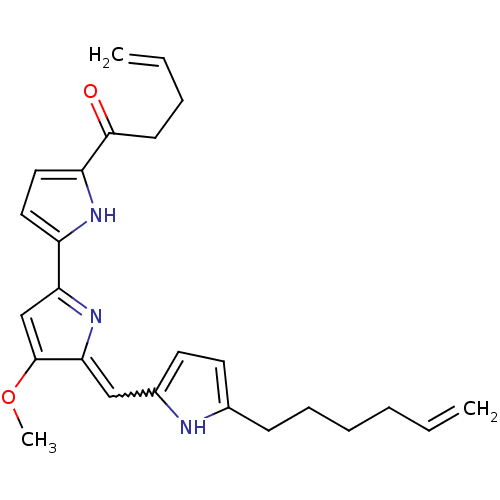 (Acyclic analogue, 11) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.20E+4 | n/a | n/a | n/a | n/a | 7.2 | 37 |
Max-Planck-Institut für Kohlenforschung | Assay Description Enzyme concentrations were chose to yield absorption changes of 0.2 OD at 405 nm within 80 min for 4-nitrophenyl phosphate (pNPP) in the absence of i... | Chembiochem 5: 1575-9 (2004) Article DOI: 10.1002/cbic.200400135 BindingDB Entry DOI: 10.7270/Q2QF8RCP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| M-phase inducer phosphatase 1 (Homo sapiens (Human)) | BDBM84498 (3-chloro-2-(5-{1-[(2Z)-2H-pyrrol-2-ylidene]ethyl}f...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.40E+4 | n/a | n/a | n/a | n/a | 8.0 | 37 |
Max-Planck-Institut für Kohlenforschung | Assay Description Enzyme concentrations were chose to yield absorption changes of 0.2 OD at 405 nm within 80 min for 4-nitrophenyl phosphate (pNPP) in the absence of i... | Chembiochem 5: 1575-9 (2004) Article DOI: 10.1002/cbic.200400135 BindingDB Entry DOI: 10.7270/Q2QF8RCP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 1 (Homo sapiens (Human)) | BDBM84495 (CHEMBL1945189 | Roseophilin, 1) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.80E+4 | n/a | n/a | n/a | n/a | 7.2 | 37 |
Max-Planck-Institut für Kohlenforschung | Assay Description Enzyme concentrations were chose to yield absorption changes of 0.2 OD at 405 nm within 80 min for 4-nitrophenyl phosphate (pNPP) in the absence of i... | Chembiochem 5: 1575-9 (2004) Article DOI: 10.1002/cbic.200400135 BindingDB Entry DOI: 10.7270/Q2QF8RCP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dual specificity protein phosphatase 3 (Homo sapiens (Human)) | BDBM84509 (Acyclic analogue, 15) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3.90E+4 | n/a | n/a | n/a | n/a | 6.5 | 37 |
Max-Planck-Institut für Kohlenforschung | Assay Description Enzyme concentrations were chose to yield absorption changes of 0.2 OD at 405 nm within 80 min for 4-nitrophenyl phosphate (pNPP) in the absence of i... | Chembiochem 5: 1575-9 (2004) Article DOI: 10.1002/cbic.200400135 BindingDB Entry DOI: 10.7270/Q2QF8RCP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| M-phase inducer phosphatase 1 (Homo sapiens (Human)) | BDBM84499 (2-(5-{1-[(2Z)-2H-pyrrol-2-ylidene]ethyl}furan-2-yl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.00E+4 | n/a | n/a | n/a | n/a | 8.0 | 37 |
Max-Planck-Institut für Kohlenforschung | Assay Description Enzyme concentrations were chose to yield absorption changes of 0.2 OD at 405 nm within 80 min for 4-nitrophenyl phosphate (pNPP) in the absence of i... | Chembiochem 5: 1575-9 (2004) Article DOI: 10.1002/cbic.200400135 BindingDB Entry DOI: 10.7270/Q2QF8RCP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dual specificity protein phosphatase 3 (Homo sapiens (Human)) | BDBM84502 (Cyclic analogue, 8) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.10E+4 | n/a | n/a | n/a | n/a | 6.5 | 37 |
Max-Planck-Institut für Kohlenforschung | Assay Description Enzyme concentrations were chose to yield absorption changes of 0.2 OD at 405 nm within 80 min for 4-nitrophenyl phosphate (pNPP) in the absence of i... | Chembiochem 5: 1575-9 (2004) Article DOI: 10.1002/cbic.200400135 BindingDB Entry DOI: 10.7270/Q2QF8RCP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 1 (Homo sapiens (Human)) | BDBM84511 (Acyclic analogue, 16) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.60E+4 | n/a | n/a | n/a | n/a | 7.2 | 37 |
Max-Planck-Institut für Kohlenforschung | Assay Description Enzyme concentrations were chose to yield absorption changes of 0.2 OD at 405 nm within 80 min for 4-nitrophenyl phosphate (pNPP) in the absence of i... | Chembiochem 5: 1575-9 (2004) Article DOI: 10.1002/cbic.200400135 BindingDB Entry DOI: 10.7270/Q2QF8RCP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dual specificity protein phosphatase 3 (Homo sapiens (Human)) | BDBM84504 (Acyclic analogue, 10) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | 6.5 | 37 |
Max-Planck-Institut für Kohlenforschung | Assay Description Enzyme concentrations were chose to yield absorption changes of 0.2 OD at 405 nm within 80 min for 4-nitrophenyl phosphate (pNPP) in the absence of i... | Chembiochem 5: 1575-9 (2004) Article DOI: 10.1002/cbic.200400135 BindingDB Entry DOI: 10.7270/Q2QF8RCP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| M-phase inducer phosphatase 1 (Homo sapiens (Human)) | BDBM84510 (Acyclic analogue, 17) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | 8.0 | 37 |
Max-Planck-Institut für Kohlenforschung | Assay Description Enzyme concentrations were chose to yield absorption changes of 0.2 OD at 405 nm within 80 min for 4-nitrophenyl phosphate (pNPP) in the absence of i... | Chembiochem 5: 1575-9 (2004) Article DOI: 10.1002/cbic.200400135 BindingDB Entry DOI: 10.7270/Q2QF8RCP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| M-phase inducer phosphatase 1 (Homo sapiens (Human)) | BDBM84509 (Acyclic analogue, 15) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | 8.0 | 37 |
Max-Planck-Institut für Kohlenforschung | Assay Description Enzyme concentrations were chose to yield absorption changes of 0.2 OD at 405 nm within 80 min for 4-nitrophenyl phosphate (pNPP) in the absence of i... | Chembiochem 5: 1575-9 (2004) Article DOI: 10.1002/cbic.200400135 BindingDB Entry DOI: 10.7270/Q2QF8RCP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| M-phase inducer phosphatase 1 (Homo sapiens (Human)) | BDBM84501 (Cyclic analogue, 7) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | 8.0 | 37 |
Max-Planck-Institut für Kohlenforschung | Assay Description Enzyme concentrations were chose to yield absorption changes of 0.2 OD at 405 nm within 80 min for 4-nitrophenyl phosphate (pNPP) in the absence of i... | Chembiochem 5: 1575-9 (2004) Article DOI: 10.1002/cbic.200400135 BindingDB Entry DOI: 10.7270/Q2QF8RCP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dual specificity protein phosphatase 3 (Homo sapiens (Human)) | BDBM84505 (Acyclic analogue, 11) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | 6.5 | 37 |
Max-Planck-Institut für Kohlenforschung | Assay Description Enzyme concentrations were chose to yield absorption changes of 0.2 OD at 405 nm within 80 min for 4-nitrophenyl phosphate (pNPP) in the absence of i... | Chembiochem 5: 1575-9 (2004) Article DOI: 10.1002/cbic.200400135 BindingDB Entry DOI: 10.7270/Q2QF8RCP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 1 (Homo sapiens (Human)) | BDBM84504 (Acyclic analogue, 10) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | 7.2 | 37 |
Max-Planck-Institut für Kohlenforschung | Assay Description Enzyme concentrations were chose to yield absorption changes of 0.2 OD at 405 nm within 80 min for 4-nitrophenyl phosphate (pNPP) in the absence of i... | Chembiochem 5: 1575-9 (2004) Article DOI: 10.1002/cbic.200400135 BindingDB Entry DOI: 10.7270/Q2QF8RCP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| M-phase inducer phosphatase 1 (Homo sapiens (Human)) | BDBM84507 (Acyclic analogue, 13) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | 8.0 | 37 |
Max-Planck-Institut für Kohlenforschung | Assay Description Enzyme concentrations were chose to yield absorption changes of 0.2 OD at 405 nm within 80 min for 4-nitrophenyl phosphate (pNPP) in the absence of i... | Chembiochem 5: 1575-9 (2004) Article DOI: 10.1002/cbic.200400135 BindingDB Entry DOI: 10.7270/Q2QF8RCP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| M-phase inducer phosphatase 1 (Homo sapiens (Human)) | BDBM84508 (Acyclic analogue, 14) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | 8.0 | 37 |
Max-Planck-Institut für Kohlenforschung | Assay Description Enzyme concentrations were chose to yield absorption changes of 0.2 OD at 405 nm within 80 min for 4-nitrophenyl phosphate (pNPP) in the absence of i... | Chembiochem 5: 1575-9 (2004) Article DOI: 10.1002/cbic.200400135 BindingDB Entry DOI: 10.7270/Q2QF8RCP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| M-phase inducer phosphatase 1 (Homo sapiens (Human)) | BDBM84503 (Cyclic analogue, 9) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | 8.0 | 37 |
Max-Planck-Institut für Kohlenforschung | Assay Description Enzyme concentrations were chose to yield absorption changes of 0.2 OD at 405 nm within 80 min for 4-nitrophenyl phosphate (pNPP) in the absence of i... | Chembiochem 5: 1575-9 (2004) Article DOI: 10.1002/cbic.200400135 BindingDB Entry DOI: 10.7270/Q2QF8RCP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| M-phase inducer phosphatase 1 (Homo sapiens (Human)) | BDBM84497 (Roseophilin analogue, 3) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | 8.0 | 37 |
Max-Planck-Institut für Kohlenforschung | Assay Description Enzyme concentrations were chose to yield absorption changes of 0.2 OD at 405 nm within 80 min for 4-nitrophenyl phosphate (pNPP) in the absence of i... | Chembiochem 5: 1575-9 (2004) Article DOI: 10.1002/cbic.200400135 BindingDB Entry DOI: 10.7270/Q2QF8RCP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| M-phase inducer phosphatase 1 (Homo sapiens (Human)) | BDBM84511 (Acyclic analogue, 16) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | 8.0 | 37 |
Max-Planck-Institut für Kohlenforschung | Assay Description Enzyme concentrations were chose to yield absorption changes of 0.2 OD at 405 nm within 80 min for 4-nitrophenyl phosphate (pNPP) in the absence of i... | Chembiochem 5: 1575-9 (2004) Article DOI: 10.1002/cbic.200400135 BindingDB Entry DOI: 10.7270/Q2QF8RCP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dual specificity protein phosphatase 3 (Homo sapiens (Human)) | BDBM84510 (Acyclic analogue, 17) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | 6.5 | 37 |
Max-Planck-Institut für Kohlenforschung | Assay Description Enzyme concentrations were chose to yield absorption changes of 0.2 OD at 405 nm within 80 min for 4-nitrophenyl phosphate (pNPP) in the absence of i... | Chembiochem 5: 1575-9 (2004) Article DOI: 10.1002/cbic.200400135 BindingDB Entry DOI: 10.7270/Q2QF8RCP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| M-phase inducer phosphatase 1 (Homo sapiens (Human)) | BDBM84504 (Acyclic analogue, 10) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | 8.0 | 37 |
Max-Planck-Institut für Kohlenforschung | Assay Description Enzyme concentrations were chose to yield absorption changes of 0.2 OD at 405 nm within 80 min for 4-nitrophenyl phosphate (pNPP) in the absence of i... | Chembiochem 5: 1575-9 (2004) Article DOI: 10.1002/cbic.200400135 BindingDB Entry DOI: 10.7270/Q2QF8RCP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| M-phase inducer phosphatase 1 (Homo sapiens (Human)) | BDBM84502 (Cyclic analogue, 8) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | 8.0 | 37 |
Max-Planck-Institut für Kohlenforschung | Assay Description Enzyme concentrations were chose to yield absorption changes of 0.2 OD at 405 nm within 80 min for 4-nitrophenyl phosphate (pNPP) in the absence of i... | Chembiochem 5: 1575-9 (2004) Article DOI: 10.1002/cbic.200400135 BindingDB Entry DOI: 10.7270/Q2QF8RCP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| M-phase inducer phosphatase 1 (Homo sapiens (Human)) | BDBM84512 (Acyclic analogue, 18) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | 8.0 | 37 |
Max-Planck-Institut für Kohlenforschung | Assay Description Enzyme concentrations were chose to yield absorption changes of 0.2 OD at 405 nm within 80 min for 4-nitrophenyl phosphate (pNPP) in the absence of i... | Chembiochem 5: 1575-9 (2004) Article DOI: 10.1002/cbic.200400135 BindingDB Entry DOI: 10.7270/Q2QF8RCP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 1 (Homo sapiens (Human)) | BDBM84510 (Acyclic analogue, 17) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | 7.2 | 37 |
Max-Planck-Institut für Kohlenforschung | Assay Description Enzyme concentrations were chose to yield absorption changes of 0.2 OD at 405 nm within 80 min for 4-nitrophenyl phosphate (pNPP) in the absence of i... | Chembiochem 5: 1575-9 (2004) Article DOI: 10.1002/cbic.200400135 BindingDB Entry DOI: 10.7270/Q2QF8RCP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 1 (Homo sapiens (Human)) | BDBM84503 (Cyclic analogue, 9) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | 7.2 | 37 |
Max-Planck-Institut für Kohlenforschung | Assay Description Enzyme concentrations were chose to yield absorption changes of 0.2 OD at 405 nm within 80 min for 4-nitrophenyl phosphate (pNPP) in the absence of i... | Chembiochem 5: 1575-9 (2004) Article DOI: 10.1002/cbic.200400135 BindingDB Entry DOI: 10.7270/Q2QF8RCP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| M-phase inducer phosphatase 1 (Homo sapiens (Human)) | BDBM84505 (Acyclic analogue, 11) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | 8.0 | 37 |
Max-Planck-Institut für Kohlenforschung | Assay Description Enzyme concentrations were chose to yield absorption changes of 0.2 OD at 405 nm within 80 min for 4-nitrophenyl phosphate (pNPP) in the absence of i... | Chembiochem 5: 1575-9 (2004) Article DOI: 10.1002/cbic.200400135 BindingDB Entry DOI: 10.7270/Q2QF8RCP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| M-phase inducer phosphatase 1 (Homo sapiens (Human)) | BDBM84500 (Nonylprodigiosin, 6) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | 8.0 | 37 |
Max-Planck-Institut für Kohlenforschung | Assay Description Enzyme concentrations were chose to yield absorption changes of 0.2 OD at 405 nm within 80 min for 4-nitrophenyl phosphate (pNPP) in the absence of i... | Chembiochem 5: 1575-9 (2004) Article DOI: 10.1002/cbic.200400135 BindingDB Entry DOI: 10.7270/Q2QF8RCP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| M-phase inducer phosphatase 1 (Homo sapiens (Human)) | BDBM84506 (Acyclic analogue, 12) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | 8.0 | 37 |
Max-Planck-Institut für Kohlenforschung | Assay Description Enzyme concentrations were chose to yield absorption changes of 0.2 OD at 405 nm within 80 min for 4-nitrophenyl phosphate (pNPP) in the absence of i... | Chembiochem 5: 1575-9 (2004) Article DOI: 10.1002/cbic.200400135 BindingDB Entry DOI: 10.7270/Q2QF8RCP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||