 Found 21 hits of Enzyme Inhibition Constant Data
Found 21 hits of Enzyme Inhibition Constant Data Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Histamine H1 receptor
(Homo sapiens (Human)) | BDBM50155336
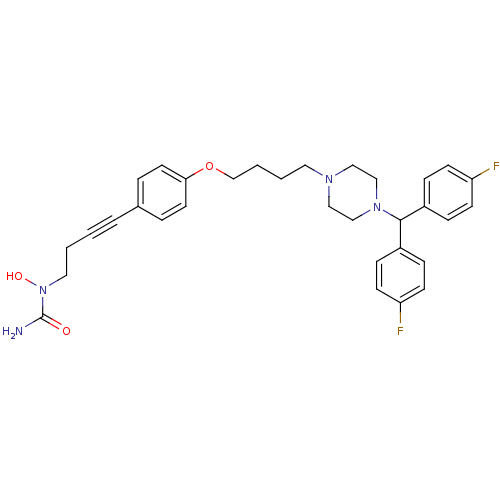
(1-{4-[4-(4-{4-[bis(4-fluorophenyl)methyl]piperazin...)Show SMILES NC(=O)N(O)CCC#Cc1ccc(OCCCCN2CCN(CC2)C(c2ccc(F)cc2)c2ccc(F)cc2)cc1 Show InChI InChI=1S/C32H36F2N4O3/c33-28-12-8-26(9-13-28)31(27-10-14-29(34)15-11-27)37-22-20-36(21-23-37)18-3-4-24-41-30-16-6-25(7-17-30)5-1-2-19-38(40)32(35)39/h6-17,31,40H,2-4,18-24H2,(H2,35,39) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 3.63 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
UCB Research
Curated by ChEMBL
| Assay Description
Binding affinity for recombinant human Histamine H1 receptor expressed in CHO K1 cells |
Bioorg Med Chem Lett 15: 1083-5 (2005)
Article DOI: 10.1016/j.bmcl.2004.12.023
BindingDB Entry DOI: 10.7270/Q2PN954N |
More data for this
Ligand-Target Pair | |
Histamine H1 receptor
(Homo sapiens (Human)) | BDBM50160828

(CHEMBL366925 | N-hydroxycarbamate derivative)Show SMILES COC(=O)N(O)CCC#Cc1ccc(OCCCCN2CCN(CC2)C(c2ccc(F)cc2)c2ccc(F)cc2)cc1 Show InChI InChI=1S/C33H37F2N3O4/c1-41-33(39)38(40)20-3-2-6-26-7-17-31(18-8-26)42-25-5-4-19-36-21-23-37(24-22-36)32(27-9-13-29(34)14-10-27)28-11-15-30(35)16-12-28/h7-18,32,40H,3-5,19-25H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 5.01 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
UCB Research
Curated by ChEMBL
| Assay Description
Binding affinity for human Histamine H1 receptor in CHO K1 cells |
Bioorg Med Chem Lett 15: 1083-5 (2005)
Article DOI: 10.1016/j.bmcl.2004.12.023
BindingDB Entry DOI: 10.7270/Q2PN954N |
More data for this
Ligand-Target Pair | |
Histamine H1 receptor
(Homo sapiens (Human)) | BDBM50160827
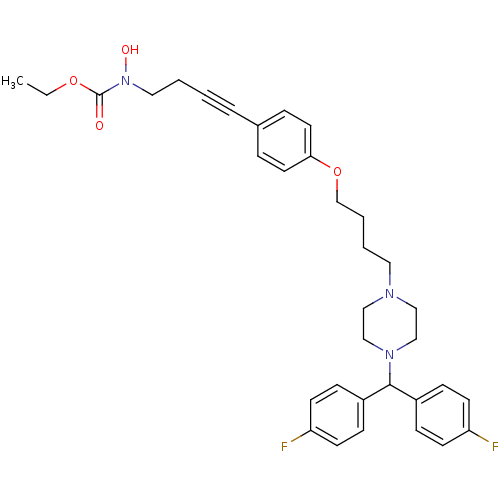
(CHEMBL179590 | N-hydroxycarbamate derivative)Show SMILES CCOC(=O)N(O)CCC#Cc1ccc(OCCCCN2CCN(CC2)C(c2ccc(F)cc2)c2ccc(F)cc2)cc1 Show InChI InChI=1S/C34H39F2N3O4/c1-2-42-34(40)39(41)21-4-3-7-27-8-18-32(19-9-27)43-26-6-5-20-37-22-24-38(25-23-37)33(28-10-14-30(35)15-11-28)29-12-16-31(36)17-13-29/h8-19,33,41H,2,4-6,20-26H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 5.89 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
UCB Research
Curated by ChEMBL
| Assay Description
Binding affinity for human Histamine H1 receptor in CHO K1 cells |
Bioorg Med Chem Lett 15: 1083-5 (2005)
Article DOI: 10.1016/j.bmcl.2004.12.023
BindingDB Entry DOI: 10.7270/Q2PN954N |
More data for this
Ligand-Target Pair | |
Histamine H1 receptor
(Homo sapiens (Human)) | BDBM22890

(2-(2-{4-[(4-chlorophenyl)(phenyl)methyl]piperazin-...)Show SMILES OC(=O)COCCN1CCN(CC1)C(c1ccccc1)c1ccc(Cl)cc1 Show InChI InChI=1S/C21H25ClN2O3/c22-19-8-6-18(7-9-19)21(17-4-2-1-3-5-17)24-12-10-23(11-13-24)14-15-27-16-20(25)26/h1-9,21H,10-16H2,(H,25,26) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
Article
PubMed
| 5.89 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
UCB Research
Curated by ChEMBL
| Assay Description
Binding affinity for human Histamine H1 receptor in CHO K1 cells |
Bioorg Med Chem Lett 15: 1083-5 (2005)
Article DOI: 10.1016/j.bmcl.2004.12.023
BindingDB Entry DOI: 10.7270/Q2PN954N |
More data for this
Ligand-Target Pair | |
Histamine H1 receptor
(Homo sapiens (Human)) | BDBM50160831

(CHEMBL181085 | N-hydroxycarbamate derivative)Show SMILES CC(C)COC(=O)N(O)CCC#Cc1ccc(OCCCCN2CCN(CC2)C(c2ccc(F)cc2)c2ccc(F)cc2)cc1 Show InChI InChI=1S/C36H43F2N3O4/c1-28(2)27-45-36(42)41(43)21-4-3-7-29-8-18-34(19-9-29)44-26-6-5-20-39-22-24-40(25-23-39)35(30-10-14-32(37)15-11-30)31-12-16-33(38)17-13-31/h8-19,28,35,43H,4-6,20-27H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 7.59 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
UCB Research
Curated by ChEMBL
| Assay Description
Binding affinity for human Histamine H1 receptor in CHO K1 cells |
Bioorg Med Chem Lett 15: 1083-5 (2005)
Article DOI: 10.1016/j.bmcl.2004.12.023
BindingDB Entry DOI: 10.7270/Q2PN954N |
More data for this
Ligand-Target Pair | |
Histamine H1 receptor
(Homo sapiens (Human)) | BDBM50160829

(CHEMBL180233 | N-hydroxycarbamate derivative)Show SMILES CC(C)OC(=O)N(O)CCC#Cc1ccc(OCCCCN2CCN(CC2)C(c2ccc(F)cc2)c2ccc(F)cc2)cc1 Show InChI InChI=1S/C35H41F2N3O4/c1-27(2)44-35(41)40(42)21-4-3-7-28-8-18-33(19-9-28)43-26-6-5-20-38-22-24-39(25-23-38)34(29-10-14-31(36)15-11-29)30-12-16-32(37)17-13-30/h8-19,27,34,42H,4-6,20-26H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 9.55 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
UCB Research
Curated by ChEMBL
| Assay Description
Binding affinity for human Histamine H1 receptor in CHO K1 cells |
Bioorg Med Chem Lett 15: 1083-5 (2005)
Article DOI: 10.1016/j.bmcl.2004.12.023
BindingDB Entry DOI: 10.7270/Q2PN954N |
More data for this
Ligand-Target Pair | |
Histamine H1 receptor
(Homo sapiens (Human)) | BDBM50160830
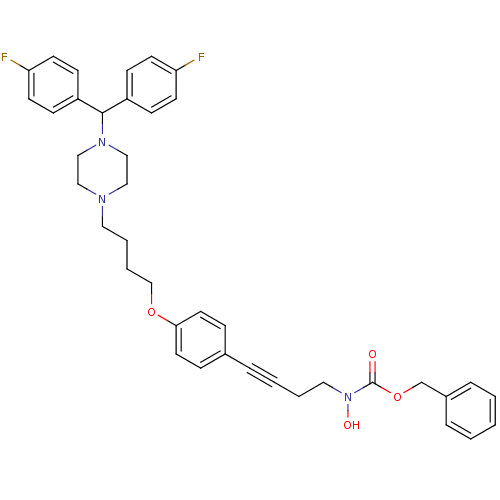
(CHEMBL360666 | N-hydroxycarbamate derivative)Show SMILES ON(CCC#Cc1ccc(OCCCCN2CCN(CC2)C(c2ccc(F)cc2)c2ccc(F)cc2)cc1)C(=O)OCc1ccccc1 Show InChI InChI=1S/C39H41F2N3O4/c40-35-17-13-33(14-18-35)38(34-15-19-36(41)20-16-34)43-27-25-42(26-28-43)23-6-7-29-47-37-21-11-31(12-22-37)8-4-5-24-44(46)39(45)48-30-32-9-2-1-3-10-32/h1-3,9-22,38,46H,5-7,23-30H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 17.8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
UCB Research
Curated by ChEMBL
| Assay Description
Binding affinity for human Histamine H1 receptor in CHO K1 cells |
Bioorg Med Chem Lett 15: 1083-5 (2005)
Article DOI: 10.1016/j.bmcl.2004.12.023
BindingDB Entry DOI: 10.7270/Q2PN954N |
More data for this
Ligand-Target Pair | |
Polyunsaturated fatty acid 5-lipoxygenase
(Homo sapiens (Human)) | BDBM50160827

(CHEMBL179590 | N-hydroxycarbamate derivative)Show SMILES CCOC(=O)N(O)CCC#Cc1ccc(OCCCCN2CCN(CC2)C(c2ccc(F)cc2)c2ccc(F)cc2)cc1 Show InChI InChI=1S/C34H39F2N3O4/c1-2-42-34(40)39(41)21-4-3-7-27-8-18-32(19-9-27)43-26-6-5-20-37-22-24-38(25-23-37)33(28-10-14-30(35)15-11-28)29-12-16-31(36)17-13-29/h8-19,33,41H,2,4-6,20-26H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 72 | n/a | n/a | n/a | n/a | n/a | n/a |
UCB Research
Curated by ChEMBL
| Assay Description
Inhibitory concentration against 5-lipoxygenase in human whole blood |
Bioorg Med Chem Lett 15: 1083-5 (2005)
Article DOI: 10.1016/j.bmcl.2004.12.023
BindingDB Entry DOI: 10.7270/Q2PN954N |
More data for this
Ligand-Target Pair | |
Polyunsaturated fatty acid 5-lipoxygenase
(Homo sapiens (Human)) | BDBM50155336

(1-{4-[4-(4-{4-[bis(4-fluorophenyl)methyl]piperazin...)Show SMILES NC(=O)N(O)CCC#Cc1ccc(OCCCCN2CCN(CC2)C(c2ccc(F)cc2)c2ccc(F)cc2)cc1 Show InChI InChI=1S/C32H36F2N4O3/c33-28-12-8-26(9-13-28)31(27-10-14-29(34)15-11-27)37-22-20-36(21-23-37)18-3-4-24-41-30-16-6-25(7-17-30)5-1-2-19-38(40)32(35)39/h6-17,31,40H,2-4,18-24H2,(H2,35,39) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 89 | n/a | n/a | n/a | n/a | n/a | n/a |
UCB Research
Curated by ChEMBL
| Assay Description
Inhibitory concentration against 5-lipoxygenase in human whole blood |
Bioorg Med Chem Lett 15: 1083-5 (2005)
Article DOI: 10.1016/j.bmcl.2004.12.023
BindingDB Entry DOI: 10.7270/Q2PN954N |
More data for this
Ligand-Target Pair | |
Polyunsaturated fatty acid 5-lipoxygenase
(Homo sapiens (Human)) | BDBM50160828

(CHEMBL366925 | N-hydroxycarbamate derivative)Show SMILES COC(=O)N(O)CCC#Cc1ccc(OCCCCN2CCN(CC2)C(c2ccc(F)cc2)c2ccc(F)cc2)cc1 Show InChI InChI=1S/C33H37F2N3O4/c1-41-33(39)38(40)20-3-2-6-26-7-17-31(18-8-26)42-25-5-4-19-36-21-23-37(24-22-36)32(27-9-13-29(34)14-10-27)28-11-15-30(35)16-12-28/h7-18,32,40H,3-5,19-25H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 90 | n/a | n/a | n/a | n/a | n/a | n/a |
UCB Research
Curated by ChEMBL
| Assay Description
Inhibitory concentration against 5-lipoxygenase in human whole blood |
Bioorg Med Chem Lett 15: 1083-5 (2005)
Article DOI: 10.1016/j.bmcl.2004.12.023
BindingDB Entry DOI: 10.7270/Q2PN954N |
More data for this
Ligand-Target Pair | |
Polyunsaturated fatty acid 5-lipoxygenase
(Homo sapiens (Human)) | BDBM50160829

(CHEMBL180233 | N-hydroxycarbamate derivative)Show SMILES CC(C)OC(=O)N(O)CCC#Cc1ccc(OCCCCN2CCN(CC2)C(c2ccc(F)cc2)c2ccc(F)cc2)cc1 Show InChI InChI=1S/C35H41F2N3O4/c1-27(2)44-35(41)40(42)21-4-3-7-28-8-18-33(19-9-28)43-26-6-5-20-38-22-24-39(25-23-38)34(29-10-14-31(36)15-11-29)30-12-16-32(37)17-13-30/h8-19,27,34,42H,4-6,20-26H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 102 | n/a | n/a | n/a | n/a | n/a | n/a |
UCB Research
Curated by ChEMBL
| Assay Description
Inhibitory concentration against 5-lipoxygenase in human whole blood |
Bioorg Med Chem Lett 15: 1083-5 (2005)
Article DOI: 10.1016/j.bmcl.2004.12.023
BindingDB Entry DOI: 10.7270/Q2PN954N |
More data for this
Ligand-Target Pair | |
Polyunsaturated fatty acid 5-lipoxygenase
(Homo sapiens (Human)) | BDBM50160830

(CHEMBL360666 | N-hydroxycarbamate derivative)Show SMILES ON(CCC#Cc1ccc(OCCCCN2CCN(CC2)C(c2ccc(F)cc2)c2ccc(F)cc2)cc1)C(=O)OCc1ccccc1 Show InChI InChI=1S/C39H41F2N3O4/c40-35-17-13-33(14-18-35)38(34-15-19-36(41)20-16-34)43-27-25-42(26-28-43)23-6-7-29-47-37-21-11-31(12-22-37)8-4-5-24-44(46)39(45)48-30-32-9-2-1-3-10-32/h1-3,9-22,38,46H,5-7,23-30H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 150 | n/a | n/a | n/a | n/a | n/a | n/a |
UCB Research
Curated by ChEMBL
| Assay Description
Inhibitory concentration against 5-lipoxygenase in human whole blood |
Bioorg Med Chem Lett 15: 1083-5 (2005)
Article DOI: 10.1016/j.bmcl.2004.12.023
BindingDB Entry DOI: 10.7270/Q2PN954N |
More data for this
Ligand-Target Pair | |
Polyunsaturated fatty acid 5-lipoxygenase
(Homo sapiens (Human)) | BDBM50160828

(CHEMBL366925 | N-hydroxycarbamate derivative)Show SMILES COC(=O)N(O)CCC#Cc1ccc(OCCCCN2CCN(CC2)C(c2ccc(F)cc2)c2ccc(F)cc2)cc1 Show InChI InChI=1S/C33H37F2N3O4/c1-41-33(39)38(40)20-3-2-6-26-7-17-31(18-8-26)42-25-5-4-19-36-21-23-37(24-22-36)32(27-9-13-29(34)14-10-27)28-11-15-30(35)16-12-28/h7-18,32,40H,3-5,19-25H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 170 | n/a | n/a | n/a | n/a | n/a | n/a |
UCB Research
Curated by ChEMBL
| Assay Description
Inhibitory concentration against human 5-lipoxygenase |
Bioorg Med Chem Lett 15: 1083-5 (2005)
Article DOI: 10.1016/j.bmcl.2004.12.023
BindingDB Entry DOI: 10.7270/Q2PN954N |
More data for this
Ligand-Target Pair | |
Polyunsaturated fatty acid 5-lipoxygenase
(Homo sapiens (Human)) | BDBM50160831

(CHEMBL181085 | N-hydroxycarbamate derivative)Show SMILES CC(C)COC(=O)N(O)CCC#Cc1ccc(OCCCCN2CCN(CC2)C(c2ccc(F)cc2)c2ccc(F)cc2)cc1 Show InChI InChI=1S/C36H43F2N3O4/c1-28(2)27-45-36(42)41(43)21-4-3-7-29-8-18-34(19-9-29)44-26-6-5-20-39-22-24-40(25-23-39)35(30-10-14-32(37)15-11-30)31-12-16-33(38)17-13-31/h8-19,28,35,43H,4-6,20-27H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 176 | n/a | n/a | n/a | n/a | n/a | n/a |
UCB Research
Curated by ChEMBL
| Assay Description
Inhibitory concentration against 5-lipoxygenase in human whole blood |
Bioorg Med Chem Lett 15: 1083-5 (2005)
Article DOI: 10.1016/j.bmcl.2004.12.023
BindingDB Entry DOI: 10.7270/Q2PN954N |
More data for this
Ligand-Target Pair | |
Polyunsaturated fatty acid 5-lipoxygenase
(Homo sapiens (Human)) | BDBM50155336

(1-{4-[4-(4-{4-[bis(4-fluorophenyl)methyl]piperazin...)Show SMILES NC(=O)N(O)CCC#Cc1ccc(OCCCCN2CCN(CC2)C(c2ccc(F)cc2)c2ccc(F)cc2)cc1 Show InChI InChI=1S/C32H36F2N4O3/c33-28-12-8-26(9-13-28)31(27-10-14-29(34)15-11-27)37-22-20-36(21-23-37)18-3-4-24-41-30-16-6-25(7-17-30)5-1-2-19-38(40)32(35)39/h6-17,31,40H,2-4,18-24H2,(H2,35,39) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 193 | n/a | n/a | n/a | n/a | n/a | n/a |
UCB Research
Curated by ChEMBL
| Assay Description
Inhibitory concentration against human 5-lipoxygenase |
Bioorg Med Chem Lett 15: 1083-5 (2005)
Article DOI: 10.1016/j.bmcl.2004.12.023
BindingDB Entry DOI: 10.7270/Q2PN954N |
More data for this
Ligand-Target Pair | |
Polyunsaturated fatty acid 5-lipoxygenase
(Homo sapiens (Human)) | BDBM50160829

(CHEMBL180233 | N-hydroxycarbamate derivative)Show SMILES CC(C)OC(=O)N(O)CCC#Cc1ccc(OCCCCN2CCN(CC2)C(c2ccc(F)cc2)c2ccc(F)cc2)cc1 Show InChI InChI=1S/C35H41F2N3O4/c1-27(2)44-35(41)40(42)21-4-3-7-28-8-18-33(19-9-28)43-26-6-5-20-38-22-24-39(25-23-38)34(29-10-14-31(36)15-11-29)30-12-16-32(37)17-13-30/h8-19,27,34,42H,4-6,20-26H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 335 | n/a | n/a | n/a | n/a | n/a | n/a |
UCB Research
Curated by ChEMBL
| Assay Description
Inhibitory concentration against human 5-lipoxygenase |
Bioorg Med Chem Lett 15: 1083-5 (2005)
Article DOI: 10.1016/j.bmcl.2004.12.023
BindingDB Entry DOI: 10.7270/Q2PN954N |
More data for this
Ligand-Target Pair | |
Polyunsaturated fatty acid 5-lipoxygenase
(Homo sapiens (Human)) | BDBM50160830

(CHEMBL360666 | N-hydroxycarbamate derivative)Show SMILES ON(CCC#Cc1ccc(OCCCCN2CCN(CC2)C(c2ccc(F)cc2)c2ccc(F)cc2)cc1)C(=O)OCc1ccccc1 Show InChI InChI=1S/C39H41F2N3O4/c40-35-17-13-33(14-18-35)38(34-15-19-36(41)20-16-34)43-27-25-42(26-28-43)23-6-7-29-47-37-21-11-31(12-22-37)8-4-5-24-44(46)39(45)48-30-32-9-2-1-3-10-32/h1-3,9-22,38,46H,5-7,23-30H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 350 | n/a | n/a | n/a | n/a | n/a | n/a |
UCB Research
Curated by ChEMBL
| Assay Description
Inhibitory concentration against human 5-lipoxygenase |
Bioorg Med Chem Lett 15: 1083-5 (2005)
Article DOI: 10.1016/j.bmcl.2004.12.023
BindingDB Entry DOI: 10.7270/Q2PN954N |
More data for this
Ligand-Target Pair | |
Polyunsaturated fatty acid 5-lipoxygenase
(Homo sapiens (Human)) | BDBM50160827

(CHEMBL179590 | N-hydroxycarbamate derivative)Show SMILES CCOC(=O)N(O)CCC#Cc1ccc(OCCCCN2CCN(CC2)C(c2ccc(F)cc2)c2ccc(F)cc2)cc1 Show InChI InChI=1S/C34H39F2N3O4/c1-2-42-34(40)39(41)21-4-3-7-27-8-18-32(19-9-27)43-26-6-5-20-37-22-24-38(25-23-37)33(28-10-14-30(35)15-11-28)29-12-16-31(36)17-13-29/h8-19,33,41H,2,4-6,20-26H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 365 | n/a | n/a | n/a | n/a | n/a | n/a |
UCB Research
Curated by ChEMBL
| Assay Description
Inhibitory concentration against human 5-lipoxygenase |
Bioorg Med Chem Lett 15: 1083-5 (2005)
Article DOI: 10.1016/j.bmcl.2004.12.023
BindingDB Entry DOI: 10.7270/Q2PN954N |
More data for this
Ligand-Target Pair | |
Polyunsaturated fatty acid 5-lipoxygenase
(Homo sapiens (Human)) | BDBM50160831

(CHEMBL181085 | N-hydroxycarbamate derivative)Show SMILES CC(C)COC(=O)N(O)CCC#Cc1ccc(OCCCCN2CCN(CC2)C(c2ccc(F)cc2)c2ccc(F)cc2)cc1 Show InChI InChI=1S/C36H43F2N3O4/c1-28(2)27-45-36(42)41(43)21-4-3-7-29-8-18-34(19-9-29)44-26-6-5-20-39-22-24-40(25-23-39)35(30-10-14-32(37)15-11-30)31-12-16-33(38)17-13-31/h8-19,28,35,43H,4-6,20-27H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 420 | n/a | n/a | n/a | n/a | n/a | n/a |
UCB Research
Curated by ChEMBL
| Assay Description
Inhibitory concentration against human 5-lipoxygenase |
Bioorg Med Chem Lett 15: 1083-5 (2005)
Article DOI: 10.1016/j.bmcl.2004.12.023
BindingDB Entry DOI: 10.7270/Q2PN954N |
More data for this
Ligand-Target Pair | |
Polyunsaturated fatty acid 5-lipoxygenase
(Homo sapiens (Human)) | BDBM50000541

((+-)-1-(1-Benzo[b]thien-2-ylethyl)-1-hydroxyurea |...)Show InChI InChI=1S/C11H12N2O2S/c1-7(13(15)11(12)14)10-6-8-4-2-3-5-9(8)16-10/h2-7,15H,1H3,(H2,12,14) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
UniChem
Patents
Similars
| DrugBank
Article
PubMed
| n/a | n/a | 518 | n/a | n/a | n/a | n/a | n/a | n/a |
UCB Research
Curated by ChEMBL
| Assay Description
Inhibitory concentration against human 5-lipoxygenase |
Bioorg Med Chem Lett 15: 1083-5 (2005)
Article DOI: 10.1016/j.bmcl.2004.12.023
BindingDB Entry DOI: 10.7270/Q2PN954N |
More data for this
Ligand-Target Pair | |
Polyunsaturated fatty acid 5-lipoxygenase
(Homo sapiens (Human)) | BDBM50000541

((+-)-1-(1-Benzo[b]thien-2-ylethyl)-1-hydroxyurea |...)Show InChI InChI=1S/C11H12N2O2S/c1-7(13(15)11(12)14)10-6-8-4-2-3-5-9(8)16-10/h2-7,15H,1H3,(H2,12,14) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
UniChem
Patents
Similars
| DrugBank
Article
PubMed
| n/a | n/a | 873 | n/a | n/a | n/a | n/a | n/a | n/a |
UCB Research
Curated by ChEMBL
| Assay Description
Inhibitory concentration against 5-lipoxygenase in human whole blood |
Bioorg Med Chem Lett 15: 1083-5 (2005)
Article DOI: 10.1016/j.bmcl.2004.12.023
BindingDB Entry DOI: 10.7270/Q2PN954N |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data