

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Prostaglandin G/H synthase 2 (Ovis aries (Sheep)) | BDBM11639 (4-[5-(4-methylphenyl)-3-(trifluoromethyl)-1H-pyraz...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 2.05E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Pharmaceutical Education and Research (NIPER) Curated by ChEMBL | Assay Description Inhibitory concentration against Prostaglandin G/H synthase 2 from sheep placenta at 100 uM | Bioorg Med Chem Lett 15: 1793-7 (2005) Article DOI: 10.1016/j.bmcl.2005.02.039 BindingDB Entry DOI: 10.7270/Q20V8DJD | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Prostaglandin G/H synthase 2 (Homo sapiens (Human)) | BDBM50163748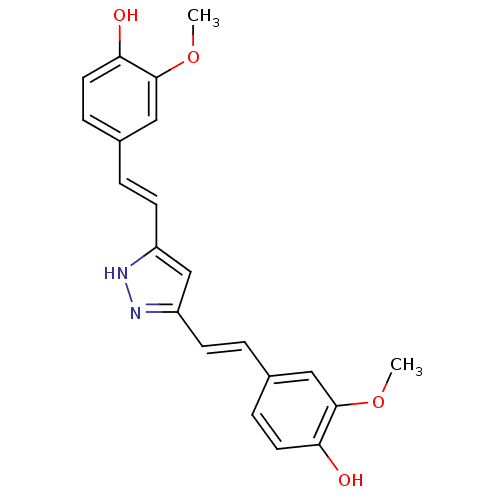 ((E)-3,5-Bis[beta-(4-Hydroxy-3-methoxyphenyl)-ethen...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 9.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Pharmaceutical Education and Research (NIPER) Curated by ChEMBL | Assay Description Anti-oxidant activity in DPPH radicak scavenging assay; n=3-4 | Bioorg Med Chem Lett 15: 1793-7 (2005) Article DOI: 10.1016/j.bmcl.2005.02.039 BindingDB Entry DOI: 10.7270/Q20V8DJD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Homo sapiens (Human)) | BDBM50163745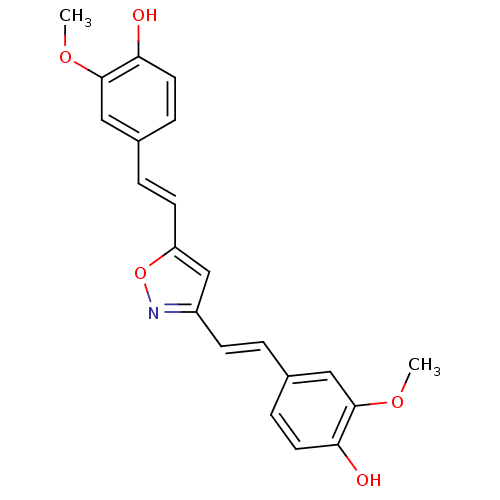 (4,40-(1E,10E)-2,20-(Isoxazole-3,5-diyl)bis(ethene-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.07E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Pharmaceutical Education and Research (NIPER) Curated by ChEMBL | Assay Description Anti-oxidant activity in DPPH radicak scavenging assay; n=3-4 | Bioorg Med Chem Lett 15: 1793-7 (2005) Article DOI: 10.1016/j.bmcl.2005.02.039 BindingDB Entry DOI: 10.7270/Q20V8DJD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Homo sapiens (Human)) | BDBM50067040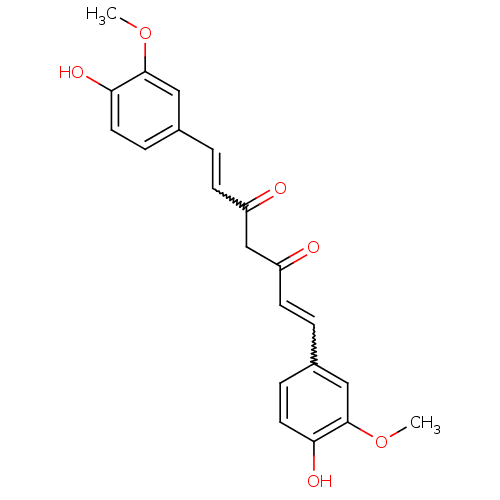 (((E,E)-1,7-bis(4-hydroxy-3-methoxyphenyl)-1,6-hept...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 1.11E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Pharmaceutical Education and Research (NIPER) Curated by ChEMBL | Assay Description Anti-oxidant activity in DPPH radicak scavenging assay; n=3-4 | Bioorg Med Chem Lett 15: 1793-7 (2005) Article DOI: 10.1016/j.bmcl.2005.02.039 BindingDB Entry DOI: 10.7270/Q20V8DJD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Homo sapiens (Human)) | BDBM50359629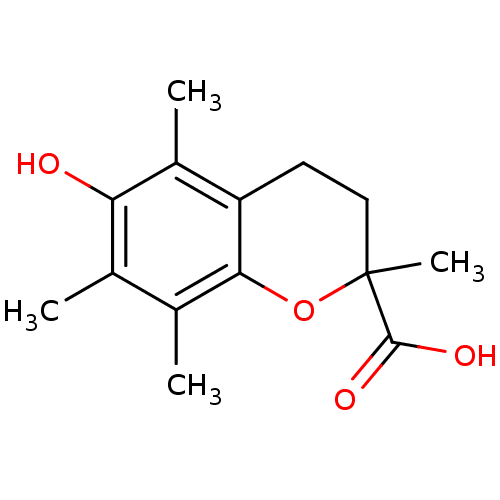 (TROLOX) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.31E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Pharmaceutical Education and Research (NIPER) Curated by ChEMBL | Assay Description Anti-oxidant activity in DPPH radical scavenging assay; n=3-4 | Bioorg Med Chem Lett 15: 1793-7 (2005) Article DOI: 10.1016/j.bmcl.2005.02.039 BindingDB Entry DOI: 10.7270/Q20V8DJD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 1 (Ovis aries (Sheep)) | BDBM50009859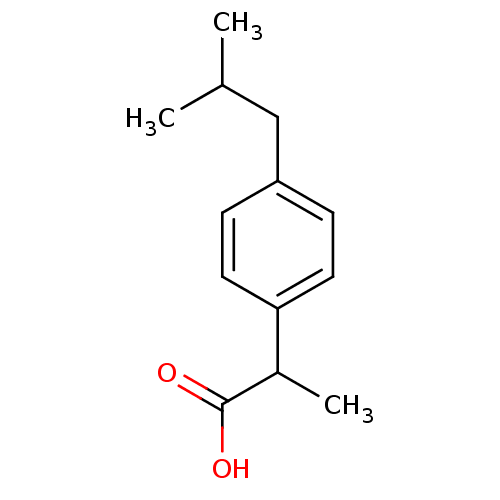 ((+-)-2-(p-isobutylphenyl)propionic acid | (+-)-alp...) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 1.44E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Pharmaceutical Education and Research (NIPER) Curated by ChEMBL | Assay Description Inhibitory concentration against Prostaglandin G/H synthase 1 of ram seminal vesicles at 100 uM | Bioorg Med Chem Lett 15: 1793-7 (2005) Article DOI: 10.1016/j.bmcl.2005.02.039 BindingDB Entry DOI: 10.7270/Q20V8DJD | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Prostaglandin G/H synthase 2 (Homo sapiens (Human)) | BDBM50163747 (4-((E)-2-{5-[(E)-2-(4-Hydroxy-phenyl)-vinyl]-1H-py...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.55E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Pharmaceutical Education and Research (NIPER) Curated by ChEMBL | Assay Description Anti-oxidant activity in DPPH radicak scavenging assay; n=3-4 | Bioorg Med Chem Lett 15: 1793-7 (2005) Article DOI: 10.1016/j.bmcl.2005.02.039 BindingDB Entry DOI: 10.7270/Q20V8DJD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Homo sapiens (Human)) | BDBM50163743 (4-((E)-2-{5-[(E)-2-(4-Hydroxy-phenyl)-vinyl]-isoxa...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.90E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Pharmaceutical Education and Research (NIPER) Curated by ChEMBL | Assay Description Anti-oxidant activity in DPPH radicak scavenging assay; n=3-4 | Bioorg Med Chem Lett 15: 1793-7 (2005) Article DOI: 10.1016/j.bmcl.2005.02.039 BindingDB Entry DOI: 10.7270/Q20V8DJD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Homo sapiens (Human)) | BDBM50163744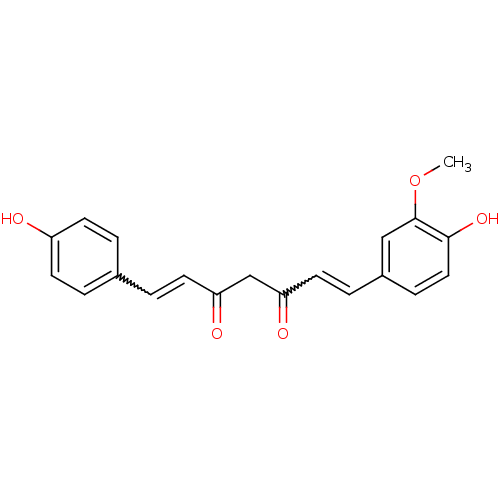 ((1E,4Z,6E)-5-Hydroxy-1-(4-hydroxy-3-methoxy-phenyl...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 2.14E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Pharmaceutical Education and Research (NIPER) Curated by ChEMBL | Assay Description Anti-oxidant activity in DPPH radicak scavenging assay; n=3-4 | Bioorg Med Chem Lett 15: 1793-7 (2005) Article DOI: 10.1016/j.bmcl.2005.02.039 BindingDB Entry DOI: 10.7270/Q20V8DJD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Homo sapiens (Human)) | BDBM50163746 (4-((E)-2-{3-[(E)-2-(4-hydroxyphenyl)vinyl]-1H-pyra...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.24E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Pharmaceutical Education and Research (NIPER) Curated by ChEMBL | Assay Description Anti-oxidant activity in DPPH radicak scavenging assay; n=3-4 | Bioorg Med Chem Lett 15: 1793-7 (2005) Article DOI: 10.1016/j.bmcl.2005.02.039 BindingDB Entry DOI: 10.7270/Q20V8DJD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Homo sapiens (Human)) | BDBM50059989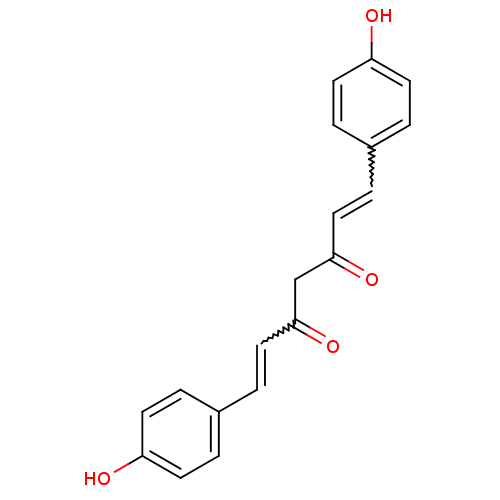 ((1E,4Z,6E)-5-Hydroxy-1,7-bis-(4-hydroxy-phenyl)-he...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 3.64E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Pharmaceutical Education and Research (NIPER) Curated by ChEMBL | Assay Description Anti-oxidant activity in DPPH radicak scavenging assay; n=3-4 | Bioorg Med Chem Lett 15: 1793-7 (2005) Article DOI: 10.1016/j.bmcl.2005.02.039 BindingDB Entry DOI: 10.7270/Q20V8DJD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Ovis aries (Sheep)) | BDBM50009859 ((+-)-2-(p-isobutylphenyl)propionic acid | (+-)-alp...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 2.66E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Pharmaceutical Education and Research (NIPER) Curated by ChEMBL | Assay Description Inhibitory concentration against Prostaglandin G/H synthase 2 from sheep placenta at 100 uM | Bioorg Med Chem Lett 15: 1793-7 (2005) Article DOI: 10.1016/j.bmcl.2005.02.039 BindingDB Entry DOI: 10.7270/Q20V8DJD | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||