

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM50163744 (1E,4Z,6E)-5-Hydroxy-1-(4-hydroxy-3-methoxy-phenyl)-7-(4-hydroxy-phenyl)-hepta-1,4,6-trien-3-one::(1E,6E)-1-(4-Hydroxy-3-methoxy-phenyl)-7-(4-hydroxy-phenyl)-hepta-1,6-diene-3,5-dione::1-(4-hydroxy-3-methoxyphenyl)-7-(4-hydroxyphenyl)-1,6-heptadiene-3,5-dione::5-hydroxy-7-(4-hydroxy-3-methoxyphenyl)-1-(4-hydroxyphenyl)hepta-1,4,6-trien-3-one::CHEMBL179512::cid_5324476::curcumin II::demethoxycurcumin
SMILES: COc1cc(C=CC(=O)CC(=O)C=Cc2ccc(O)cc2)ccc1O
InChI Key: InChIKey=HJTVQHVGMGKONQ-UHFFFAOYSA-N
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Serine/threonine-protein kinase 33 (Homo sapiens (Human)) | BDBM50163744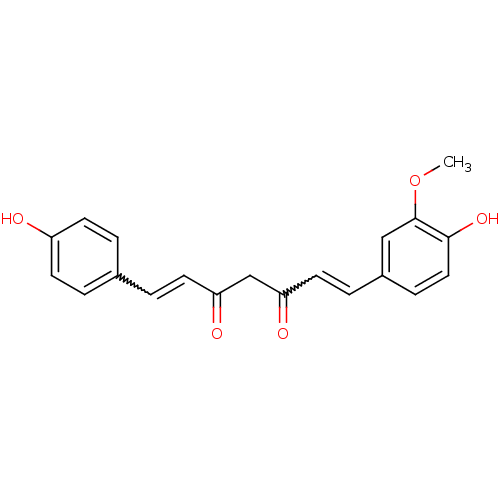 ((1E,4Z,6E)-5-Hydroxy-1-(4-hydroxy-3-methoxy-phenyl...) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | PCBioAssay | n/a | n/a | n/a | n/a | 1.75E+3 | n/a | n/a | n/a | n/a |
Broad Institute Curated by PubChem BioAssay | Assay Description Keywords: STK33 Kinase, Non-ATP Competitive Inhibitor Assay Overview: Purified STK33 Kinase is preincubated with potential inhibitors and allowed to ... | PubChem Bioassay (2010) BindingDB Entry DOI: 10.7270/Q2B27SRF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM50163744 ((1E,4Z,6E)-5-Hydroxy-1-(4-hydroxy-3-methoxy-phenyl...) | PDB MMDB KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.60E+6 | n/a | n/a | n/a | n/a | n/a | n/a |
Yamagata University Curated by ChEMBL | Assay Description Inhibition of recombinant BACE1 (unknown origin) | Bioorg Med Chem Lett 24: 685-90 (2014) Article DOI: 10.1016/j.bmcl.2013.11.039 BindingDB Entry DOI: 10.7270/Q26T0QMH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alkaline phosphatase placental-like (Homo sapiens (Human)) | BDBM50163744 ((1E,4Z,6E)-5-Hydroxy-1-(4-hydroxy-3-methoxy-phenyl...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | PCBioAssay | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Burnham Center for Chemical Genomics Curated by PubChem BioAssay | Assay Description Data Source: Sanford-Burnham Center for Chemical Genomics (SBCCG) Source Affiliation: Sanford-Burnham Medical Research Institute (SBMRI, San Diego, C... | PubChem Bioassay (2010) BindingDB Entry DOI: 10.7270/Q2NS0SCW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alkaline phosphatase, tissue-nonspecific isozyme (Homo sapiens (Human)) | BDBM50163744 ((1E,4Z,6E)-5-Hydroxy-1-(4-hydroxy-3-methoxy-phenyl...) | KEGG UniProtKB/SwissProt UniProtKB/TrEMBL DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | PCBioAssay | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Burnham Center for Chemical Genomics Curated by PubChem BioAssay | Assay Description Data Source: Sanford-Burnham Center for Chemical Genomics (SBCCG) Source Affiliation: Sanford-Burnham Medical Research Institute (SBMRI, San Diego, C... | PubChem Bioassay (2010) BindingDB Entry DOI: 10.7270/Q2H130G1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpi (Rattus norvegicus (Rat)) | BDBM50163744 ((1E,4Z,6E)-5-Hydroxy-1-(4-hydroxy-3-methoxy-phenyl...) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | PCBioAssay | n/a | n/a | 8.38E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Burnham Center for Chemical Genomics Curated by PubChem BioAssay | Assay Description Data Source: Sanford-Burnham Center for Chemical Genomics (SBCCG) Source Affiliation: Sanford-Burnham Medical Research Institute (SBMRI, San Diego, C... | PubChem Bioassay (2010) BindingDB Entry DOI: 10.7270/Q2DB809V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta lactamase (Pseudomonas aeruginosa) | BDBM50163744 ((1E,4Z,6E)-5-Hydroxy-1-(4-hydroxy-3-methoxy-phenyl...) | PDB MMDB KEGG UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | PCBioAssay | n/a | n/a | 8.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute Molecular Screening Center Curated by PubChem BioAssay | Assay Description Source (MLPCN Center Name): The Scripps Research Institute Molecular Screening Center (SRIMSC) Affiliation: The Scripps Research Institute, TSRI Assa... | PubChem Bioassay (2011) BindingDB Entry DOI: 10.7270/Q2G15ZB8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Toll-like receptor 9 (Homo sapiens (Human)) | BDBM50163744 ((1E,4Z,6E)-5-Hydroxy-1-(4-hydroxy-3-methoxy-phenyl...) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | PCBioAssay | n/a | n/a | 9.13E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute Molecular Screening Center Curated by PubChem BioAssay | Assay Description Source (MLPCN Center Name): The Scripps Research Institute Molecular Screening Center (SRIMSC) Affiliation: The Scripps Research Institute, TSRI Assa... | PubChem Bioassay (2011) BindingDB Entry DOI: 10.7270/Q2KS6Q2P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Human immunodeficiency virus type 1 integrase (Human immunodeficiency virus 1) | BDBM50163744 ((1E,4Z,6E)-5-Hydroxy-1-(4-hydroxy-3-methoxy-phenyl...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.40E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
National Cancer Institute Curated by ChEMBL | Assay Description Inhibition of 3'- processing activity of HIV-1 integrase | J Med Chem 40: 3057-63 (1997) Article DOI: 10.1021/jm970190x BindingDB Entry DOI: 10.7270/Q22Z166F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| CAMK2A (Homo sapiens (Human)) | BDBM50163744 ((1E,4Z,6E)-5-Hydroxy-1-(4-hydroxy-3-methoxy-phenyl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 2.40E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Rajiv Gandhi Centre for Biotechnology Curated by ChEMBL | Assay Description Inhibition of alphaCaMK2 using GST-NR2A as substrate incubated for 1 min prior to substrate addition measured after 1 min | Bioorg Med Chem 20: 6040-7 (2012) Article DOI: 10.1016/j.bmc.2012.08.029 BindingDB Entry DOI: 10.7270/Q28916ZM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Multidrug resistance protein CDR1 (Candida albicans) | BDBM50163744 ((1E,4Z,6E)-5-Hydroxy-1-(4-hydroxy-3-methoxy-phenyl...) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 4.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Jawaharlal Nehru University Curated by ChEMBL | Assay Description Inhibition of GFP-tagged Candida albicans CDR1 expressed in Saccharomyces cerevisiae assessed as inhibition of R6G efflux | Antimicrob Agents Chemother 53: 3256-65 (2009) Article DOI: 10.1128/AAC.01497-08 BindingDB Entry DOI: 10.7270/Q2BP032R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Human immunodeficiency virus type 1 integrase (Human immunodeficiency virus 1) | BDBM50163744 ((1E,4Z,6E)-5-Hydroxy-1-(4-hydroxy-3-methoxy-phenyl...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.20E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
National Cancer Institute Curated by ChEMBL | Assay Description Inhibition of strand transfer activity of HIV-1 integrase | J Med Chem 40: 3057-63 (1997) Article DOI: 10.1021/jm970190x BindingDB Entry DOI: 10.7270/Q22Z166F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclooxygenase (Homo sapiens (Human)) | BDBM50163744 ((1E,4Z,6E)-5-Hydroxy-1-(4-hydroxy-3-methoxy-phenyl...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 2.14E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Pharmaceutical Education and Research (NIPER) Curated by ChEMBL | Assay Description Anti-oxidant activity in DPPH radicak scavenging assay; n=3-4 | Bioorg Med Chem Lett 15: 1793-7 (2005) Article DOI: 10.1016/j.bmcl.2005.02.039 BindingDB Entry DOI: 10.7270/Q20V8DJD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50163744 ((1E,4Z,6E)-5-Hydroxy-1-(4-hydroxy-3-methoxy-phenyl...) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 3.77E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Ramkhamhaeng University Curated by ChEMBL | Assay Description Inhibition of electric eel AChE using acetylthiocholine iodide as substrate after 15 mins by Ellman's method | Bioorg Med Chem Lett 23: 2880-2 (2013) Article DOI: 10.1016/j.bmcl.2013.03.069 BindingDB Entry DOI: 10.7270/Q22Z16WD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| M.SssI methyltransferase (Spiroplasma monobiae strain MQ-1) | BDBM50163744 ((1E,4Z,6E)-5-Hydroxy-1-(4-hydroxy-3-methoxy-phenyl...) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
The Ohio State University Curated by ChEMBL | Assay Description Inhibition of Spiroplasma sp. MQ-1 M.SssI | Bioorg Med Chem Lett 19: 706-9 (2009) Article DOI: 10.1016/j.bmcl.2008.12.041 BindingDB Entry DOI: 10.7270/Q2PV6M8R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Intestinal alkaline phosphatase (Homo sapiens (Human)) | BDBM50163744 ((1E,4Z,6E)-5-Hydroxy-1-(4-hydroxy-3-methoxy-phenyl...) | PDB MMDB KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | PCBioAssay | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Burnham Center for Chemical Genomics Curated by PubChem BioAssay | Assay Description Data Source: Sanford-Burnham Center for Chemical Genomics (SBCCG) Source Affiliation: Sanford-Burnham Medical Research Institute(SBMRI, San Diego, CA... | PubChem Bioassay (2010) BindingDB Entry DOI: 10.7270/Q2X63KDM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||