

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Neuropeptide Y receptor type 5 (Homo sapiens (Human)) | BDBM50166561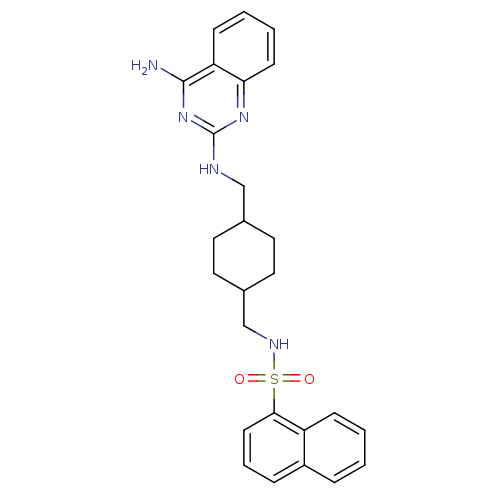 (CHEMBL195380 | Naphthalene-1-sulfonic acid {4-[(4-...) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Taisho Pharmaceutical Co. Ltd Curated by ChEMBL | Assay Description Inhibitory activity against constitutively activated human Neuropeptide Y receptor Y5 stransiently expressed in COS-1 cells using [125I]-PYY | Bioorg Med Chem Lett 15: 2565-9 (2005) Article DOI: 10.1016/j.bmcl.2005.03.052 BindingDB Entry DOI: 10.7270/Q2Z89BXQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropeptide Y receptor type 5 (Homo sapiens (Human)) | BDBM50166554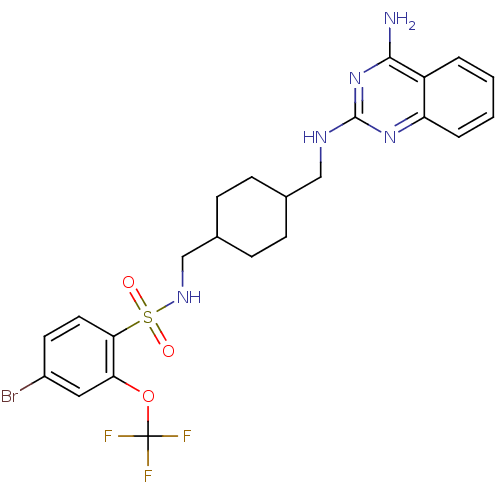 (CHEMBL192266 | N-{4-[(4-Amino-quinazolin-2-ylamino...) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Taisho Pharmaceutical Co. Ltd Curated by ChEMBL | Assay Description Inhibitory activity against constitutively activated human Neuropeptide Y receptor Y5 stransiently expressed in COS-1 cells using [125I]-PYY | Bioorg Med Chem Lett 15: 2565-9 (2005) Article DOI: 10.1016/j.bmcl.2005.03.052 BindingDB Entry DOI: 10.7270/Q2Z89BXQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropeptide Y receptor type 5 (Homo sapiens (Human)) | BDBM50166552 (4-Bromo-N-{4-[(4-dimethylamino-quinazolin-2-ylamin...) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 2.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Taisho Pharmaceutical Co. Ltd Curated by ChEMBL | Assay Description Inhibitory activity against constitutively activated human Neuropeptide Y receptor Y5 stransiently expressed in COS-1 cells using [125I]-PYY | Bioorg Med Chem Lett 15: 2565-9 (2005) Article DOI: 10.1016/j.bmcl.2005.03.052 BindingDB Entry DOI: 10.7270/Q2Z89BXQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropeptide Y receptor type 5 (Homo sapiens (Human)) | BDBM50166555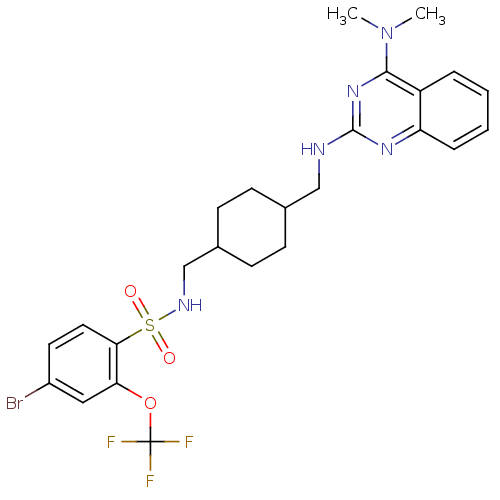 (4-Bromo-N-{4-[(4-dimethylamino-quinazolin-2-ylamin...) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 2.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Taisho Pharmaceutical Co. Ltd Curated by ChEMBL | Assay Description Inhibitory activity against constitutively activated human Neuropeptide Y receptor Y5 stransiently expressed in COS-1 cells using [125I]-PYY | Bioorg Med Chem Lett 15: 2565-9 (2005) Article DOI: 10.1016/j.bmcl.2005.03.052 BindingDB Entry DOI: 10.7270/Q2Z89BXQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropeptide Y receptor type 5 (Homo sapiens (Human)) | BDBM50166559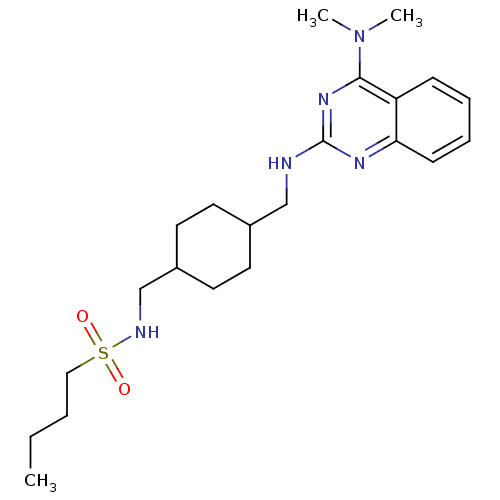 (Butane-1-sulfonic acid {4-[(4-dimethylamino-quinaz...) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 4.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Taisho Pharmaceutical Co. Ltd Curated by ChEMBL | Assay Description Inhibitory activity against constitutively activated human Neuropeptide Y receptor Y5 stransiently expressed in COS-1 cells using [125I]-PYY | Bioorg Med Chem Lett 15: 2565-9 (2005) Article DOI: 10.1016/j.bmcl.2005.03.052 BindingDB Entry DOI: 10.7270/Q2Z89BXQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-2A adrenergic receptor (Homo sapiens (Human)) | BDBM50166551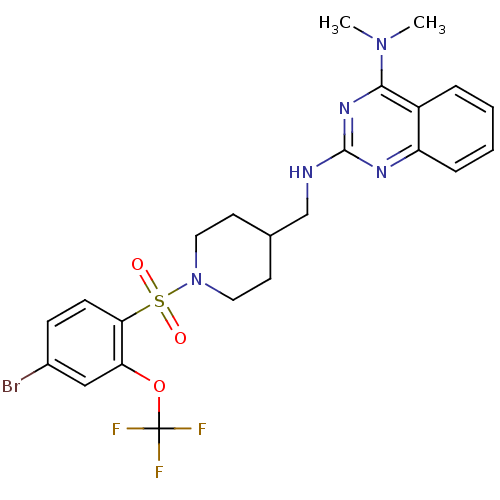 (CHEMBL194173 | N*2*-[1-(4-Bromo-2-trifluoromethoxy...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 5.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Taisho Pharmaceutical Co. Ltd Curated by ChEMBL | Assay Description Inhibitory activity against constitutively activated human Alpha-2A adrenergic receptor transiently expressed in COS-1 cells using [3H]-MK912 | Bioorg Med Chem Lett 15: 2565-9 (2005) Article DOI: 10.1016/j.bmcl.2005.03.052 BindingDB Entry DOI: 10.7270/Q2Z89BXQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-2A adrenergic receptor (Homo sapiens (Human)) | BDBM50166555 (4-Bromo-N-{4-[(4-dimethylamino-quinazolin-2-ylamin...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 7.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Taisho Pharmaceutical Co. Ltd Curated by ChEMBL | Assay Description Inhibitory activity against constitutively activated human Alpha-2A adrenergic receptor transiently expressed in COS-1 cells using [3H]-MK912 | Bioorg Med Chem Lett 15: 2565-9 (2005) Article DOI: 10.1016/j.bmcl.2005.03.052 BindingDB Entry DOI: 10.7270/Q2Z89BXQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-2A adrenergic receptor (Homo sapiens (Human)) | BDBM50166552 (4-Bromo-N-{4-[(4-dimethylamino-quinazolin-2-ylamin...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 21 | n/a | n/a | n/a | n/a | n/a | n/a |
Taisho Pharmaceutical Co. Ltd Curated by ChEMBL | Assay Description Inhibitory activity against constitutively activated human Alpha-2A adrenergic receptor transiently expressed in COS-1 cells using [3H]-MK912 | Bioorg Med Chem Lett 15: 2565-9 (2005) Article DOI: 10.1016/j.bmcl.2005.03.052 BindingDB Entry DOI: 10.7270/Q2Z89BXQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropeptide Y receptor type 5 (Homo sapiens (Human)) | BDBM50166563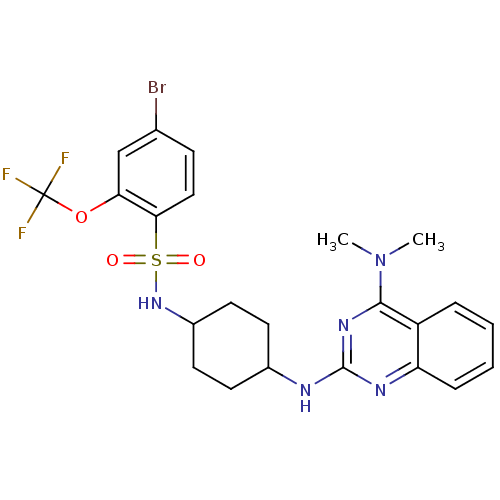 (4-Bromo-N-[4-(4-dimethylamino-quinazolin-2-ylamino...) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 31 | n/a | n/a | n/a | n/a | n/a | n/a |
Taisho Pharmaceutical Co. Ltd Curated by ChEMBL | Assay Description Inhibitory activity against constitutively activated human Neuropeptide Y receptor Y5 stransiently expressed in COS-1 cells using [125I]-PYY | Bioorg Med Chem Lett 15: 2565-9 (2005) Article DOI: 10.1016/j.bmcl.2005.03.052 BindingDB Entry DOI: 10.7270/Q2Z89BXQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropeptide Y receptor type 5 (Homo sapiens (Human)) | BDBM50166564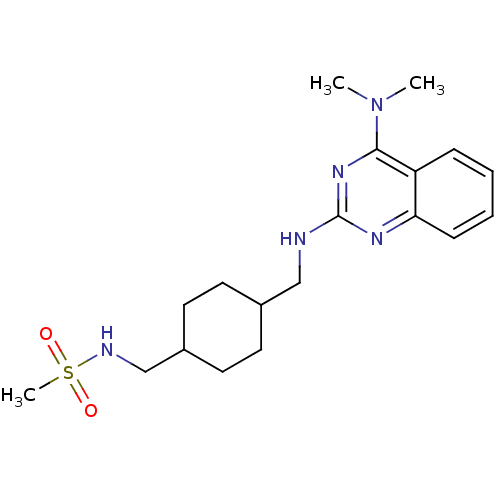 (CHEMBL195123 | N-{4-[(4-Dimethylamino-quinazolin-2...) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 41 | n/a | n/a | n/a | n/a | n/a | n/a |
Taisho Pharmaceutical Co. Ltd Curated by ChEMBL | Assay Description Inhibitory activity against constitutively activated human Neuropeptide Y receptor Y5 stransiently expressed in COS-1 cells using [125I]-PYY | Bioorg Med Chem Lett 15: 2565-9 (2005) Article DOI: 10.1016/j.bmcl.2005.03.052 BindingDB Entry DOI: 10.7270/Q2Z89BXQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-2A adrenergic receptor (Homo sapiens (Human)) | BDBM50166563 (4-Bromo-N-[4-(4-dimethylamino-quinazolin-2-ylamino...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 47 | n/a | n/a | n/a | n/a | n/a | n/a |
Taisho Pharmaceutical Co. Ltd Curated by ChEMBL | Assay Description Inhibitory activity against constitutively activated human Alpha-2A adrenergic receptor transiently expressed in COS-1 cells using [3H]-MK912 | Bioorg Med Chem Lett 15: 2565-9 (2005) Article DOI: 10.1016/j.bmcl.2005.03.052 BindingDB Entry DOI: 10.7270/Q2Z89BXQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-2A adrenergic receptor (Homo sapiens (Human)) | BDBM50166565 (4-Bromo-N-[4-(quinazolin-2-ylamino)-cyclohexyl]-2-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 57 | n/a | n/a | n/a | n/a | n/a | n/a |
Taisho Pharmaceutical Co. Ltd Curated by ChEMBL | Assay Description Inhibitory activity against constitutively activated human Alpha-2A adrenergic receptor transiently expressed in COS-1 cells using [3H]-MK912 | Bioorg Med Chem Lett 15: 2565-9 (2005) Article DOI: 10.1016/j.bmcl.2005.03.052 BindingDB Entry DOI: 10.7270/Q2Z89BXQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-2A adrenergic receptor (Homo sapiens (Human)) | BDBM50166559 (Butane-1-sulfonic acid {4-[(4-dimethylamino-quinaz...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 58 | n/a | n/a | n/a | n/a | n/a | n/a |
Taisho Pharmaceutical Co. Ltd Curated by ChEMBL | Assay Description Inhibitory activity against constitutively activated human Alpha-2A adrenergic receptor transiently expressed in COS-1 cells using [3H]-MK912 | Bioorg Med Chem Lett 15: 2565-9 (2005) Article DOI: 10.1016/j.bmcl.2005.03.052 BindingDB Entry DOI: 10.7270/Q2Z89BXQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropeptide Y receptor type 5 (Homo sapiens (Human)) | BDBM50166562 (4-Bromo-N-[4-(quinazolin-2-ylaminomethyl)-cyclohex...) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 78 | n/a | n/a | n/a | n/a | n/a | n/a |
Taisho Pharmaceutical Co. Ltd Curated by ChEMBL | Assay Description Inhibitory activity against constitutively activated human Neuropeptide Y receptor Y5 stransiently expressed in COS-1 cells using [125I]-PYY | Bioorg Med Chem Lett 15: 2565-9 (2005) Article DOI: 10.1016/j.bmcl.2005.03.052 BindingDB Entry DOI: 10.7270/Q2Z89BXQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melanin-concentrating hormone receptor 1 (Homo sapiens (Human)) | BDBM50166565 (4-Bromo-N-[4-(quinazolin-2-ylamino)-cyclohexyl]-2-...) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 83 | n/a | n/a | n/a | n/a | n/a | n/a |
Taisho Pharmaceutical Co. Ltd Curated by ChEMBL | Assay Description Inhibitory activity against mutated constitutively activated human Melanin concentrating hormone receptor 1 (CA-MCH-R1) stably expressed in HEK293 ce... | Bioorg Med Chem Lett 15: 2565-9 (2005) Article DOI: 10.1016/j.bmcl.2005.03.052 BindingDB Entry DOI: 10.7270/Q2Z89BXQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-2A adrenergic receptor (Homo sapiens (Human)) | BDBM50166564 (CHEMBL195123 | N-{4-[(4-Dimethylamino-quinazolin-2...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 86 | n/a | n/a | n/a | n/a | n/a | n/a |
Taisho Pharmaceutical Co. Ltd Curated by ChEMBL | Assay Description Inhibitory activity against constitutively activated human Alpha-2A adrenergic receptor transiently expressed in COS-1 cells using [3H]-MK912 | Bioorg Med Chem Lett 15: 2565-9 (2005) Article DOI: 10.1016/j.bmcl.2005.03.052 BindingDB Entry DOI: 10.7270/Q2Z89BXQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-2A adrenergic receptor (Homo sapiens (Human)) | BDBM50166561 (CHEMBL195380 | Naphthalene-1-sulfonic acid {4-[(4-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 97 | n/a | n/a | n/a | n/a | n/a | n/a |
Taisho Pharmaceutical Co. Ltd Curated by ChEMBL | Assay Description Inhibitory activity against constitutively activated human Alpha-2A adrenergic receptor transiently expressed in COS-1 cells using [3H]-MK912 | Bioorg Med Chem Lett 15: 2565-9 (2005) Article DOI: 10.1016/j.bmcl.2005.03.052 BindingDB Entry DOI: 10.7270/Q2Z89BXQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-2A adrenergic receptor (Homo sapiens (Human)) | BDBM50166554 (CHEMBL192266 | N-{4-[(4-Amino-quinazolin-2-ylamino...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
Taisho Pharmaceutical Co. Ltd Curated by ChEMBL | Assay Description Inhibitory activity against constitutively activated human Alpha-2A adrenergic receptor transiently expressed in COS-1 cells using [3H]-MK912 | Bioorg Med Chem Lett 15: 2565-9 (2005) Article DOI: 10.1016/j.bmcl.2005.03.052 BindingDB Entry DOI: 10.7270/Q2Z89BXQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-2A adrenergic receptor (Homo sapiens (Human)) | BDBM50166560 (4-Bromo-N-[4-(4-dimethylamino-quinazolin-2-ylamino...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 110 | n/a | n/a | n/a | n/a | n/a | n/a |
Taisho Pharmaceutical Co. Ltd Curated by ChEMBL | Assay Description Inhibitory activity against constitutively activated human Alpha-2A adrenergic receptor transiently expressed in COS-1 cells using [3H]-MK912 | Bioorg Med Chem Lett 15: 2565-9 (2005) Article DOI: 10.1016/j.bmcl.2005.03.052 BindingDB Entry DOI: 10.7270/Q2Z89BXQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropeptide Y receptor type 5 (Homo sapiens (Human)) | BDBM50166557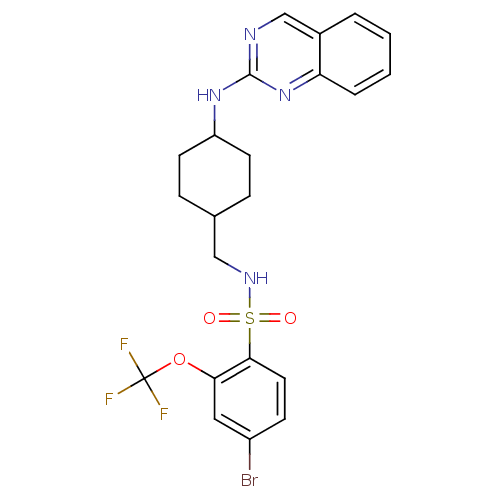 (4-Bromo-N-[4-(quinazolin-2-ylamino)-cyclohexylmeth...) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 120 | n/a | n/a | n/a | n/a | n/a | n/a |
Taisho Pharmaceutical Co. Ltd Curated by ChEMBL | Assay Description Inhibitory activity against constitutively activated human Neuropeptide Y receptor Y5 stransiently expressed in COS-1 cells using [125I]-PYY | Bioorg Med Chem Lett 15: 2565-9 (2005) Article DOI: 10.1016/j.bmcl.2005.03.052 BindingDB Entry DOI: 10.7270/Q2Z89BXQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-2A adrenergic receptor (Homo sapiens (Human)) | BDBM50166557 (4-Bromo-N-[4-(quinazolin-2-ylamino)-cyclohexylmeth...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 130 | n/a | n/a | n/a | n/a | n/a | n/a |
Taisho Pharmaceutical Co. Ltd Curated by ChEMBL | Assay Description Inhibitory activity against constitutively activated human Alpha-2A adrenergic receptor transiently expressed in COS-1 cells using [3H]-MK912 | Bioorg Med Chem Lett 15: 2565-9 (2005) Article DOI: 10.1016/j.bmcl.2005.03.052 BindingDB Entry DOI: 10.7270/Q2Z89BXQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-2A adrenergic receptor (Homo sapiens (Human)) | BDBM50166562 (4-Bromo-N-[4-(quinazolin-2-ylaminomethyl)-cyclohex...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 140 | n/a | n/a | n/a | n/a | n/a | n/a |
Taisho Pharmaceutical Co. Ltd Curated by ChEMBL | Assay Description Inhibitory activity against constitutively activated human Alpha-2A adrenergic receptor transiently expressed in COS-1 cells using [3H]-MK912 | Bioorg Med Chem Lett 15: 2565-9 (2005) Article DOI: 10.1016/j.bmcl.2005.03.052 BindingDB Entry DOI: 10.7270/Q2Z89BXQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melanin-concentrating hormone receptor 1 (Homo sapiens (Human)) | BDBM50166557 (4-Bromo-N-[4-(quinazolin-2-ylamino)-cyclohexylmeth...) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 140 | n/a | n/a | n/a | n/a | n/a | n/a |
Taisho Pharmaceutical Co. Ltd Curated by ChEMBL | Assay Description Inhibitory activity against mutated constitutively activated human Melanin concentrating hormone receptor 1 (CA-MCH-R1) stably expressed in HEK293 ce... | Bioorg Med Chem Lett 15: 2565-9 (2005) Article DOI: 10.1016/j.bmcl.2005.03.052 BindingDB Entry DOI: 10.7270/Q2Z89BXQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melanin-concentrating hormone receptor 1 (Homo sapiens (Human)) | BDBM50166555 (4-Bromo-N-{4-[(4-dimethylamino-quinazolin-2-ylamin...) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 140 | n/a | n/a | n/a | n/a | n/a | n/a |
Taisho Pharmaceutical Co. Ltd Curated by ChEMBL | Assay Description Inhibitory activity against human wild type Melanin concentrating hormone receptor 1 induced calcium flux stably expressed in HEK293 cells (n=6) | Bioorg Med Chem Lett 15: 2565-9 (2005) Article DOI: 10.1016/j.bmcl.2005.03.052 BindingDB Entry DOI: 10.7270/Q2Z89BXQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropeptide Y receptor type 5 (Homo sapiens (Human)) | BDBM50166560 (4-Bromo-N-[4-(4-dimethylamino-quinazolin-2-ylamino...) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 150 | n/a | n/a | n/a | n/a | n/a | n/a |
Taisho Pharmaceutical Co. Ltd Curated by ChEMBL | Assay Description Inhibitory activity against constitutively activated human Neuropeptide Y receptor Y5 stransiently expressed in COS-1 cells using [125I]-PYY | Bioorg Med Chem Lett 15: 2565-9 (2005) Article DOI: 10.1016/j.bmcl.2005.03.052 BindingDB Entry DOI: 10.7270/Q2Z89BXQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melanin-concentrating hormone receptor 1 (Homo sapiens (Human)) | BDBM50166555 (4-Bromo-N-{4-[(4-dimethylamino-quinazolin-2-ylamin...) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 160 | n/a | n/a | n/a | n/a | n/a | n/a |
Taisho Pharmaceutical Co. Ltd Curated by ChEMBL | Assay Description Inhibitory activity against mutated constitutively activated human Melanin concentrating hormone receptor 1 (CA-MCH-R1) stably expressed in HEK293 ce... | Bioorg Med Chem Lett 15: 2565-9 (2005) Article DOI: 10.1016/j.bmcl.2005.03.052 BindingDB Entry DOI: 10.7270/Q2Z89BXQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-2A adrenergic receptor (Homo sapiens (Human)) | BDBM50166556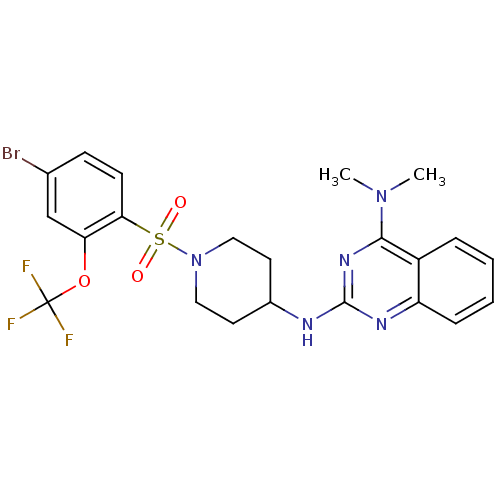 (CHEMBL360671 | N*2*-[1-(4-Bromo-2-trifluoromethoxy...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 170 | n/a | n/a | n/a | n/a | n/a | n/a |
Taisho Pharmaceutical Co. Ltd Curated by ChEMBL | Assay Description Inhibitory activity against constitutively activated human Alpha-2A adrenergic receptor transiently expressed in COS-1 cells using [3H]-MK912 | Bioorg Med Chem Lett 15: 2565-9 (2005) Article DOI: 10.1016/j.bmcl.2005.03.052 BindingDB Entry DOI: 10.7270/Q2Z89BXQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melanin-concentrating hormone receptor 1 (Homo sapiens (Human)) | BDBM50166555 (4-Bromo-N-{4-[(4-dimethylamino-quinazolin-2-ylamin...) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 200 | n/a | n/a | n/a | n/a | n/a | n/a |
Taisho Pharmaceutical Co. Ltd Curated by ChEMBL | Assay Description Inhibitory activity against human wild type Melanin concentrating hormone receptor 1 (CA-MCH-R1) stably expressed in HEK293 cells using [125I](Phe13,... | Bioorg Med Chem Lett 15: 2565-9 (2005) Article DOI: 10.1016/j.bmcl.2005.03.052 BindingDB Entry DOI: 10.7270/Q2Z89BXQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melanin-concentrating hormone receptor 1 (Homo sapiens (Human)) | BDBM50166556 (CHEMBL360671 | N*2*-[1-(4-Bromo-2-trifluoromethoxy...) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 250 | n/a | n/a | n/a | n/a | n/a | n/a |
Taisho Pharmaceutical Co. Ltd Curated by ChEMBL | Assay Description Inhibitory activity against mutated constitutively activated human Melanin concentrating hormone receptor 1 (CA-MCH-R1) stably expressed in HEK293 ce... | Bioorg Med Chem Lett 15: 2565-9 (2005) Article DOI: 10.1016/j.bmcl.2005.03.052 BindingDB Entry DOI: 10.7270/Q2Z89BXQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melanin-concentrating hormone receptor 1 (Homo sapiens (Human)) | BDBM50166552 (4-Bromo-N-{4-[(4-dimethylamino-quinazolin-2-ylamin...) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 810 | n/a | n/a | n/a | n/a | n/a | n/a |
Taisho Pharmaceutical Co. Ltd Curated by ChEMBL | Assay Description Inhibitory activity against mutated constitutively activated human Melanin concentrating hormone receptor 1 (CA-MCH-R1) stably expressed in HEK293 ce... | Bioorg Med Chem Lett 15: 2565-9 (2005) Article DOI: 10.1016/j.bmcl.2005.03.052 BindingDB Entry DOI: 10.7270/Q2Z89BXQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropeptide Y receptor type 5 (Homo sapiens (Human)) | BDBM50166565 (4-Bromo-N-[4-(quinazolin-2-ylamino)-cyclohexyl]-2-...) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 880 | n/a | n/a | n/a | n/a | n/a | n/a |
Taisho Pharmaceutical Co. Ltd Curated by ChEMBL | Assay Description Inhibitory activity against constitutively activated human Neuropeptide Y receptor Y5 stransiently expressed in COS-1 cells using [125I]-PYY | Bioorg Med Chem Lett 15: 2565-9 (2005) Article DOI: 10.1016/j.bmcl.2005.03.052 BindingDB Entry DOI: 10.7270/Q2Z89BXQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melanin-concentrating hormone receptor 1 (Homo sapiens (Human)) | BDBM50166560 (4-Bromo-N-[4-(4-dimethylamino-quinazolin-2-ylamino...) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Taisho Pharmaceutical Co. Ltd Curated by ChEMBL | Assay Description Inhibitory activity against mutated constitutively activated human Melanin concentrating hormone receptor 1 (CA-MCH-R1) stably expressed in HEK293 ce... | Bioorg Med Chem Lett 15: 2565-9 (2005) Article DOI: 10.1016/j.bmcl.2005.03.052 BindingDB Entry DOI: 10.7270/Q2Z89BXQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melanin-concentrating hormone receptor 1 (Homo sapiens (Human)) | BDBM50166554 (CHEMBL192266 | N-{4-[(4-Amino-quinazolin-2-ylamino...) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Taisho Pharmaceutical Co. Ltd Curated by ChEMBL | Assay Description Inhibitory activity against mutated constitutively activated human Melanin concentrating hormone receptor 1 (CA-MCH-R1) stably expressed in HEK293 ce... | Bioorg Med Chem Lett 15: 2565-9 (2005) Article DOI: 10.1016/j.bmcl.2005.03.052 BindingDB Entry DOI: 10.7270/Q2Z89BXQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melanin-concentrating hormone receptor 1 (Homo sapiens (Human)) | BDBM50166563 (4-Bromo-N-[4-(4-dimethylamino-quinazolin-2-ylamino...) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 2.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Taisho Pharmaceutical Co. Ltd Curated by ChEMBL | Assay Description Inhibitory activity against mutated constitutively activated human Melanin concentrating hormone receptor 1 (CA-MCH-R1) stably expressed in HEK293 ce... | Bioorg Med Chem Lett 15: 2565-9 (2005) Article DOI: 10.1016/j.bmcl.2005.03.052 BindingDB Entry DOI: 10.7270/Q2Z89BXQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melanin-concentrating hormone receptor 1 (Homo sapiens (Human)) | BDBM50166558 (4-Bromo-N-[4-(quinazolin-2-ylaminomethyl)-cyclohex...) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Taisho Pharmaceutical Co. Ltd Curated by ChEMBL | Assay Description Inhibitory activity against mutated constitutively activated human Melanin concentrating hormone receptor 1 (CA-MCH-R1) stably expressed in HEK293 ce... | Bioorg Med Chem Lett 15: 2565-9 (2005) Article DOI: 10.1016/j.bmcl.2005.03.052 BindingDB Entry DOI: 10.7270/Q2Z89BXQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melanin-concentrating hormone receptor 1 (Homo sapiens (Human)) | BDBM50166559 (Butane-1-sulfonic acid {4-[(4-dimethylamino-quinaz...) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 2.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Taisho Pharmaceutical Co. Ltd Curated by ChEMBL | Assay Description Inhibitory activity against mutated constitutively activated human Melanin concentrating hormone receptor 1 (CA-MCH-R1) stably expressed in HEK293 ce... | Bioorg Med Chem Lett 15: 2565-9 (2005) Article DOI: 10.1016/j.bmcl.2005.03.052 BindingDB Entry DOI: 10.7270/Q2Z89BXQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melanin-concentrating hormone receptor 1 (Homo sapiens (Human)) | BDBM50166562 (4-Bromo-N-[4-(quinazolin-2-ylaminomethyl)-cyclohex...) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Taisho Pharmaceutical Co. Ltd Curated by ChEMBL | Assay Description Inhibitory activity against mutated constitutively activated human Melanin concentrating hormone receptor 1 (CA-MCH-R1) stably expressed in HEK293 ce... | Bioorg Med Chem Lett 15: 2565-9 (2005) Article DOI: 10.1016/j.bmcl.2005.03.052 BindingDB Entry DOI: 10.7270/Q2Z89BXQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropeptide Y receptor type 5 (Homo sapiens (Human)) | BDBM50166551 (CHEMBL194173 | N*2*-[1-(4-Bromo-2-trifluoromethoxy...) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 3.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Taisho Pharmaceutical Co. Ltd Curated by ChEMBL | Assay Description Inhibitory activity against constitutively activated human Neuropeptide Y receptor Y5 stransiently expressed in COS-1 cells using [125I]-PYY | Bioorg Med Chem Lett 15: 2565-9 (2005) Article DOI: 10.1016/j.bmcl.2005.03.052 BindingDB Entry DOI: 10.7270/Q2Z89BXQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melanin-concentrating hormone receptor 1 (Homo sapiens (Human)) | BDBM50166561 (CHEMBL195380 | Naphthalene-1-sulfonic acid {4-[(4-...) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 3.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Taisho Pharmaceutical Co. Ltd Curated by ChEMBL | Assay Description Inhibitory activity against mutated constitutively activated human Melanin concentrating hormone receptor 1 (CA-MCH-R1) stably expressed in HEK293 ce... | Bioorg Med Chem Lett 15: 2565-9 (2005) Article DOI: 10.1016/j.bmcl.2005.03.052 BindingDB Entry DOI: 10.7270/Q2Z89BXQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melanin-concentrating hormone receptor 1 (Homo sapiens (Human)) | BDBM50166551 (CHEMBL194173 | N*2*-[1-(4-Bromo-2-trifluoromethoxy...) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 6.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Taisho Pharmaceutical Co. Ltd Curated by ChEMBL | Assay Description Inhibitory activity against mutated constitutively activated human Melanin concentrating hormone receptor 1 (CA-MCH-R1) stably expressed in HEK293 ce... | Bioorg Med Chem Lett 15: 2565-9 (2005) Article DOI: 10.1016/j.bmcl.2005.03.052 BindingDB Entry DOI: 10.7270/Q2Z89BXQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melanin-concentrating hormone receptor 1 (Homo sapiens (Human)) | BDBM50166550 (CHEMBL194122 | Octane-1-sulfonic acid {4-[(4-dimet...) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 6.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Taisho Pharmaceutical Co. Ltd Curated by ChEMBL | Assay Description Inhibitory activity against mutated constitutively activated human Melanin concentrating hormone receptor 1 (CA-MCH-R1) stably expressed in HEK293 ce... | Bioorg Med Chem Lett 15: 2565-9 (2005) Article DOI: 10.1016/j.bmcl.2005.03.052 BindingDB Entry DOI: 10.7270/Q2Z89BXQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropeptide Y receptor type 5 (Homo sapiens (Human)) | BDBM50166556 (CHEMBL360671 | N*2*-[1-(4-Bromo-2-trifluoromethoxy...) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 8.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Taisho Pharmaceutical Co. Ltd Curated by ChEMBL | Assay Description Inhibitory activity against constitutively activated human Neuropeptide Y receptor Y5 stransiently expressed in COS-1 cells using [125I]-PYY | Bioorg Med Chem Lett 15: 2565-9 (2005) Article DOI: 10.1016/j.bmcl.2005.03.052 BindingDB Entry DOI: 10.7270/Q2Z89BXQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melanin-concentrating hormone receptor 1 (Homo sapiens (Human)) | BDBM50166562 (4-Bromo-N-[4-(quinazolin-2-ylaminomethyl)-cyclohex...) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Taisho Pharmaceutical Co. Ltd Curated by ChEMBL | Assay Description Inhibitory activity against mutated constitutively activated human Melanin concentrating hormone receptor 1 (CA-MCH-R1) stably expressed in HEK293 ce... | Bioorg Med Chem Lett 15: 2565-9 (2005) Article DOI: 10.1016/j.bmcl.2005.03.052 BindingDB Entry DOI: 10.7270/Q2Z89BXQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melanin-concentrating hormone receptor 1 (Homo sapiens (Human)) | BDBM50166553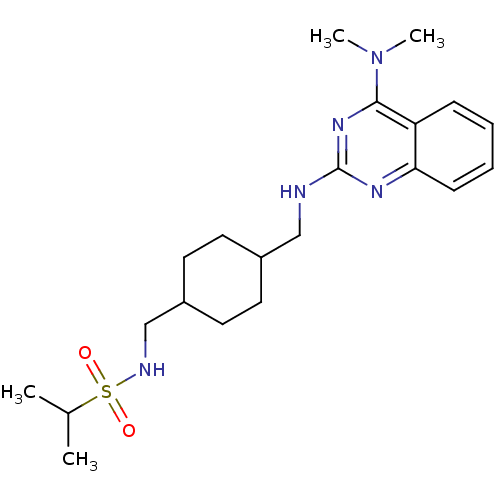 (CHEMBL427539 | Propane-2-sulfonic acid {4-[(4-dime...) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Taisho Pharmaceutical Co. Ltd Curated by ChEMBL | Assay Description Inhibitory activity against mutated constitutively activated human Melanin concentrating hormone receptor 1 (CA-MCH-R1) stably expressed in HEK293 ce... | Bioorg Med Chem Lett 15: 2565-9 (2005) Article DOI: 10.1016/j.bmcl.2005.03.052 BindingDB Entry DOI: 10.7270/Q2Z89BXQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melanin-concentrating hormone receptor 1 (Homo sapiens (Human)) | BDBM50166564 (CHEMBL195123 | N-{4-[(4-Dimethylamino-quinazolin-2...) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Taisho Pharmaceutical Co. Ltd Curated by ChEMBL | Assay Description Inhibitory activity against mutated constitutively activated human Melanin concentrating hormone receptor 1 (CA-MCH-R1) stably expressed in HEK293 ce... | Bioorg Med Chem Lett 15: 2565-9 (2005) Article DOI: 10.1016/j.bmcl.2005.03.052 BindingDB Entry DOI: 10.7270/Q2Z89BXQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||