

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Oxysterols receptor LXR-alpha (Homo sapiens (Human)) | BDBM50167697 ((4aR,9S)-6-Hydroxy-1,4a-dimethyl-1,2,3,4,4a,9,10,1...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibitory concentration in LXRSPA alpha binding assay | Bioorg Med Chem Lett 15: 4574-8 (2005) Article DOI: 10.1016/j.bmcl.2005.06.100 BindingDB Entry DOI: 10.7270/Q2154GKK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxysterols receptor LXR-beta (Homo sapiens (Human)) | BDBM50167697 ((4aR,9S)-6-Hydroxy-1,4a-dimethyl-1,2,3,4,4a,9,10,1...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibitory concentration in LXRSPA beta binding assay | Bioorg Med Chem Lett 15: 4574-8 (2005) Article DOI: 10.1016/j.bmcl.2005.06.100 BindingDB Entry DOI: 10.7270/Q2154GKK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxysterols receptor LXR-beta (Homo sapiens (Human)) | BDBM50167694 (2,6-dimethyl-13-methylcarbonyloxy-(2S)-tricyclo[8....) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibitory concentration in LXRSPA beta binding assay | Bioorg Med Chem Lett 15: 4574-8 (2005) Article DOI: 10.1016/j.bmcl.2005.06.100 BindingDB Entry DOI: 10.7270/Q2154GKK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxysterols receptor LXR-alpha (Homo sapiens (Human)) | BDBM50167694 (2,6-dimethyl-13-methylcarbonyloxy-(2S)-tricyclo[8....) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibitory concentration in LXRSPA alpha binding assay | Bioorg Med Chem Lett 15: 4574-8 (2005) Article DOI: 10.1016/j.bmcl.2005.06.100 BindingDB Entry DOI: 10.7270/Q2154GKK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxysterols receptor LXR-beta (Homo sapiens (Human)) | BDBM50172204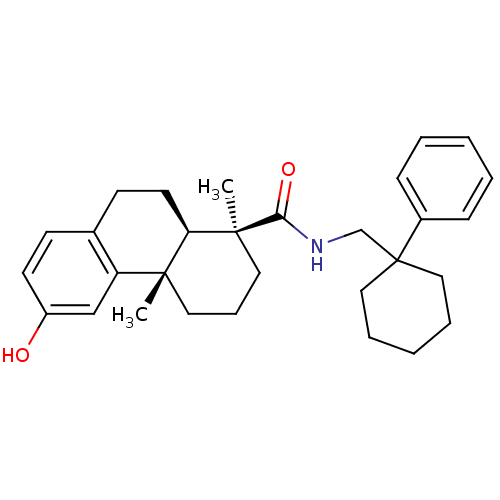 ((1S,4aS,10aR)-6-Hydroxy-1,4a-dimethyl-1,2,3,4,4a,9...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibitory concentration in LXRSPA beta binding assay | Bioorg Med Chem Lett 15: 4574-8 (2005) Article DOI: 10.1016/j.bmcl.2005.06.100 BindingDB Entry DOI: 10.7270/Q2154GKK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxysterols receptor LXR-alpha (Homo sapiens (Human)) | BDBM50172191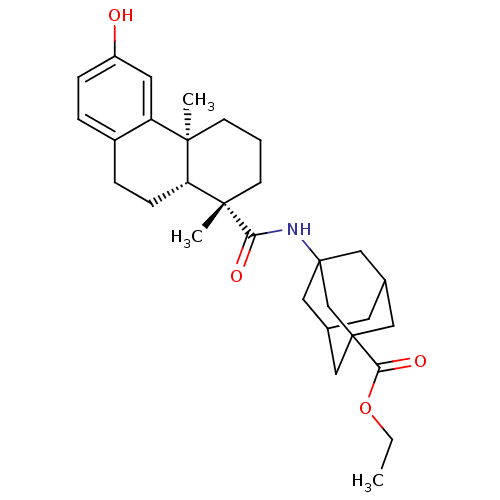 (3-[((4S,4aR)-6-Hydroxy-4a-methyl-1-(S)-methyl-1,2,...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibitory concentration in LXRSPA alpha binding assay | Bioorg Med Chem Lett 15: 4574-8 (2005) Article DOI: 10.1016/j.bmcl.2005.06.100 BindingDB Entry DOI: 10.7270/Q2154GKK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxysterols receptor LXR-alpha (Homo sapiens (Human)) | BDBM50172204 ((1S,4aS,10aR)-6-Hydroxy-1,4a-dimethyl-1,2,3,4,4a,9...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibitory concentration in LXRSPA alpha binding assay | Bioorg Med Chem Lett 15: 4574-8 (2005) Article DOI: 10.1016/j.bmcl.2005.06.100 BindingDB Entry DOI: 10.7270/Q2154GKK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxysterols receptor LXR-alpha (Homo sapiens (Human)) | BDBM50172206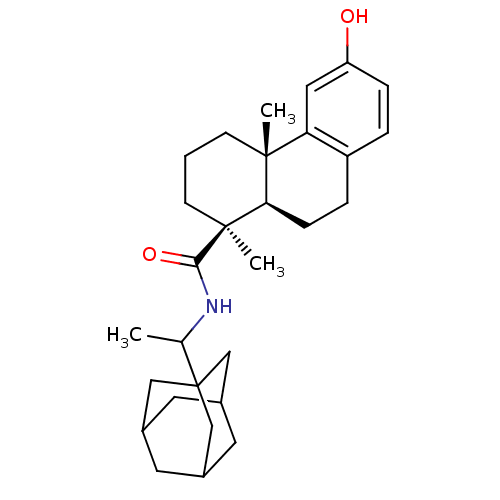 ((4S,4aR)-6-Hydroxy-4a-methyl-1-(S)-methyl-1,2,3,4,...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 90 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibitory concentration in LXRSPA alpha binding assay | Bioorg Med Chem Lett 15: 4574-8 (2005) Article DOI: 10.1016/j.bmcl.2005.06.100 BindingDB Entry DOI: 10.7270/Q2154GKK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxysterols receptor LXR-beta (Homo sapiens (Human)) | BDBM50172188 ((4S,4aR)-6-Hydroxy-4a-methyl-1-(S)-methyl-1,2,3,4,...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibitory concentration in LXRSPA beta binding assay | Bioorg Med Chem Lett 15: 4574-8 (2005) Article DOI: 10.1016/j.bmcl.2005.06.100 BindingDB Entry DOI: 10.7270/Q2154GKK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxysterols receptor LXR-alpha (Homo sapiens (Human)) | BDBM50172185 ((1S,4aS,10aR)-6-Hydroxy-1,4a-dimethyl-1,2,3,4,4a,9...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibitory concentration in LXRSPA alpha binding assay | Bioorg Med Chem Lett 15: 4574-8 (2005) Article DOI: 10.1016/j.bmcl.2005.06.100 BindingDB Entry DOI: 10.7270/Q2154GKK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxysterols receptor LXR-beta (Homo sapiens (Human)) | BDBM50172199 (1-Phenyl-cyclohexanecarboxylic acid ((1S,4aS,10aR)...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 120 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibitory concentration in LXRSPA beta binding assay | Bioorg Med Chem Lett 15: 4574-8 (2005) Article DOI: 10.1016/j.bmcl.2005.06.100 BindingDB Entry DOI: 10.7270/Q2154GKK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxysterols receptor LXR-alpha (Homo sapiens (Human)) | BDBM50172188 ((4S,4aR)-6-Hydroxy-4a-methyl-1-(S)-methyl-1,2,3,4,...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 130 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibitory concentration in LXRSPA alpha binding assay | Bioorg Med Chem Lett 15: 4574-8 (2005) Article DOI: 10.1016/j.bmcl.2005.06.100 BindingDB Entry DOI: 10.7270/Q2154GKK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxysterols receptor LXR-beta (Homo sapiens (Human)) | BDBM50172186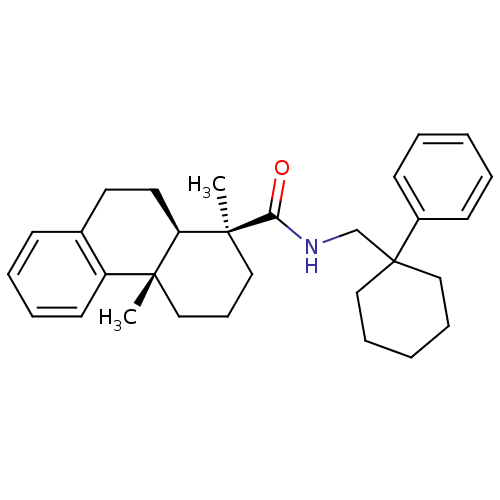 ((1S,4aS,10aR)-1,4a-Dimethyl-1,2,3,4,4a,9,10,10a-oc...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 130 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibitory concentration in LXRSPA beta binding assay | Bioorg Med Chem Lett 15: 4574-8 (2005) Article DOI: 10.1016/j.bmcl.2005.06.100 BindingDB Entry DOI: 10.7270/Q2154GKK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxysterols receptor LXR-alpha (Homo sapiens (Human)) | BDBM50172200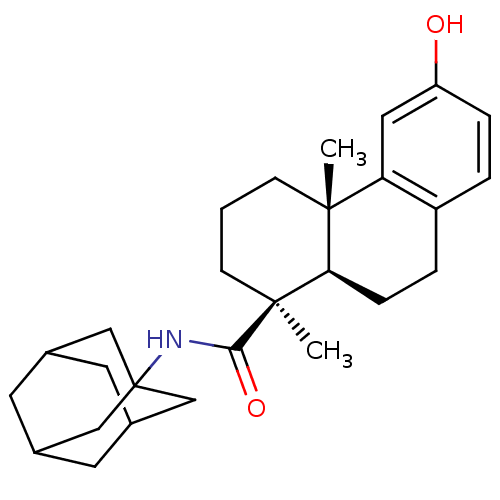 ((4S,4aR)-6-Hydroxy-4a-methyl-1-(S)-methyl-1,2,3,4,...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 140 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibitory concentration in LXRSPA alpha binding assay | Bioorg Med Chem Lett 15: 4574-8 (2005) Article DOI: 10.1016/j.bmcl.2005.06.100 BindingDB Entry DOI: 10.7270/Q2154GKK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxysterols receptor LXR-beta (Homo sapiens (Human)) | BDBM50172200 ((4S,4aR)-6-Hydroxy-4a-methyl-1-(S)-methyl-1,2,3,4,...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 170 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibitory concentration in LXRSPA beta binding assay | Bioorg Med Chem Lett 15: 4574-8 (2005) Article DOI: 10.1016/j.bmcl.2005.06.100 BindingDB Entry DOI: 10.7270/Q2154GKK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxysterols receptor LXR-beta (Homo sapiens (Human)) | BDBM50172206 ((4S,4aR)-6-Hydroxy-4a-methyl-1-(S)-methyl-1,2,3,4,...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 180 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibitory concentration in LXRSPA beta binding assay | Bioorg Med Chem Lett 15: 4574-8 (2005) Article DOI: 10.1016/j.bmcl.2005.06.100 BindingDB Entry DOI: 10.7270/Q2154GKK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxysterols receptor LXR-beta (Homo sapiens (Human)) | BDBM50172195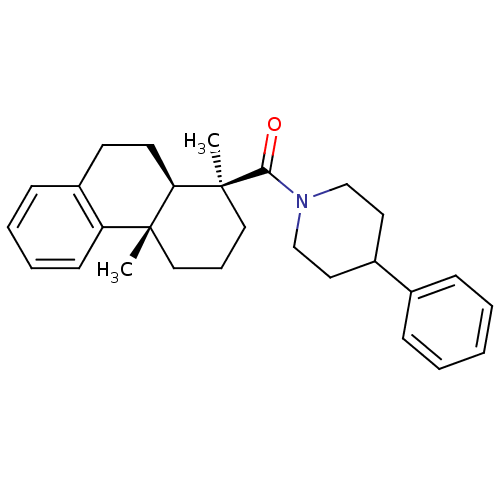 (((1S,4aS,10aR)-1,4a-Dimethyl-1,2,3,4,4a,9,10,10a-o...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 180 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibitory concentration in LXRSPA beta binding assay | Bioorg Med Chem Lett 15: 4574-8 (2005) Article DOI: 10.1016/j.bmcl.2005.06.100 BindingDB Entry DOI: 10.7270/Q2154GKK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxysterols receptor LXR-beta (Homo sapiens (Human)) | BDBM50172185 ((1S,4aS,10aR)-6-Hydroxy-1,4a-dimethyl-1,2,3,4,4a,9...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 190 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibitory concentration in LXRSPA beta binding assay | Bioorg Med Chem Lett 15: 4574-8 (2005) Article DOI: 10.1016/j.bmcl.2005.06.100 BindingDB Entry DOI: 10.7270/Q2154GKK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxysterols receptor LXR-beta (Homo sapiens (Human)) | BDBM50172192 ((Dodecahydro-carbazol-9-yl)-((1S,4aS,10aR)-6-hydro...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 190 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibitory concentration in LXRSPA beta binding assay | Bioorg Med Chem Lett 15: 4574-8 (2005) Article DOI: 10.1016/j.bmcl.2005.06.100 BindingDB Entry DOI: 10.7270/Q2154GKK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxysterols receptor LXR-beta (Homo sapiens (Human)) | BDBM50172190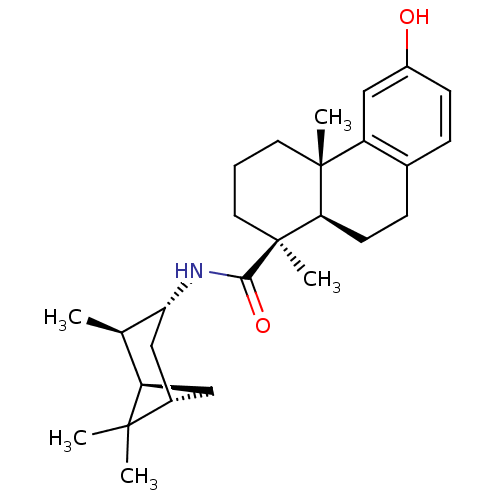 ((4S,4aR)-6-Hydroxy-4a-methyl-1-(S)-methyl-1,2,3,4,...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 200 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibitory concentration in LXRSPA beta binding assay | Bioorg Med Chem Lett 15: 4574-8 (2005) Article DOI: 10.1016/j.bmcl.2005.06.100 BindingDB Entry DOI: 10.7270/Q2154GKK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxysterols receptor LXR-alpha (Homo sapiens (Human)) | BDBM50172195 (((1S,4aS,10aR)-1,4a-Dimethyl-1,2,3,4,4a,9,10,10a-o...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 200 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibitory concentration in LXRSPA alpha binding assay | Bioorg Med Chem Lett 15: 4574-8 (2005) Article DOI: 10.1016/j.bmcl.2005.06.100 BindingDB Entry DOI: 10.7270/Q2154GKK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxysterols receptor LXR-alpha (Homo sapiens (Human)) | BDBM50172186 ((1S,4aS,10aR)-1,4a-Dimethyl-1,2,3,4,4a,9,10,10a-oc...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 200 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibitory concentration in LXRSPA alpha binding assay | Bioorg Med Chem Lett 15: 4574-8 (2005) Article DOI: 10.1016/j.bmcl.2005.06.100 BindingDB Entry DOI: 10.7270/Q2154GKK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxysterols receptor LXR-alpha (Homo sapiens (Human)) | BDBM50172190 ((4S,4aR)-6-Hydroxy-4a-methyl-1-(S)-methyl-1,2,3,4,...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 270 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibitory concentration in LXRSPA alpha binding assay | Bioorg Med Chem Lett 15: 4574-8 (2005) Article DOI: 10.1016/j.bmcl.2005.06.100 BindingDB Entry DOI: 10.7270/Q2154GKK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxysterols receptor LXR-beta (Homo sapiens (Human)) | BDBM50172207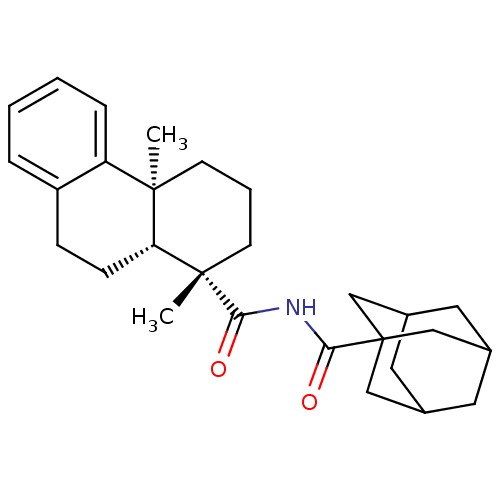 (Adamantane-1-carboxylic acid ((1S,4aS,10aR)-1,4a-d...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 290 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibitory concentration in LXRSPA beta binding assay | Bioorg Med Chem Lett 15: 4574-8 (2005) Article DOI: 10.1016/j.bmcl.2005.06.100 BindingDB Entry DOI: 10.7270/Q2154GKK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxysterols receptor LXR-alpha (Homo sapiens (Human)) | BDBM50172207 (Adamantane-1-carboxylic acid ((1S,4aS,10aR)-1,4a-d...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 290 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibitory concentration in LXRSPA alpha binding assay | Bioorg Med Chem Lett 15: 4574-8 (2005) Article DOI: 10.1016/j.bmcl.2005.06.100 BindingDB Entry DOI: 10.7270/Q2154GKK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxysterols receptor LXR-alpha (Homo sapiens (Human)) | BDBM50172192 ((Dodecahydro-carbazol-9-yl)-((1S,4aS,10aR)-6-hydro...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 300 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibitory concentration in LXRSPA alpha binding assay | Bioorg Med Chem Lett 15: 4574-8 (2005) Article DOI: 10.1016/j.bmcl.2005.06.100 BindingDB Entry DOI: 10.7270/Q2154GKK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxysterols receptor LXR-beta (Homo sapiens (Human)) | BDBM50172197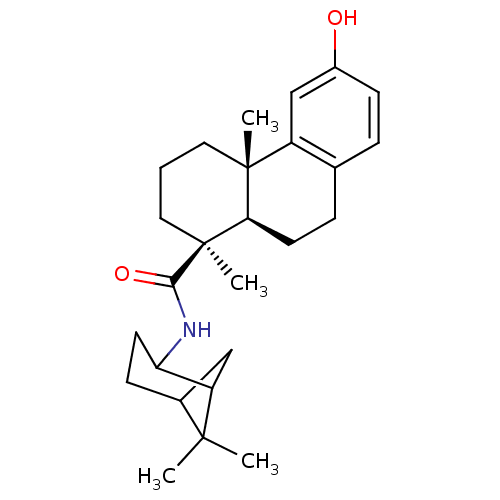 ((4S,4aR)-6-Hydroxy-4a-methyl-1-(S)-methyl-1,2,3,4,...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 310 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibitory concentration in LXRSPA beta binding assay | Bioorg Med Chem Lett 15: 4574-8 (2005) Article DOI: 10.1016/j.bmcl.2005.06.100 BindingDB Entry DOI: 10.7270/Q2154GKK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxysterols receptor LXR-alpha (Homo sapiens (Human)) | BDBM50172197 ((4S,4aR)-6-Hydroxy-4a-methyl-1-(S)-methyl-1,2,3,4,...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 350 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibitory concentration in LXRSPA alpha binding assay | Bioorg Med Chem Lett 15: 4574-8 (2005) Article DOI: 10.1016/j.bmcl.2005.06.100 BindingDB Entry DOI: 10.7270/Q2154GKK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxysterols receptor LXR-alpha (Homo sapiens (Human)) | BDBM50172189 (((1S,4aS,10aR)-1,4a-Dimethyl-1,2,3,4,4a,9,10,10a-o...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 360 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibitory concentration in LXRSPA alpha binding assay | Bioorg Med Chem Lett 15: 4574-8 (2005) Article DOI: 10.1016/j.bmcl.2005.06.100 BindingDB Entry DOI: 10.7270/Q2154GKK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxysterols receptor LXR-beta (Homo sapiens (Human)) | BDBM50172189 (((1S,4aS,10aR)-1,4a-Dimethyl-1,2,3,4,4a,9,10,10a-o...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 370 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibitory concentration in LXRSPA beta binding assay | Bioorg Med Chem Lett 15: 4574-8 (2005) Article DOI: 10.1016/j.bmcl.2005.06.100 BindingDB Entry DOI: 10.7270/Q2154GKK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxysterols receptor LXR-alpha (Homo sapiens (Human)) | BDBM50172199 (1-Phenyl-cyclohexanecarboxylic acid ((1S,4aS,10aR)...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 420 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibitory concentration in LXRSPA alpha binding assay | Bioorg Med Chem Lett 15: 4574-8 (2005) Article DOI: 10.1016/j.bmcl.2005.06.100 BindingDB Entry DOI: 10.7270/Q2154GKK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxysterols receptor LXR-beta (Homo sapiens (Human)) | BDBM50172203 ((1S,4aS,10aR)-1,4a-Dimethyl-1,2,3,4,4a,9,10,10a-oc...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 420 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibitory concentration in LXRSPA beta binding assay | Bioorg Med Chem Lett 15: 4574-8 (2005) Article DOI: 10.1016/j.bmcl.2005.06.100 BindingDB Entry DOI: 10.7270/Q2154GKK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxysterols receptor LXR-alpha (Homo sapiens (Human)) | BDBM50172203 ((1S,4aS,10aR)-1,4a-Dimethyl-1,2,3,4,4a,9,10,10a-oc...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 490 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibitory concentration in LXRSPA alpha binding assay | Bioorg Med Chem Lett 15: 4574-8 (2005) Article DOI: 10.1016/j.bmcl.2005.06.100 BindingDB Entry DOI: 10.7270/Q2154GKK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxysterols receptor LXR-beta (Homo sapiens (Human)) | BDBM50172198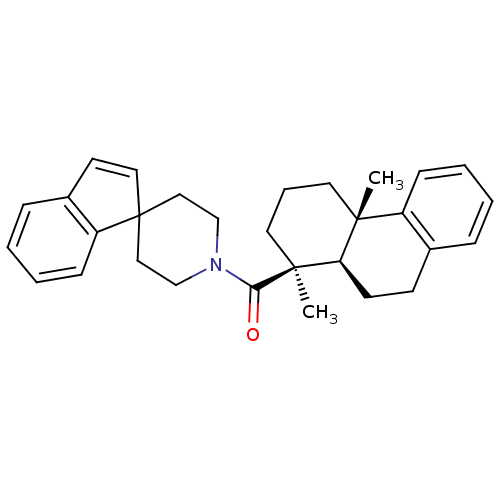 (2,6-dimethyl-(2S,6S,7R)-tricyclo[8.4.0.02,7]tetrad...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 500 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibitory concentration in LXRSPA beta binding assay | Bioorg Med Chem Lett 15: 4574-8 (2005) Article DOI: 10.1016/j.bmcl.2005.06.100 BindingDB Entry DOI: 10.7270/Q2154GKK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxysterols receptor LXR-beta (Homo sapiens (Human)) | BDBM50172191 (3-[((4S,4aR)-6-Hydroxy-4a-methyl-1-(S)-methyl-1,2,...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 530 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibitory concentration in LXRSPA beta binding assay | Bioorg Med Chem Lett 15: 4574-8 (2005) Article DOI: 10.1016/j.bmcl.2005.06.100 BindingDB Entry DOI: 10.7270/Q2154GKK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxysterols receptor LXR-alpha (Homo sapiens (Human)) | BDBM50172198 (2,6-dimethyl-(2S,6S,7R)-tricyclo[8.4.0.02,7]tetrad...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 750 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibitory concentration in LXRSPA alpha binding assay | Bioorg Med Chem Lett 15: 4574-8 (2005) Article DOI: 10.1016/j.bmcl.2005.06.100 BindingDB Entry DOI: 10.7270/Q2154GKK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxysterols receptor LXR-beta (Homo sapiens (Human)) | BDBM50172187 (1-Adamantan-1-yl-3-((1S,4aS,10aR)-1,4a-dimethyl-1,...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.01E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibitory concentration in LXRSPA beta binding assay | Bioorg Med Chem Lett 15: 4574-8 (2005) Article DOI: 10.1016/j.bmcl.2005.06.100 BindingDB Entry DOI: 10.7270/Q2154GKK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxysterols receptor LXR-alpha (Homo sapiens (Human)) | BDBM50172187 (1-Adamantan-1-yl-3-((1S,4aS,10aR)-1,4a-dimethyl-1,...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.67E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibitory concentration in LXRSPA alpha binding assay | Bioorg Med Chem Lett 15: 4574-8 (2005) Article DOI: 10.1016/j.bmcl.2005.06.100 BindingDB Entry DOI: 10.7270/Q2154GKK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxysterols receptor LXR-beta (Homo sapiens (Human)) | BDBM50172193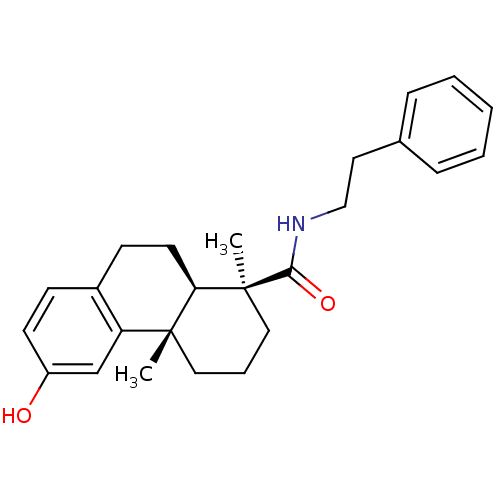 ((4S,4aR)-6-Hydroxy-4a-methyl-1-(S)-methyl-1,2,3,4,...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.22E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibitory concentration in LXRSPA beta binding assay | Bioorg Med Chem Lett 15: 4574-8 (2005) Article DOI: 10.1016/j.bmcl.2005.06.100 BindingDB Entry DOI: 10.7270/Q2154GKK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxysterols receptor LXR-alpha (Homo sapiens (Human)) | BDBM50172196 ((1S,4aS,10aR)-6-Hydroxy-1,4a-dimethyl-1,2,3,4,4a,9...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibitory concentration in LXRSPA alpha binding assay | Bioorg Med Chem Lett 15: 4574-8 (2005) Article DOI: 10.1016/j.bmcl.2005.06.100 BindingDB Entry DOI: 10.7270/Q2154GKK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxysterols receptor LXR-beta (Homo sapiens (Human)) | BDBM50172196 ((1S,4aS,10aR)-6-Hydroxy-1,4a-dimethyl-1,2,3,4,4a,9...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibitory concentration in LXRSPA beta binding assay | Bioorg Med Chem Lett 15: 4574-8 (2005) Article DOI: 10.1016/j.bmcl.2005.06.100 BindingDB Entry DOI: 10.7270/Q2154GKK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxysterols receptor LXR-alpha (Homo sapiens (Human)) | BDBM50172193 ((4S,4aR)-6-Hydroxy-4a-methyl-1-(S)-methyl-1,2,3,4,...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.93E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibitory concentration in LXRSPA alpha binding assay | Bioorg Med Chem Lett 15: 4574-8 (2005) Article DOI: 10.1016/j.bmcl.2005.06.100 BindingDB Entry DOI: 10.7270/Q2154GKK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxysterols receptor LXR-alpha (Homo sapiens (Human)) | BDBM50172202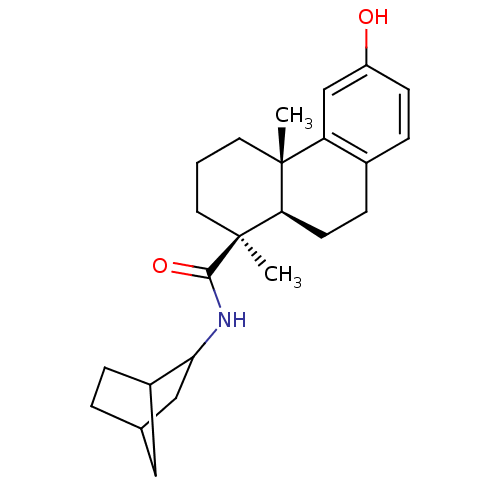 ((4S,4aR)-6-Hydroxy-4a-methyl-1-(S)-methyl-1,2,3,4,...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibitory concentration in LXRSPA alpha binding assay | Bioorg Med Chem Lett 15: 4574-8 (2005) Article DOI: 10.1016/j.bmcl.2005.06.100 BindingDB Entry DOI: 10.7270/Q2154GKK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxysterols receptor LXR-alpha (Homo sapiens (Human)) | BDBM50172205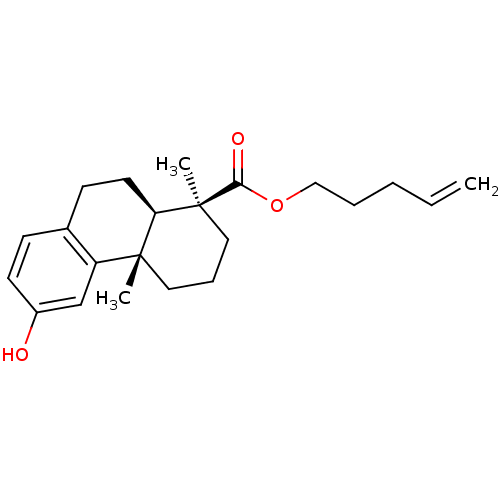 ((1S,4aS,10aR)-6-Hydroxy-1,4a-dimethyl-1,2,3,4,4a,9...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibitory concentration in LXRSPA alpha binding assay | Bioorg Med Chem Lett 15: 4574-8 (2005) Article DOI: 10.1016/j.bmcl.2005.06.100 BindingDB Entry DOI: 10.7270/Q2154GKK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxysterols receptor LXR-beta (Homo sapiens (Human)) | BDBM50172194 ((1S,4aS,10aR)-6-Hydroxy-1,4a-dimethyl-1,2,3,4,4a,9...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.85E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibitory concentration in LXRSPA beta binding assay | Bioorg Med Chem Lett 15: 4574-8 (2005) Article DOI: 10.1016/j.bmcl.2005.06.100 BindingDB Entry DOI: 10.7270/Q2154GKK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxysterols receptor LXR-beta (Homo sapiens (Human)) | BDBM50172202 ((4S,4aR)-6-Hydroxy-4a-methyl-1-(S)-methyl-1,2,3,4,...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.12E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibitory concentration in LXRSPA beta binding assay | Bioorg Med Chem Lett 15: 4574-8 (2005) Article DOI: 10.1016/j.bmcl.2005.06.100 BindingDB Entry DOI: 10.7270/Q2154GKK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxysterols receptor LXR-beta (Homo sapiens (Human)) | BDBM50172205 ((1S,4aS,10aR)-6-Hydroxy-1,4a-dimethyl-1,2,3,4,4a,9...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibitory concentration in LXRSPA beta binding assay | Bioorg Med Chem Lett 15: 4574-8 (2005) Article DOI: 10.1016/j.bmcl.2005.06.100 BindingDB Entry DOI: 10.7270/Q2154GKK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxysterols receptor LXR-alpha (Homo sapiens (Human)) | BDBM50172194 ((1S,4aS,10aR)-6-Hydroxy-1,4a-dimethyl-1,2,3,4,4a,9...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.06E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibitory concentration in LXRSPA alpha binding assay | Bioorg Med Chem Lett 15: 4574-8 (2005) Article DOI: 10.1016/j.bmcl.2005.06.100 BindingDB Entry DOI: 10.7270/Q2154GKK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxysterols receptor LXR-beta (Homo sapiens (Human)) | BDBM50172201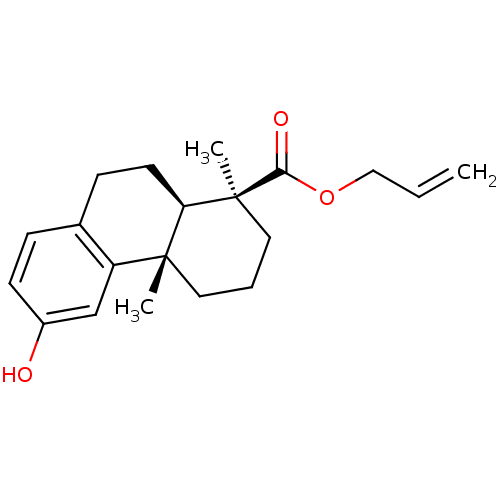 ((1S,4aS,10aR)-6-Hydroxy-1,4a-dimethyl-1,2,3,4,4a,9...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibitory concentration in LXRSPA beta binding assay | Bioorg Med Chem Lett 15: 4574-8 (2005) Article DOI: 10.1016/j.bmcl.2005.06.100 BindingDB Entry DOI: 10.7270/Q2154GKK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxysterols receptor LXR-alpha (Homo sapiens (Human)) | BDBM50172201 ((1S,4aS,10aR)-6-Hydroxy-1,4a-dimethyl-1,2,3,4,4a,9...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibitory concentration in LXRSPA alpha binding assay | Bioorg Med Chem Lett 15: 4574-8 (2005) Article DOI: 10.1016/j.bmcl.2005.06.100 BindingDB Entry DOI: 10.7270/Q2154GKK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxysterols receptor LXR-beta (Homo sapiens (Human)) | BDBM50172186 ((1S,4aS,10aR)-1,4a-Dimethyl-1,2,3,4,4a,9,10,10a-oc...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Effective concentration in recombinant human LXRbeta ligand binding domain in homogeneous time-resolved fluorescence assay | Bioorg Med Chem Lett 15: 4574-8 (2005) Article DOI: 10.1016/j.bmcl.2005.06.100 BindingDB Entry DOI: 10.7270/Q2154GKK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxysterols receptor LXR-alpha (Homo sapiens (Human)) | BDBM50167697 ((4aR,9S)-6-Hydroxy-1,4a-dimethyl-1,2,3,4,4a,9,10,1...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | <3 | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Effective concentration in transactivation assay using a chimeric LXR construct in HEK-293 cells for LXRalpha receptor | Bioorg Med Chem Lett 15: 4574-8 (2005) Article DOI: 10.1016/j.bmcl.2005.06.100 BindingDB Entry DOI: 10.7270/Q2154GKK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxysterols receptor LXR-beta (Homo sapiens (Human)) | BDBM50172198 (2,6-dimethyl-(2S,6S,7R)-tricyclo[8.4.0.02,7]tetrad...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 1.50E+3 | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Effective concentration in recombinant human LXRbeta ligand binding domain in homogeneous time-resolved fluorescence assay | Bioorg Med Chem Lett 15: 4574-8 (2005) Article DOI: 10.1016/j.bmcl.2005.06.100 BindingDB Entry DOI: 10.7270/Q2154GKK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxysterols receptor LXR-alpha (Homo sapiens (Human)) | BDBM50167694 (2,6-dimethyl-13-methylcarbonyloxy-(2S)-tricyclo[8....) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 10 | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Effective concentration in transactivation assay using a chimeric LXR construct in HEK-293 cells for LXRalpha receptor | Bioorg Med Chem Lett 15: 4574-8 (2005) Article DOI: 10.1016/j.bmcl.2005.06.100 BindingDB Entry DOI: 10.7270/Q2154GKK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxysterols receptor LXR-alpha (Homo sapiens (Human)) | BDBM50172188 ((4S,4aR)-6-Hydroxy-4a-methyl-1-(S)-methyl-1,2,3,4,...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 900 | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Effective concentration in transactivation assay using a chimeric LXR construct in HEK-293 cells for LXRalpha receptor | Bioorg Med Chem Lett 15: 4574-8 (2005) Article DOI: 10.1016/j.bmcl.2005.06.100 BindingDB Entry DOI: 10.7270/Q2154GKK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxysterols receptor LXR-beta (Homo sapiens (Human)) | BDBM50172206 ((4S,4aR)-6-Hydroxy-4a-methyl-1-(S)-methyl-1,2,3,4,...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Effective concentration in recombinant human LXRbeta ligand binding domain in homogeneous time-resolved fluorescence assay | Bioorg Med Chem Lett 15: 4574-8 (2005) Article DOI: 10.1016/j.bmcl.2005.06.100 BindingDB Entry DOI: 10.7270/Q2154GKK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxysterols receptor LXR-alpha (Homo sapiens (Human)) | BDBM50172196 ((1S,4aS,10aR)-6-Hydroxy-1,4a-dimethyl-1,2,3,4,4a,9...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | >1.50E+4 | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Effective concentration in transactivation assay using a chimeric LXR construct in HEK293 cells for LXRalpha receptor | Bioorg Med Chem Lett 15: 4574-8 (2005) Article DOI: 10.1016/j.bmcl.2005.06.100 BindingDB Entry DOI: 10.7270/Q2154GKK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxysterols receptor LXR-alpha (Homo sapiens (Human)) | BDBM50172189 (((1S,4aS,10aR)-1,4a-Dimethyl-1,2,3,4,4a,9,10,10a-o...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 150 | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Effective concentration in transactivation assay using a chimeric LXR construct in HEK293 cells for LXRalpha receptor | Bioorg Med Chem Lett 15: 4574-8 (2005) Article DOI: 10.1016/j.bmcl.2005.06.100 BindingDB Entry DOI: 10.7270/Q2154GKK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxysterols receptor LXR-alpha (Homo sapiens (Human)) | BDBM50172195 (((1S,4aS,10aR)-1,4a-Dimethyl-1,2,3,4,4a,9,10,10a-o...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 70 | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Effective concentration in transactivation assay using a chimeric LXR construct in HEK293 cells for LXRalpha receptor | Bioorg Med Chem Lett 15: 4574-8 (2005) Article DOI: 10.1016/j.bmcl.2005.06.100 BindingDB Entry DOI: 10.7270/Q2154GKK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxysterols receptor LXR-alpha (Homo sapiens (Human)) | BDBM50172205 ((1S,4aS,10aR)-6-Hydroxy-1,4a-dimethyl-1,2,3,4,4a,9...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Effective concentration in transactivation assay using a chimeric LXR construct in HEK293 cells for LXRalpha receptor | Bioorg Med Chem Lett 15: 4574-8 (2005) Article DOI: 10.1016/j.bmcl.2005.06.100 BindingDB Entry DOI: 10.7270/Q2154GKK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxysterols receptor LXR-alpha (Homo sapiens (Human)) | BDBM50172192 ((Dodecahydro-carbazol-9-yl)-((1S,4aS,10aR)-6-hydro...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 320 | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Effective concentration in transactivation assay using a chimeric LXR construct in HEK293 cells for LXRalpha receptor | Bioorg Med Chem Lett 15: 4574-8 (2005) Article DOI: 10.1016/j.bmcl.2005.06.100 BindingDB Entry DOI: 10.7270/Q2154GKK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxysterols receptor LXR-beta (Homo sapiens (Human)) | BDBM50172196 ((1S,4aS,10aR)-6-Hydroxy-1,4a-dimethyl-1,2,3,4,4a,9...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | >1.50E+4 | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Effective concentration in recombinant human LXRbeta ligand binding domain in homogeneous time-resolved fluorescence assay | Bioorg Med Chem Lett 15: 4574-8 (2005) Article DOI: 10.1016/j.bmcl.2005.06.100 BindingDB Entry DOI: 10.7270/Q2154GKK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxysterols receptor LXR-alpha (Homo sapiens (Human)) | BDBM50172187 (1-Adamantan-1-yl-3-((1S,4aS,10aR)-1,4a-dimethyl-1,...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Effective concentration in recombinant human LXRalpha ligand binding domain in homogeneous time-resolved fluorescence assay | Bioorg Med Chem Lett 15: 4574-8 (2005) Article DOI: 10.1016/j.bmcl.2005.06.100 BindingDB Entry DOI: 10.7270/Q2154GKK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxysterols receptor LXR-alpha (Homo sapiens (Human)) | BDBM50172199 (1-Phenyl-cyclohexanecarboxylic acid ((1S,4aS,10aR)...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 170 | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Effective concentration in recombinant human LXRalpha ligand binding domain in homogeneous time-resolved fluorescence assay | Bioorg Med Chem Lett 15: 4574-8 (2005) Article DOI: 10.1016/j.bmcl.2005.06.100 BindingDB Entry DOI: 10.7270/Q2154GKK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxysterols receptor LXR-beta (Homo sapiens (Human)) | BDBM50172205 ((1S,4aS,10aR)-6-Hydroxy-1,4a-dimethyl-1,2,3,4,4a,9...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Effective concentration in recombinant human LXRbeta ligand binding domain in homogeneous time-resolved fluorescence assay | Bioorg Med Chem Lett 15: 4574-8 (2005) Article DOI: 10.1016/j.bmcl.2005.06.100 BindingDB Entry DOI: 10.7270/Q2154GKK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxysterols receptor LXR-beta (Homo sapiens (Human)) | BDBM50172197 ((4S,4aR)-6-Hydroxy-4a-methyl-1-(S)-methyl-1,2,3,4,...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Effective concentration in recombinant human LXRbeta ligand binding domain in homogeneous time-resolved fluorescence assay | Bioorg Med Chem Lett 15: 4574-8 (2005) Article DOI: 10.1016/j.bmcl.2005.06.100 BindingDB Entry DOI: 10.7270/Q2154GKK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxysterols receptor LXR-alpha (Homo sapiens (Human)) | BDBM50172191 (3-[((4S,4aR)-6-Hydroxy-4a-methyl-1-(S)-methyl-1,2,...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | 760 | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Effective concentration in transactivation assay using a chimeric LXR construct in HEK293 cells for LXRalpha receptor | Bioorg Med Chem Lett 15: 4574-8 (2005) Article DOI: 10.1016/j.bmcl.2005.06.100 BindingDB Entry DOI: 10.7270/Q2154GKK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxysterols receptor LXR-alpha (Homo sapiens (Human)) | BDBM50172200 ((4S,4aR)-6-Hydroxy-4a-methyl-1-(S)-methyl-1,2,3,4,...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 220 | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Effective concentration in transactivation assay using a chimeric LXR construct in HEK293 cells for LXRalpha receptor | Bioorg Med Chem Lett 15: 4574-8 (2005) Article DOI: 10.1016/j.bmcl.2005.06.100 BindingDB Entry DOI: 10.7270/Q2154GKK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxysterols receptor LXR-beta (Homo sapiens (Human)) | BDBM50172190 ((4S,4aR)-6-Hydroxy-4a-methyl-1-(S)-methyl-1,2,3,4,...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Effective concentration in recombinant human LXRbeta ligand binding domain in homogeneous time-resolved fluorescence assay | Bioorg Med Chem Lett 15: 4574-8 (2005) Article DOI: 10.1016/j.bmcl.2005.06.100 BindingDB Entry DOI: 10.7270/Q2154GKK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxysterols receptor LXR-alpha (Homo sapiens (Human)) | BDBM50172190 ((4S,4aR)-6-Hydroxy-4a-methyl-1-(S)-methyl-1,2,3,4,...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 1.70E+3 | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Effective concentration in transactivation assay using a chimeric LXR construct in HEK-293 cells for LXRalpha receptor | Bioorg Med Chem Lett 15: 4574-8 (2005) Article DOI: 10.1016/j.bmcl.2005.06.100 BindingDB Entry DOI: 10.7270/Q2154GKK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxysterols receptor LXR-beta (Homo sapiens (Human)) | BDBM50172203 ((1S,4aS,10aR)-1,4a-Dimethyl-1,2,3,4,4a,9,10,10a-oc...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Effective concentration in recombinant human LXRbeta ligand binding domain in homogeneous time-resolved fluorescence assay | Bioorg Med Chem Lett 15: 4574-8 (2005) Article DOI: 10.1016/j.bmcl.2005.06.100 BindingDB Entry DOI: 10.7270/Q2154GKK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxysterols receptor LXR-beta (Homo sapiens (Human)) | BDBM50172195 (((1S,4aS,10aR)-1,4a-Dimethyl-1,2,3,4,4a,9,10,10a-o...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 70 | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Effective concentration in recombinant human LXRbeta ligand binding domain in homogeneous time-resolved fluorescence assay | Bioorg Med Chem Lett 15: 4574-8 (2005) Article DOI: 10.1016/j.bmcl.2005.06.100 BindingDB Entry DOI: 10.7270/Q2154GKK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxysterols receptor LXR-beta (Homo sapiens (Human)) | BDBM50172188 ((4S,4aR)-6-Hydroxy-4a-methyl-1-(S)-methyl-1,2,3,4,...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 3.80E+3 | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Effective concentration in transactivation assay using a chimeric LXR construct in HEK-293 cells for LXRbeta receptor | Bioorg Med Chem Lett 15: 4574-8 (2005) Article DOI: 10.1016/j.bmcl.2005.06.100 BindingDB Entry DOI: 10.7270/Q2154GKK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxysterols receptor LXR-beta (Homo sapiens (Human)) | BDBM50172188 ((4S,4aR)-6-Hydroxy-4a-methyl-1-(S)-methyl-1,2,3,4,...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Effective concentration in recombinant human LXRbeta ligand binding domain in homogeneous time-resolved fluorescence assay | Bioorg Med Chem Lett 15: 4574-8 (2005) Article DOI: 10.1016/j.bmcl.2005.06.100 BindingDB Entry DOI: 10.7270/Q2154GKK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxysterols receptor LXR-beta (Homo sapiens (Human)) | BDBM50172185 ((1S,4aS,10aR)-6-Hydroxy-1,4a-dimethyl-1,2,3,4,4a,9...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Effective concentration in recombinant human LXRbeta ligand binding domain in homogeneous time-resolved fluorescence assay | Bioorg Med Chem Lett 15: 4574-8 (2005) Article DOI: 10.1016/j.bmcl.2005.06.100 BindingDB Entry DOI: 10.7270/Q2154GKK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxysterols receptor LXR-beta (Homo sapiens (Human)) | BDBM50172189 (((1S,4aS,10aR)-1,4a-Dimethyl-1,2,3,4,4a,9,10,10a-o...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 100 | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Effective concentration in recombinant human LXRbeta ligand binding domain in homogeneous time-resolved fluorescence assay | Bioorg Med Chem Lett 15: 4574-8 (2005) Article DOI: 10.1016/j.bmcl.2005.06.100 BindingDB Entry DOI: 10.7270/Q2154GKK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxysterols receptor LXR-beta (Homo sapiens (Human)) | BDBM50167697 ((4aR,9S)-6-Hydroxy-1,4a-dimethyl-1,2,3,4,4a,9,10,1...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 1 | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Effective concentration in recombinant human LXRbeta ligand binding domain in homogeneous time-resolved fluorescence assay | Bioorg Med Chem Lett 15: 4574-8 (2005) Article DOI: 10.1016/j.bmcl.2005.06.100 BindingDB Entry DOI: 10.7270/Q2154GKK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxysterols receptor LXR-alpha (Homo sapiens (Human)) | BDBM50172203 ((1S,4aS,10aR)-1,4a-Dimethyl-1,2,3,4,4a,9,10,10a-oc...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 360 | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Effective concentration in transactivation assay using a chimeric LXR construct in HEK293 cells for LXRalpha receptor | Bioorg Med Chem Lett 15: 4574-8 (2005) Article DOI: 10.1016/j.bmcl.2005.06.100 BindingDB Entry DOI: 10.7270/Q2154GKK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxysterols receptor LXR-alpha (Homo sapiens (Human)) | BDBM50172198 (2,6-dimethyl-(2S,6S,7R)-tricyclo[8.4.0.02,7]tetrad...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 430 | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Effective concentration in transactivation assay using a chimeric LXR construct in HEK293 cells for LXRalpha receptor | Bioorg Med Chem Lett 15: 4574-8 (2005) Article DOI: 10.1016/j.bmcl.2005.06.100 BindingDB Entry DOI: 10.7270/Q2154GKK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxysterols receptor LXR-alpha (Homo sapiens (Human)) | BDBM50167694 (2,6-dimethyl-13-methylcarbonyloxy-(2S)-tricyclo[8....) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 2 | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Effective concentration in transactivation assay using a chimeric LXR construct in HEK293 cells for LXRalpha receptor | Bioorg Med Chem Lett 15: 4574-8 (2005) Article DOI: 10.1016/j.bmcl.2005.06.100 BindingDB Entry DOI: 10.7270/Q2154GKK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxysterols receptor LXR-alpha (Homo sapiens (Human)) | BDBM50172201 ((1S,4aS,10aR)-6-Hydroxy-1,4a-dimethyl-1,2,3,4,4a,9...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Effective concentration in transactivation assay using a chimeric LXR construct in HEK293 cells for LXRalpha receptor | Bioorg Med Chem Lett 15: 4574-8 (2005) Article DOI: 10.1016/j.bmcl.2005.06.100 BindingDB Entry DOI: 10.7270/Q2154GKK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxysterols receptor LXR-alpha (Homo sapiens (Human)) | BDBM50172188 ((4S,4aR)-6-Hydroxy-4a-methyl-1-(S)-methyl-1,2,3,4,...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 320 | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Effective concentration in transactivation assay using a chimeric LXR construct in HEK293 cells for LXRalpha receptor | Bioorg Med Chem Lett 15: 4574-8 (2005) Article DOI: 10.1016/j.bmcl.2005.06.100 BindingDB Entry DOI: 10.7270/Q2154GKK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxysterols receptor LXR-alpha (Homo sapiens (Human)) | BDBM50172193 ((4S,4aR)-6-Hydroxy-4a-methyl-1-(S)-methyl-1,2,3,4,...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 5.20E+3 | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Effective concentration in transactivation assay using a chimeric LXR construct in HEK293 cells for LXRalpha receptor | Bioorg Med Chem Lett 15: 4574-8 (2005) Article DOI: 10.1016/j.bmcl.2005.06.100 BindingDB Entry DOI: 10.7270/Q2154GKK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxysterols receptor LXR-alpha (Homo sapiens (Human)) | BDBM50172204 ((1S,4aS,10aR)-6-Hydroxy-1,4a-dimethyl-1,2,3,4,4a,9...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 50 | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Effective concentration in transactivation assay using a chimeric LXR construct in HEK293 cells for LXRalpha receptor | Bioorg Med Chem Lett 15: 4574-8 (2005) Article DOI: 10.1016/j.bmcl.2005.06.100 BindingDB Entry DOI: 10.7270/Q2154GKK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxysterols receptor LXR-alpha (Homo sapiens (Human)) | BDBM50172186 ((1S,4aS,10aR)-1,4a-Dimethyl-1,2,3,4,4a,9,10,10a-oc...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 170 | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Effective concentration in transactivation assay using a chimeric LXR construct in HEK293 cells for LXRalpha receptor | Bioorg Med Chem Lett 15: 4574-8 (2005) Article DOI: 10.1016/j.bmcl.2005.06.100 BindingDB Entry DOI: 10.7270/Q2154GKK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxysterols receptor LXR-beta (Homo sapiens (Human)) | BDBM50172204 ((1S,4aS,10aR)-6-Hydroxy-1,4a-dimethyl-1,2,3,4,4a,9...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Effective concentration in recombinant human LXRbeta ligand binding domain in homogeneous time-resolved fluorescence assay | Bioorg Med Chem Lett 15: 4574-8 (2005) Article DOI: 10.1016/j.bmcl.2005.06.100 BindingDB Entry DOI: 10.7270/Q2154GKK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxysterols receptor LXR-alpha (Homo sapiens (Human)) | BDBM50172207 (Adamantane-1-carboxylic acid ((1S,4aS,10aR)-1,4a-d...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 1.50E+3 | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Effective concentration in recombinant human LXRalpha ligand binding domain in homogeneous time-resolved fluorescence assay | Bioorg Med Chem Lett 15: 4574-8 (2005) Article DOI: 10.1016/j.bmcl.2005.06.100 BindingDB Entry DOI: 10.7270/Q2154GKK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxysterols receptor LXR-alpha (Homo sapiens (Human)) | BDBM50172191 (3-[((4S,4aR)-6-Hydroxy-4a-methyl-1-(S)-methyl-1,2,...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | 830 | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Effective concentration in transactivation assay using a chimeric LXR construct in HEK-293 cells for LXRalpha receptor | Bioorg Med Chem Lett 15: 4574-8 (2005) Article DOI: 10.1016/j.bmcl.2005.06.100 BindingDB Entry DOI: 10.7270/Q2154GKK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxysterols receptor LXR-beta (Homo sapiens (Human)) | BDBM50172192 ((Dodecahydro-carbazol-9-yl)-((1S,4aS,10aR)-6-hydro...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Effective concentration in recombinant human LXRbeta ligand binding domain in homogeneous time-resolved fluorescence assay | Bioorg Med Chem Lett 15: 4574-8 (2005) Article DOI: 10.1016/j.bmcl.2005.06.100 BindingDB Entry DOI: 10.7270/Q2154GKK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxysterols receptor LXR-beta (Homo sapiens (Human)) | BDBM50167694 (2,6-dimethyl-13-methylcarbonyloxy-(2S)-tricyclo[8....) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 10 | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Effective concentration in transactivation assay using a chimeric LXR construct in HEK-293 cells for LXRbeta receptor | Bioorg Med Chem Lett 15: 4574-8 (2005) Article DOI: 10.1016/j.bmcl.2005.06.100 BindingDB Entry DOI: 10.7270/Q2154GKK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxysterols receptor LXR-beta (Homo sapiens (Human)) | BDBM50172199 (1-Phenyl-cyclohexanecarboxylic acid ((1S,4aS,10aR)...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Effective concentration in recombinant human LXRbeta ligand binding domain in homogeneous time-resolved fluorescence assay | Bioorg Med Chem Lett 15: 4574-8 (2005) Article DOI: 10.1016/j.bmcl.2005.06.100 BindingDB Entry DOI: 10.7270/Q2154GKK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxysterols receptor LXR-alpha (Homo sapiens (Human)) | BDBM50172202 ((4S,4aR)-6-Hydroxy-4a-methyl-1-(S)-methyl-1,2,3,4,...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 3.10E+3 | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Effective concentration in transactivation assay using a chimeric LXR construct in HEK293 cells for LXRalpha receptor | Bioorg Med Chem Lett 15: 4574-8 (2005) Article DOI: 10.1016/j.bmcl.2005.06.100 BindingDB Entry DOI: 10.7270/Q2154GKK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxysterols receptor LXR-beta (Homo sapiens (Human)) | BDBM50172200 ((4S,4aR)-6-Hydroxy-4a-methyl-1-(S)-methyl-1,2,3,4,...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 220 | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Effective concentration in recombinant human LXRbeta ligand binding domain in homogeneous time-resolved fluorescence assay | Bioorg Med Chem Lett 15: 4574-8 (2005) Article DOI: 10.1016/j.bmcl.2005.06.100 BindingDB Entry DOI: 10.7270/Q2154GKK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxysterols receptor LXR-alpha (Homo sapiens (Human)) | BDBM50167697 ((4aR,9S)-6-Hydroxy-1,4a-dimethyl-1,2,3,4,4a,9,10,1...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 1 | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Effective concentration in recombinant human LXRalpha ligand binding domain in homogeneous time-resolved fluorescence assay | Bioorg Med Chem Lett 15: 4574-8 (2005) Article DOI: 10.1016/j.bmcl.2005.06.100 BindingDB Entry DOI: 10.7270/Q2154GKK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxysterols receptor LXR-beta (Homo sapiens (Human)) | BDBM50167694 (2,6-dimethyl-13-methylcarbonyloxy-(2S)-tricyclo[8....) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 2 | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Effective concentration in recombinant human LXRbeta ligand binding domain in homogeneous time-resolved fluorescence assay | Bioorg Med Chem Lett 15: 4574-8 (2005) Article DOI: 10.1016/j.bmcl.2005.06.100 BindingDB Entry DOI: 10.7270/Q2154GKK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxysterols receptor LXR-beta (Homo sapiens (Human)) | BDBM50172191 (3-[((4S,4aR)-6-Hydroxy-4a-methyl-1-(S)-methyl-1,2,...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Effective concentration in recombinant human LXRbeta ligand binding domain in homogeneous time-resolved fluorescence assay | Bioorg Med Chem Lett 15: 4574-8 (2005) Article DOI: 10.1016/j.bmcl.2005.06.100 BindingDB Entry DOI: 10.7270/Q2154GKK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxysterols receptor LXR-alpha (Homo sapiens (Human)) | BDBM50172185 ((1S,4aS,10aR)-6-Hydroxy-1,4a-dimethyl-1,2,3,4,4a,9...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 860 | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Effective concentration in transactivation assay using a chimeric LXR construct in HEK-293 cells for LXRalpha receptor | Bioorg Med Chem Lett 15: 4574-8 (2005) Article DOI: 10.1016/j.bmcl.2005.06.100 BindingDB Entry DOI: 10.7270/Q2154GKK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxysterols receptor LXR-beta (Homo sapiens (Human)) | BDBM50167697 ((4aR,9S)-6-Hydroxy-1,4a-dimethyl-1,2,3,4,4a,9,10,1...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | <3 | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Effective concentration in transactivation assay using a chimeric LXR construct in HEK-293 cells for LXRbeta receptor | Bioorg Med Chem Lett 15: 4574-8 (2005) Article DOI: 10.1016/j.bmcl.2005.06.100 BindingDB Entry DOI: 10.7270/Q2154GKK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxysterols receptor LXR-alpha (Homo sapiens (Human)) | BDBM50172194 ((1S,4aS,10aR)-6-Hydroxy-1,4a-dimethyl-1,2,3,4,4a,9...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Effective concentration in transactivation assay using a chimeric LXR construct in HEK293 cells for LXRalpha receptor | Bioorg Med Chem Lett 15: 4574-8 (2005) Article DOI: 10.1016/j.bmcl.2005.06.100 BindingDB Entry DOI: 10.7270/Q2154GKK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxysterols receptor LXR-beta (Homo sapiens (Human)) | BDBM50172193 ((4S,4aR)-6-Hydroxy-4a-methyl-1-(S)-methyl-1,2,3,4,...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Effective concentration in recombinant human LXRbeta ligand binding domain in homogeneous time-resolved fluorescence assay | Bioorg Med Chem Lett 15: 4574-8 (2005) Article DOI: 10.1016/j.bmcl.2005.06.100 BindingDB Entry DOI: 10.7270/Q2154GKK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxysterols receptor LXR-alpha (Homo sapiens (Human)) | BDBM50172185 ((1S,4aS,10aR)-6-Hydroxy-1,4a-dimethyl-1,2,3,4,4a,9...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 230 | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Effective concentration in transactivation assay using a chimeric LXR construct in HEK293 cells for LXRalpha receptor | Bioorg Med Chem Lett 15: 4574-8 (2005) Article DOI: 10.1016/j.bmcl.2005.06.100 BindingDB Entry DOI: 10.7270/Q2154GKK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxysterols receptor LXR-alpha (Homo sapiens (Human)) | BDBM50172190 ((4S,4aR)-6-Hydroxy-4a-methyl-1-(S)-methyl-1,2,3,4,...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 280 | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Effective concentration in transactivation assay using a chimeric LXR construct in HEK293 cells for LXRalpha receptor | Bioorg Med Chem Lett 15: 4574-8 (2005) Article DOI: 10.1016/j.bmcl.2005.06.100 BindingDB Entry DOI: 10.7270/Q2154GKK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxysterols receptor LXR-beta (Homo sapiens (Human)) | BDBM50172187 (1-Adamantan-1-yl-3-((1S,4aS,10aR)-1,4a-dimethyl-1,...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Effective concentration in recombinant human LXRbeta ligand binding domain in homogeneous time-resolved fluorescence assay | Bioorg Med Chem Lett 15: 4574-8 (2005) Article DOI: 10.1016/j.bmcl.2005.06.100 BindingDB Entry DOI: 10.7270/Q2154GKK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxysterols receptor LXR-beta (Homo sapiens (Human)) | BDBM50172194 ((1S,4aS,10aR)-6-Hydroxy-1,4a-dimethyl-1,2,3,4,4a,9...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Effective concentration in recombinant human LXRbeta ligand binding domain in homogeneous time-resolved fluorescence assay | Bioorg Med Chem Lett 15: 4574-8 (2005) Article DOI: 10.1016/j.bmcl.2005.06.100 BindingDB Entry DOI: 10.7270/Q2154GKK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxysterols receptor LXR-beta (Homo sapiens (Human)) | BDBM50172201 ((1S,4aS,10aR)-6-Hydroxy-1,4a-dimethyl-1,2,3,4,4a,9...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Effective concentration in recombinant human LXRbeta ligand binding domain in homogeneous time-resolved fluorescence assay | Bioorg Med Chem Lett 15: 4574-8 (2005) Article DOI: 10.1016/j.bmcl.2005.06.100 BindingDB Entry DOI: 10.7270/Q2154GKK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxysterols receptor LXR-beta (Homo sapiens (Human)) | BDBM50172202 ((4S,4aR)-6-Hydroxy-4a-methyl-1-(S)-methyl-1,2,3,4,...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Effective concentration in recombinant human LXRbeta ligand binding domain in homogeneous time-resolved fluorescence assay | Bioorg Med Chem Lett 15: 4574-8 (2005) Article DOI: 10.1016/j.bmcl.2005.06.100 BindingDB Entry DOI: 10.7270/Q2154GKK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxysterols receptor LXR-beta (Homo sapiens (Human)) | BDBM50172207 (Adamantane-1-carboxylic acid ((1S,4aS,10aR)-1,4a-d...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Effective concentration in recombinant human LXRbeta ligand binding domain in homogeneous time-resolved fluorescence assay | Bioorg Med Chem Lett 15: 4574-8 (2005) Article DOI: 10.1016/j.bmcl.2005.06.100 BindingDB Entry DOI: 10.7270/Q2154GKK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxysterols receptor LXR-alpha (Homo sapiens (Human)) | BDBM50172197 ((4S,4aR)-6-Hydroxy-4a-methyl-1-(S)-methyl-1,2,3,4,...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 240 | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Effective concentration in transactivation assay using a chimeric LXR construct in HEK293 cells for LXRalpha receptor | Bioorg Med Chem Lett 15: 4574-8 (2005) Article DOI: 10.1016/j.bmcl.2005.06.100 BindingDB Entry DOI: 10.7270/Q2154GKK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxysterols receptor LXR-alpha (Homo sapiens (Human)) | BDBM50172206 ((4S,4aR)-6-Hydroxy-4a-methyl-1-(S)-methyl-1,2,3,4,...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 140 | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Effective concentration in transactivation assay using a chimeric LXR construct in HEK293 cells for LXRalpha receptor | Bioorg Med Chem Lett 15: 4574-8 (2005) Article DOI: 10.1016/j.bmcl.2005.06.100 BindingDB Entry DOI: 10.7270/Q2154GKK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||