| Reaction Details |
|---|
 | Report a problem with these data |
| Target | Oxysterols receptor LXR-alpha |
|---|
| Ligand | BDBM50172193 |
|---|
| Substrate/Competitor | n/a |
|---|
| Meas. Tech. | ChEMBL_321113 (CHEMBL882879) |
|---|
| EC50 | 5200±n/a nM |
|---|
| Citation |  Liu, W; Chen, S; Dropinski, J; Colwell, L; Robins, M; Szymonifka, M; Hayes, N; Sharma, N; MacNaul, K; Hernandez, M; Burton, C; Sparrow, CP; Menke, JG; Singh, SB Design, synthesis, and structure-activity relationship of podocarpic acid amides as liver X receptor agonists for potential treatment of atherosclerosis. Bioorg Med Chem Lett15:4574-8 (2005) [PubMed] Article Liu, W; Chen, S; Dropinski, J; Colwell, L; Robins, M; Szymonifka, M; Hayes, N; Sharma, N; MacNaul, K; Hernandez, M; Burton, C; Sparrow, CP; Menke, JG; Singh, SB Design, synthesis, and structure-activity relationship of podocarpic acid amides as liver X receptor agonists for potential treatment of atherosclerosis. Bioorg Med Chem Lett15:4574-8 (2005) [PubMed] Article |
|---|
| More Info.: | Get all data from this article, Assay Method |
|---|
| |
| Oxysterols receptor LXR-alpha |
|---|
| Name: | Oxysterols receptor LXR-alpha |
|---|
| Synonyms: | LXRA | Liver X Receptor alpha (LXR-alpha) | Liver X receptor alpha | Liver X receptor alpha (LXRA) | Liver X receptor alpha (NR1H3) | Liver X, LXR alpha | NR1H3 | NR1H3_HUMAN | Nuclear orphan receptor LXR-alpha | Nuclear receptor subfamily 1 group H member 3 |
|---|
| Type: | Enzyme Catalytic Domain |
|---|
| Mol. Mass.: | 50403.85 |
|---|
| Organism: | Homo sapiens (Human) |
|---|
| Description: | Q13133 |
|---|
| Residue: | 447 |
|---|
| Sequence: | MSLWLGAPVPDIPPDSAVELWKPGAQDASSQAQGGSSCILREEARMPHSAGGTAGVGLEA
AEPTALLTRAEPPSEPTEIRPQKRKKGPAPKMLGNELCSVCGDKASGFHYNVLSCEGCKG
FFRRSVIKGAHYICHSGGHCPMDTYMRRKCQECRLRKCRQAGMREECVLSEEQIRLKKLK
RQEEEQAHATSLPPRASSPPQILPQLSPEQLGMIEKLVAAQQQCNRRSFSDRLRVTPWPM
APDPHSREARQQRFAHFTELAIVSVQEIVDFAKQLPGFLQLSREDQIALLKTSAIEVMLL
ETSRRYNPGSESITFLKDFSYNREDFAKAGLQVEFINPIFEFSRAMNELQLNDAEFALLI
AISIFSADRPNVQDQLQVERLQHTYVEALHAYVSIHHPHDRLMFPRMLMKLVSLRTLSSV
HSEQVFALRLQDKKLPPLLSEIWDVHE
|
|
|
|---|
| BDBM50172193 |
|---|
| n/a |
|---|
| Name | BDBM50172193 |
|---|
| Synonyms: | (4S,4aR)-6-Hydroxy-4a-methyl-1-(S)-methyl-1,2,3,4,4a,9,10,10a-octahydro-phenanthrene-1-carboxylic acid phenethyl-amide | CHEMBL196145 |
|---|
| Type | Small organic molecule |
|---|
| Emp. Form. | C25H31NO2 |
|---|
| Mol. Mass. | 377.5191 |
|---|
| SMILES | C[C@@]1(CCC[C@@]2(C)[C@H]1CCc1ccc(O)cc21)C(=O)NCCc1ccccc1 |
|---|
| Structure | 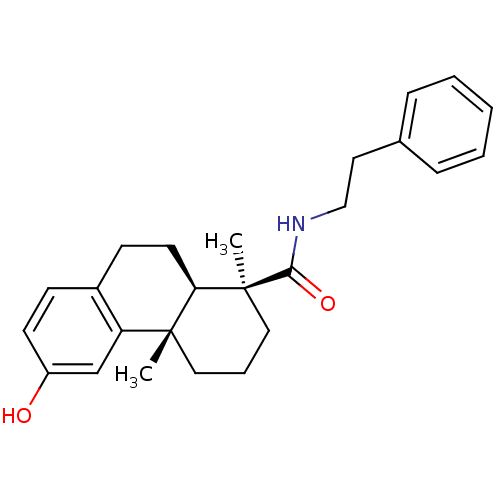
|
|---|


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data

 Liu, W; Chen, S; Dropinski, J; Colwell, L; Robins, M; Szymonifka, M; Hayes, N; Sharma, N; MacNaul, K; Hernandez, M; Burton, C; Sparrow, CP; Menke, JG; Singh, SB Design, synthesis, and structure-activity relationship of podocarpic acid amides as liver X receptor agonists for potential treatment of atherosclerosis. Bioorg Med Chem Lett15:4574-8 (2005) [PubMed] Article
Liu, W; Chen, S; Dropinski, J; Colwell, L; Robins, M; Szymonifka, M; Hayes, N; Sharma, N; MacNaul, K; Hernandez, M; Burton, C; Sparrow, CP; Menke, JG; Singh, SB Design, synthesis, and structure-activity relationship of podocarpic acid amides as liver X receptor agonists for potential treatment of atherosclerosis. Bioorg Med Chem Lett15:4574-8 (2005) [PubMed] Article