

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| D(2) dopamine receptor (Homo sapiens (Human)) | BDBM50048302 (4-[(R)-5-(4-Phenyl-3,6-dihydro-2H-pyridin-1-ylmeth...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company Curated by ChEMBL | Assay Description Binding affinity of the compound towards human Dopamine receptor D2L evaluated using [3H]-N-0437 | J Med Chem 38: 5007-14 (1996) BindingDB Entry DOI: 10.7270/Q2VQ31SG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(3) dopamine receptor (Homo sapiens (Human)) | BDBM50048302 (4-[(R)-5-(4-Phenyl-3,6-dihydro-2H-pyridin-1-ylmeth...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.520 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company Curated by ChEMBL | Assay Description Binding affinity of the compound towards human Dopamine receptor D3 evaluated using [3H]-spiperone | J Med Chem 38: 5007-14 (1996) BindingDB Entry DOI: 10.7270/Q2VQ31SG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Homo sapiens (Human)) | BDBM50048300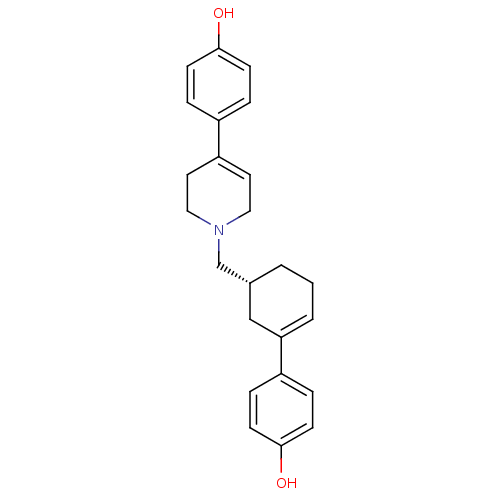 (4-(1-[methyl cyclohex-3-en-3-yl phenol]-1,2,3,6-te...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 2.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company Curated by ChEMBL | Assay Description Binding affinity of the compound towards human Dopamine receptor D2L evaluated using [3H]-N-0437 | J Med Chem 38: 5007-14 (1996) BindingDB Entry DOI: 10.7270/Q2VQ31SG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(3) dopamine receptor (Homo sapiens (Human)) | BDBM50048300 (4-(1-[methyl cyclohex-3-en-3-yl phenol]-1,2,3,6-te...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 3.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company Curated by ChEMBL | Assay Description Binding affinity of the compound towards human Dopamine receptor D3 evaluated using [3H]-spiperone | J Med Chem 38: 5007-14 (1996) BindingDB Entry DOI: 10.7270/Q2VQ31SG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Homo sapiens (Human)) | BDBM50048302 (4-[(R)-5-(4-Phenyl-3,6-dihydro-2H-pyridin-1-ylmeth...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 3.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company Curated by ChEMBL | Assay Description Binding affinity of the compound towards human Dopamine receptor D2L evaluated using [3H]-spiperone | J Med Chem 38: 5007-14 (1996) BindingDB Entry DOI: 10.7270/Q2VQ31SG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Homo sapiens (Human)) | BDBM50048301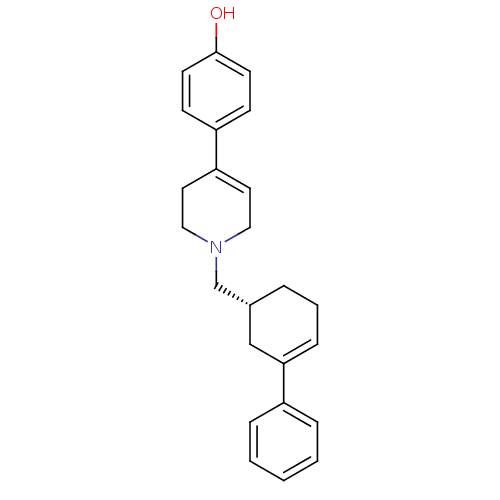 (4-[1-((R)-3-Phenyl-cyclohex-3-enylmethyl)-1,2,3,6-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 5.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company Curated by ChEMBL | Assay Description Binding affinity of the compound towards human Dopamine receptor D2L evaluated using [3H]-N-0437 | J Med Chem 38: 5007-14 (1996) BindingDB Entry DOI: 10.7270/Q2VQ31SG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Homo sapiens (Human)) | BDBM50048299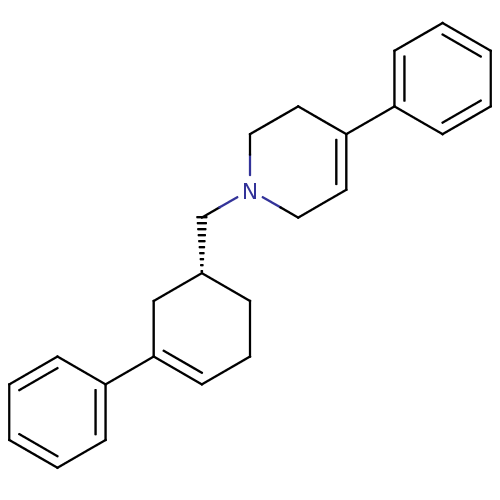 (4-Phenyl-1-((R)-3-phenyl-cyclohex-3-enylmethyl)-1,...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 8.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company Curated by ChEMBL | Assay Description Binding affinity of the compound towards human Dopamine receptor D2L evaluated using [3H]-N-0437 | J Med Chem 38: 5007-14 (1996) BindingDB Entry DOI: 10.7270/Q2VQ31SG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Homo sapiens (Human)) | BDBM50048300 (4-(1-[methyl cyclohex-3-en-3-yl phenol]-1,2,3,6-te...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 10.3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company Curated by ChEMBL | Assay Description Binding affinity of the compound towards human Dopamine receptor D2L evaluated using [3H]-spiperone | J Med Chem 38: 5007-14 (1996) BindingDB Entry DOI: 10.7270/Q2VQ31SG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(3) dopamine receptor (Homo sapiens (Human)) | BDBM50048299 (4-Phenyl-1-((R)-3-phenyl-cyclohex-3-enylmethyl)-1,...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 16.6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company Curated by ChEMBL | Assay Description Binding affinity of the compound towards human Dopamine receptor D3 evaluated using [3H]-spiperone | J Med Chem 38: 5007-14 (1996) BindingDB Entry DOI: 10.7270/Q2VQ31SG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Homo sapiens (Human)) | BDBM50048299 (4-Phenyl-1-((R)-3-phenyl-cyclohex-3-enylmethyl)-1,...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 25.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company Curated by ChEMBL | Assay Description Binding affinity of the compound towards human Dopamine receptor D2L evaluated using [3H]-spiperone | J Med Chem 38: 5007-14 (1996) BindingDB Entry DOI: 10.7270/Q2VQ31SG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(3) dopamine receptor (Homo sapiens (Human)) | BDBM50048301 (4-[1-((R)-3-Phenyl-cyclohex-3-enylmethyl)-1,2,3,6-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 56.7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company Curated by ChEMBL | Assay Description Binding affinity of the compound towards human Dopamine receptor D3 evaluated using [3H]-spiperone | J Med Chem 38: 5007-14 (1996) BindingDB Entry DOI: 10.7270/Q2VQ31SG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(4) dopamine receptor (Homo sapiens (Human)) | BDBM50048302 (4-[(R)-5-(4-Phenyl-3,6-dihydro-2H-pyridin-1-ylmeth...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 61.4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company Curated by ChEMBL | Assay Description Binding affinity of the compound towards human Dopamine receptor D4.2 evaluated using [3H]-spiperone | J Med Chem 38: 5007-14 (1996) BindingDB Entry DOI: 10.7270/Q2VQ31SG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(4) dopamine receptor (Homo sapiens (Human)) | BDBM50048300 (4-(1-[methyl cyclohex-3-en-3-yl phenol]-1,2,3,6-te...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 68 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company Curated by ChEMBL | Assay Description Binding affinity of the compound towards human Dopamine receptor D4.2 evaluated using [3H]-spiperone | J Med Chem 38: 5007-14 (1996) BindingDB Entry DOI: 10.7270/Q2VQ31SG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(4) dopamine receptor (Homo sapiens (Human)) | BDBM50048299 (4-Phenyl-1-((R)-3-phenyl-cyclohex-3-enylmethyl)-1,...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 90.9 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company Curated by ChEMBL | Assay Description Binding affinity of the compound towards human Dopamine receptor D4.2 evaluated using [3H]-spiperone | J Med Chem 38: 5007-14 (1996) BindingDB Entry DOI: 10.7270/Q2VQ31SG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Homo sapiens (Human)) | BDBM50048301 (4-[1-((R)-3-Phenyl-cyclohex-3-enylmethyl)-1,2,3,6-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 94 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company Curated by ChEMBL | Assay Description Binding affinity of the compound towards human Dopamine receptor D2L evaluated using [3H]-spiperone | J Med Chem 38: 5007-14 (1996) BindingDB Entry DOI: 10.7270/Q2VQ31SG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(4) dopamine receptor (Homo sapiens (Human)) | BDBM50048301 (4-[1-((R)-3-Phenyl-cyclohex-3-enylmethyl)-1,2,3,6-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company Curated by ChEMBL | Assay Description Binding affinity of the compound towards human Dopamine receptor D4.2 evaluated using [3H]-spiperone | J Med Chem 38: 5007-14 (1996) BindingDB Entry DOI: 10.7270/Q2VQ31SG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50000293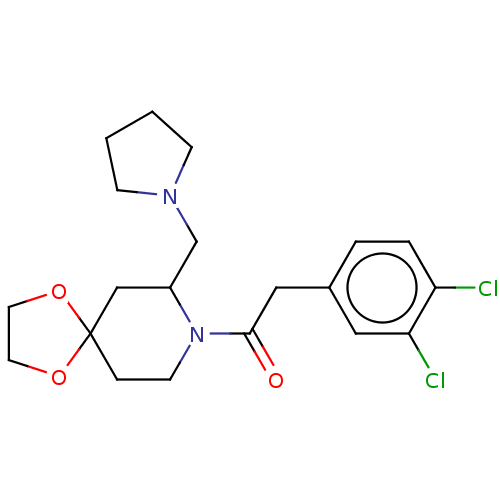 (2-(3,4-Dichloro-phenyl)-1-(7-pyrrolidin-1-ylmethyl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Group Research Ltd. Curated by ChEMBL | Assay Description Opioid receptor kappa 1 agonist potency was determined in vitro using rabbit vas deferens (LVD) preparation | J Med Chem 35: 490-501 (1992) BindingDB Entry DOI: 10.7270/Q2TQ60GV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50000288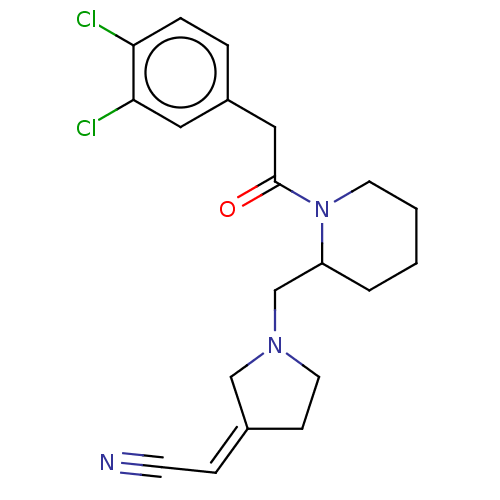 ((1-{1-[2-(3,4-Dichloro-phenyl)-acetyl]-piperidin-2...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.120 | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Group Research Ltd. Curated by ChEMBL | Assay Description Opioid receptor kappa 1 agonist potency of the compound was determined in vitro using rabbit vas deferens(LVD) preparation | J Med Chem 35: 490-501 (1992) BindingDB Entry DOI: 10.7270/Q2TQ60GV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50000271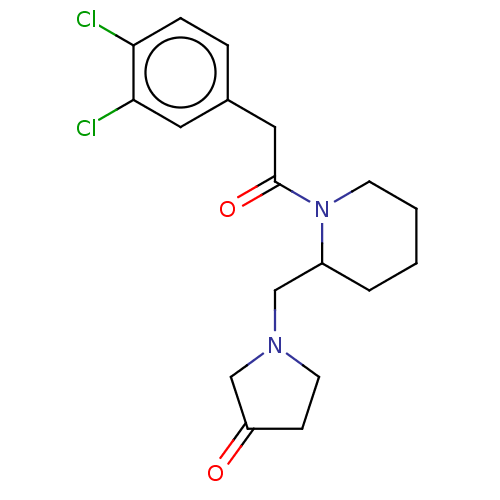 (1-{1-[2-(3,4-Dichloro-phenyl)-acetyl]-piperidin-2-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Group Research Ltd. Curated by ChEMBL | Assay Description Opioid receptor kappa 1 agonist potency of the compound was determined in vitro using rabbit vas deferens(LVD) preparation | J Med Chem 35: 490-501 (1992) BindingDB Entry DOI: 10.7270/Q2TQ60GV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Arachidonate 5-lipoxygenase-activating protein (Homo sapiens (Human)) | BDBM50383916 (CHEMBL2031657) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of [125I]L-691,831 from 5-lipoxygenase-activating protein in human polymorphonuclear cells | Bioorg Med Chem Lett 22: 4133-8 (2012) Article DOI: 10.1016/j.bmcl.2012.04.064 BindingDB Entry DOI: 10.7270/Q2348MCB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50000260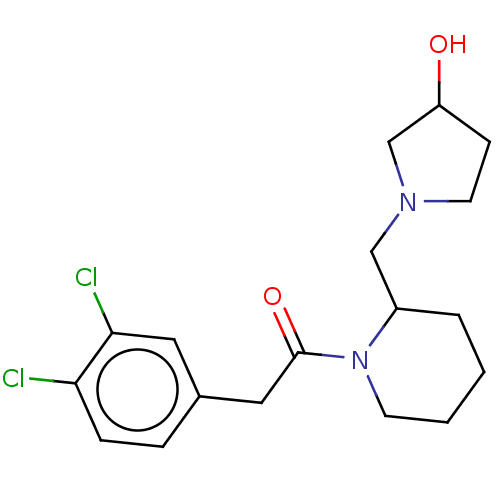 (2-(3,4-Dichloro-phenyl)-1-[2-(3-hydroxy-pyrrolidin...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.880 | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Group Research Ltd. Curated by ChEMBL | Assay Description Opioid receptor kappa agonist potency was determined in vitro using rabbit vas deferens(LVD) preparation | J Med Chem 35: 490-501 (1992) BindingDB Entry DOI: 10.7270/Q2TQ60GV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxysterols receptor LXR-beta (Homo sapiens (Human)) | BDBM50167697 ((4aR,9S)-6-Hydroxy-1,4a-dimethyl-1,2,3,4,4a,9,10,1...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of [3H2]-F3-methyl AA (1) from liver X receptor-beta in SPA assay | Bioorg Med Chem Lett 15: 2824-8 (2005) Article DOI: 10.1016/j.bmcl.2005.03.100 BindingDB Entry DOI: 10.7270/Q29G5NM2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxysterols receptor LXR-alpha (Homo sapiens (Human)) | BDBM50167697 ((4aR,9S)-6-Hydroxy-1,4a-dimethyl-1,2,3,4,4a,9,10,1...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibitory concentration in LXRSPA alpha binding assay | Bioorg Med Chem Lett 15: 4574-8 (2005) Article DOI: 10.1016/j.bmcl.2005.06.100 BindingDB Entry DOI: 10.7270/Q2154GKK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxysterols receptor LXR-alpha (Homo sapiens (Human)) | BDBM50167697 ((4aR,9S)-6-Hydroxy-1,4a-dimethyl-1,2,3,4,4a,9,10,1...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of [3H2]-F3-methyl AA (1) from liver X receptor-alpha in SPA assay | Bioorg Med Chem Lett 15: 2824-8 (2005) Article DOI: 10.1016/j.bmcl.2005.03.100 BindingDB Entry DOI: 10.7270/Q29G5NM2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxysterols receptor LXR-beta (Homo sapiens (Human)) | BDBM50167698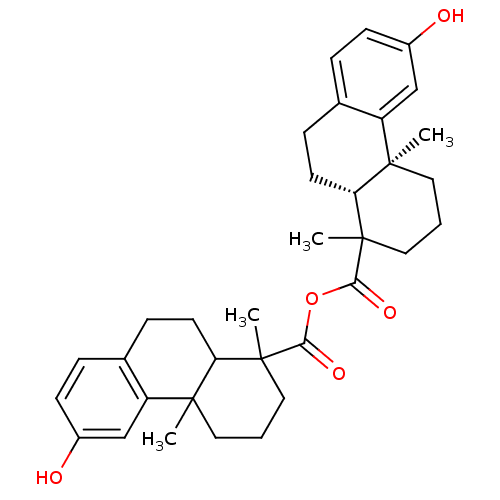 (13-hydroxy-2,6-dimethyl-(2S,7R)-tricyclo[8.4.0.02,...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of [3H2]-F3-methyl AA (1) from liver X receptor-beta in SPA assay | Bioorg Med Chem Lett 15: 2824-8 (2005) Article DOI: 10.1016/j.bmcl.2005.03.100 BindingDB Entry DOI: 10.7270/Q29G5NM2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxysterols receptor LXR-beta (Homo sapiens (Human)) | BDBM50167697 ((4aR,9S)-6-Hydroxy-1,4a-dimethyl-1,2,3,4,4a,9,10,1...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibitory concentration in LXRSPA beta binding assay | Bioorg Med Chem Lett 15: 4574-8 (2005) Article DOI: 10.1016/j.bmcl.2005.06.100 BindingDB Entry DOI: 10.7270/Q2154GKK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Arachidonate 5-lipoxygenase-activating protein (Homo sapiens (Human)) | BDBM50383906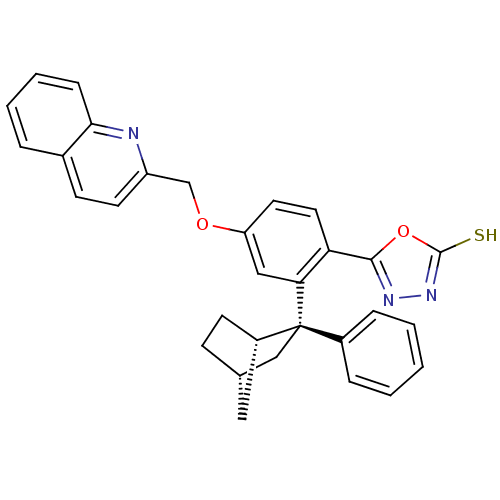 (CHEMBL2031647) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of [125I]L-691,831 from 5-lipoxygenase-activating protein in human polymorphonuclear cells | Bioorg Med Chem Lett 22: 4133-8 (2012) Article DOI: 10.1016/j.bmcl.2012.04.064 BindingDB Entry DOI: 10.7270/Q2348MCB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Arachidonate 5-lipoxygenase-activating protein (Homo sapiens (Human)) | BDBM50383917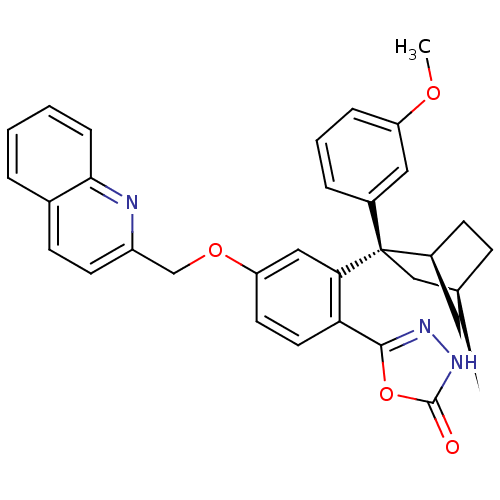 (CHEMBL2031658) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of [125I]L-691,831 from 5-lipoxygenase-activating protein in human polymorphonuclear cells | Bioorg Med Chem Lett 22: 4133-8 (2012) Article DOI: 10.1016/j.bmcl.2012.04.064 BindingDB Entry DOI: 10.7270/Q2348MCB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Arachidonate 5-lipoxygenase-activating protein (Homo sapiens (Human)) | BDBM50383910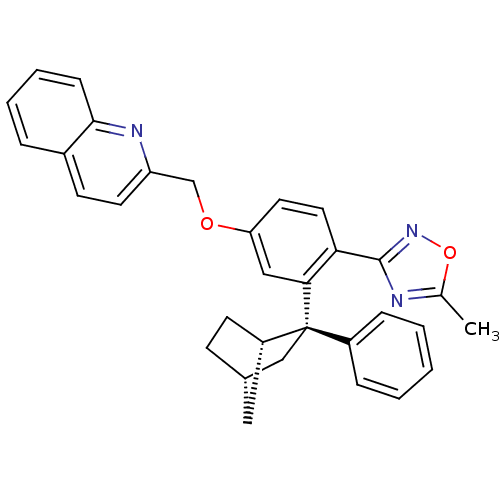 (CHEMBL2031651) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of [125I]L-691,831 from 5-lipoxygenase-activating protein in human polymorphonuclear cells | Bioorg Med Chem Lett 22: 4133-8 (2012) Article DOI: 10.1016/j.bmcl.2012.04.064 BindingDB Entry DOI: 10.7270/Q2348MCB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50000284 (2-(3,4-Dichloro-phenyl)-1-(2-methyl-6-pyrrolidin-1...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Group Research Ltd. Curated by ChEMBL | Assay Description Opioid receptor kappa agonist potency was determined in vitro using rabbit vas deferens(LVD) preparation | J Med Chem 35: 490-501 (1992) BindingDB Entry DOI: 10.7270/Q2TQ60GV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Arachidonate 5-lipoxygenase-activating protein (Homo sapiens (Human)) | BDBM50383914 (CHEMBL2031655) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of [125I]L-691,831 from 5-lipoxygenase-activating protein in human polymorphonuclear cells | Bioorg Med Chem Lett 22: 4133-8 (2012) Article DOI: 10.1016/j.bmcl.2012.04.064 BindingDB Entry DOI: 10.7270/Q2348MCB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Arachidonate 5-lipoxygenase-activating protein (Homo sapiens (Human)) | BDBM50383918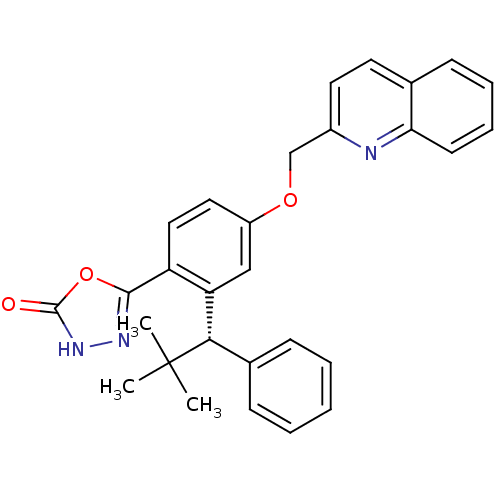 (CHEMBL2031659) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of [125I]L-691,831 from 5-lipoxygenase-activating protein in human polymorphonuclear cells | Bioorg Med Chem Lett 22: 4133-8 (2012) Article DOI: 10.1016/j.bmcl.2012.04.064 BindingDB Entry DOI: 10.7270/Q2348MCB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxysterols receptor LXR-alpha (Homo sapiens (Human)) | BDBM50167698 (13-hydroxy-2,6-dimethyl-(2S,7R)-tricyclo[8.4.0.02,...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of [3H2]-F3-methyl AA (1) from liver X receptor-alpha in SPA assay | Bioorg Med Chem Lett 15: 2824-8 (2005) Article DOI: 10.1016/j.bmcl.2005.03.100 BindingDB Entry DOI: 10.7270/Q29G5NM2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxysterols receptor LXR-alpha (Homo sapiens (Human)) | BDBM50167694 (2,6-dimethyl-13-methylcarbonyloxy-(2S)-tricyclo[8....) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of [3H2]-F3-methyl AA (1) from liver X receptor-alpha in SPA assay | Bioorg Med Chem Lett 15: 2824-8 (2005) Article DOI: 10.1016/j.bmcl.2005.03.100 BindingDB Entry DOI: 10.7270/Q29G5NM2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxysterols receptor LXR-alpha (Homo sapiens (Human)) | BDBM50167694 (2,6-dimethyl-13-methylcarbonyloxy-(2S)-tricyclo[8....) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibitory concentration in LXRSPA alpha binding assay | Bioorg Med Chem Lett 15: 4574-8 (2005) Article DOI: 10.1016/j.bmcl.2005.06.100 BindingDB Entry DOI: 10.7270/Q2154GKK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxysterols receptor LXR-beta (Homo sapiens (Human)) | BDBM50167694 (2,6-dimethyl-13-methylcarbonyloxy-(2S)-tricyclo[8....) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of [3H2]-F3-methyl AA (1) from liver X receptor-beta in SPA assay | Bioorg Med Chem Lett 15: 2824-8 (2005) Article DOI: 10.1016/j.bmcl.2005.03.100 BindingDB Entry DOI: 10.7270/Q29G5NM2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxysterols receptor LXR-beta (Homo sapiens (Human)) | BDBM50167694 (2,6-dimethyl-13-methylcarbonyloxy-(2S)-tricyclo[8....) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibitory concentration in LXRSPA beta binding assay | Bioorg Med Chem Lett 15: 4574-8 (2005) Article DOI: 10.1016/j.bmcl.2005.06.100 BindingDB Entry DOI: 10.7270/Q2154GKK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Arachidonate 5-lipoxygenase-activating protein (Homo sapiens (Human)) | BDBM50383883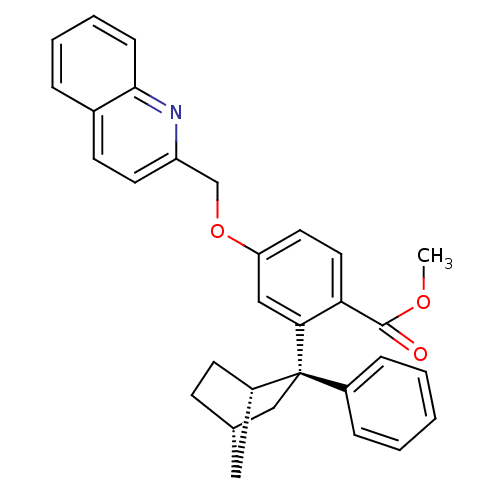 (CHEMBL2029369) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of [125I]L-691,831 from 5-lipoxygenase-activating protein in human polymorphonuclear cells | Bioorg Med Chem Lett 22: 4133-8 (2012) Article DOI: 10.1016/j.bmcl.2012.04.064 BindingDB Entry DOI: 10.7270/Q2348MCB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Arachidonate 5-lipoxygenase-activating protein (Homo sapiens (Human)) | BDBM50383879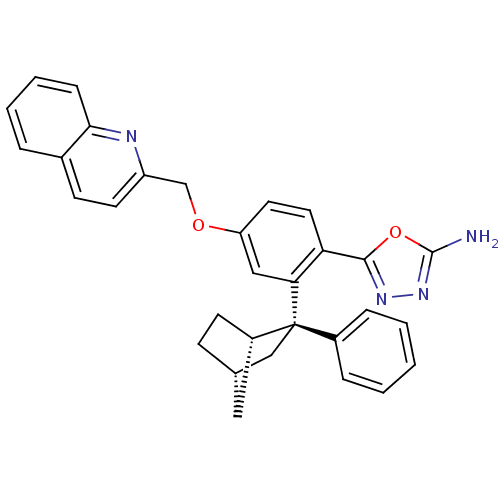 (CHEMBL2031645) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of [125I]L-691,831 from 5-lipoxygenase-activating protein in human polymorphonuclear cells | Bioorg Med Chem Lett 22: 4133-8 (2012) Article DOI: 10.1016/j.bmcl.2012.04.064 BindingDB Entry DOI: 10.7270/Q2348MCB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Arachidonate 5-lipoxygenase-activating protein (Homo sapiens (Human)) | BDBM50383885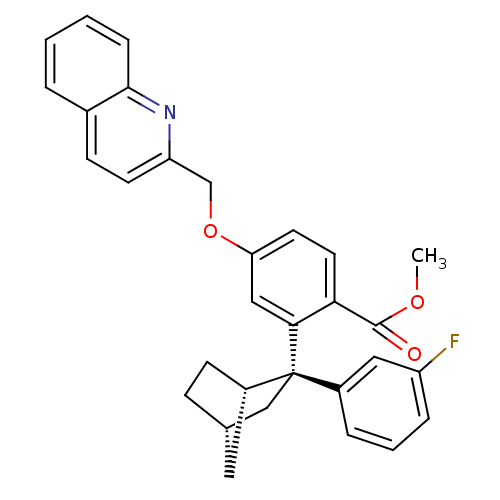 (CHEMBL2031437) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of [125I]L-691,831 from 5-lipoxygenase-activating protein in human polymorphonuclear cells | Bioorg Med Chem Lett 22: 4133-8 (2012) Article DOI: 10.1016/j.bmcl.2012.04.064 BindingDB Entry DOI: 10.7270/Q2348MCB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Arachidonate 5-lipoxygenase-activating protein (Homo sapiens (Human)) | BDBM50383915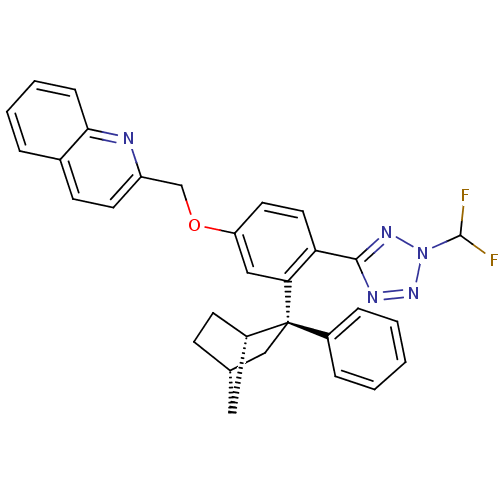 (CHEMBL2031656) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of [125I]L-691,831 from 5-lipoxygenase-activating protein in human polymorphonuclear cells | Bioorg Med Chem Lett 22: 4133-8 (2012) Article DOI: 10.1016/j.bmcl.2012.04.064 BindingDB Entry DOI: 10.7270/Q2348MCB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Arachidonate 5-lipoxygenase-activating protein (Homo sapiens (Human)) | BDBM50383913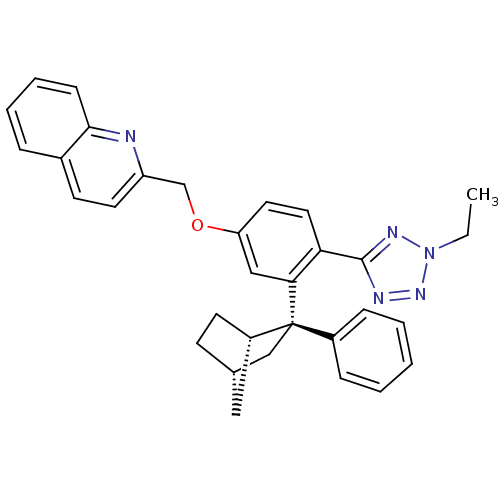 (CHEMBL2031654) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of [125I]L-691,831 from 5-lipoxygenase-activating protein in human polymorphonuclear cells | Bioorg Med Chem Lett 22: 4133-8 (2012) Article DOI: 10.1016/j.bmcl.2012.04.064 BindingDB Entry DOI: 10.7270/Q2348MCB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Arachidonate 5-lipoxygenase-activating protein (Homo sapiens (Human)) | BDBM50383878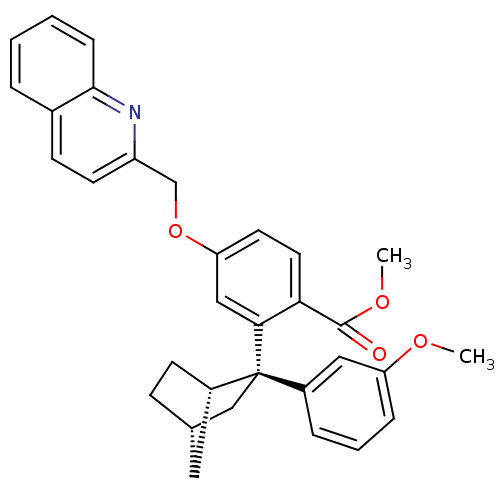 (CHEMBL2031441) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of [125I]L-691,831 from 5-lipoxygenase-activating protein in human polymorphonuclear cells | Bioorg Med Chem Lett 22: 4133-8 (2012) Article DOI: 10.1016/j.bmcl.2012.04.064 BindingDB Entry DOI: 10.7270/Q2348MCB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Arachidonate 5-lipoxygenase-activating protein (Homo sapiens (Human)) | BDBM50383905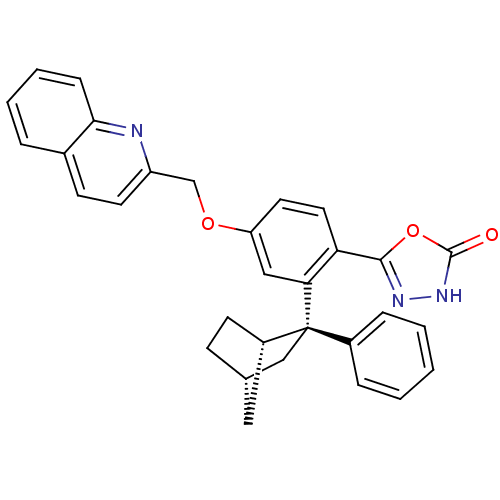 (CHEMBL2031646) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of [125I]L-691,831 from 5-lipoxygenase-activating protein in human polymorphonuclear cells | Bioorg Med Chem Lett 22: 4133-8 (2012) Article DOI: 10.1016/j.bmcl.2012.04.064 BindingDB Entry DOI: 10.7270/Q2348MCB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Arachidonate 5-lipoxygenase-activating protein (Homo sapiens (Human)) | BDBM50383882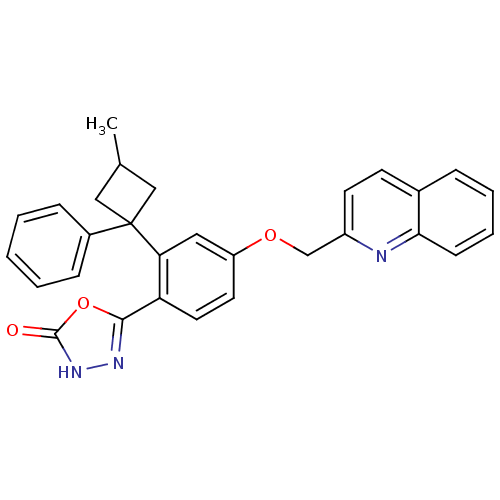 (CHEMBL2031661) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of [125I]L-691,831 from 5-lipoxygenase-activating protein in human polymorphonuclear cells | Bioorg Med Chem Lett 22: 4133-8 (2012) Article DOI: 10.1016/j.bmcl.2012.04.064 BindingDB Entry DOI: 10.7270/Q2348MCB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Arachidonate 5-lipoxygenase-activating protein (Homo sapiens (Human)) | BDBM50383912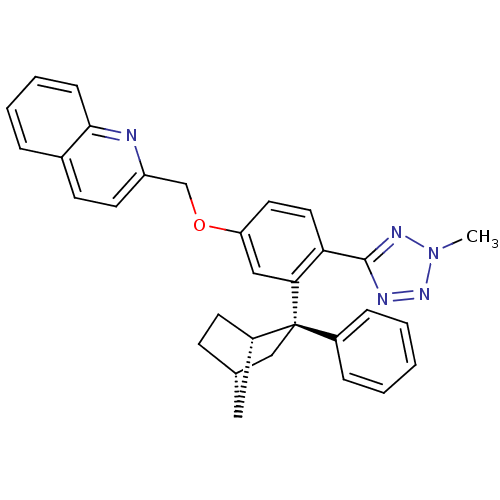 (CHEMBL2031653) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of [125I]L-691,831 from 5-lipoxygenase-activating protein in human polymorphonuclear cells | Bioorg Med Chem Lett 22: 4133-8 (2012) Article DOI: 10.1016/j.bmcl.2012.04.064 BindingDB Entry DOI: 10.7270/Q2348MCB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Arachidonate 5-lipoxygenase-activating protein (Homo sapiens (Human)) | BDBM50383911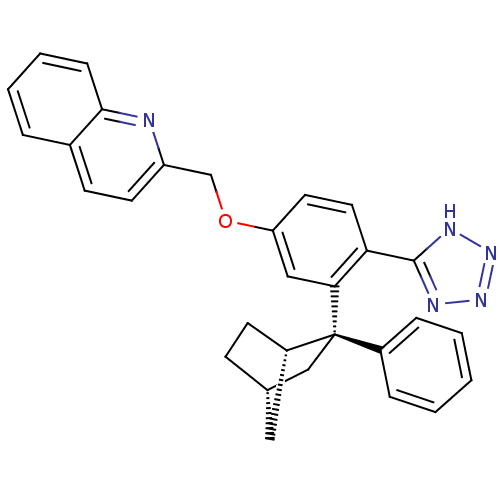 (CHEMBL2031652) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of [125I]L-691,831 from 5-lipoxygenase-activating protein in human polymorphonuclear cells | Bioorg Med Chem Lett 22: 4133-8 (2012) Article DOI: 10.1016/j.bmcl.2012.04.064 BindingDB Entry DOI: 10.7270/Q2348MCB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Arachidonate 5-lipoxygenase-activating protein (Homo sapiens (Human)) | BDBM50383908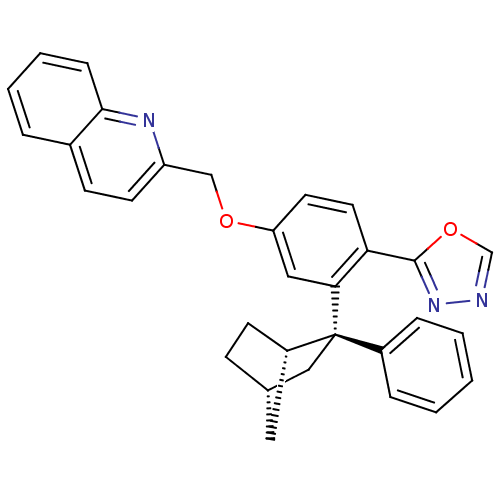 (CHEMBL2031649) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of [125I]L-691,831 from 5-lipoxygenase-activating protein in human polymorphonuclear cells | Bioorg Med Chem Lett 22: 4133-8 (2012) Article DOI: 10.1016/j.bmcl.2012.04.064 BindingDB Entry DOI: 10.7270/Q2348MCB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Arachidonate 5-lipoxygenase-activating protein (Homo sapiens (Human)) | BDBM50383894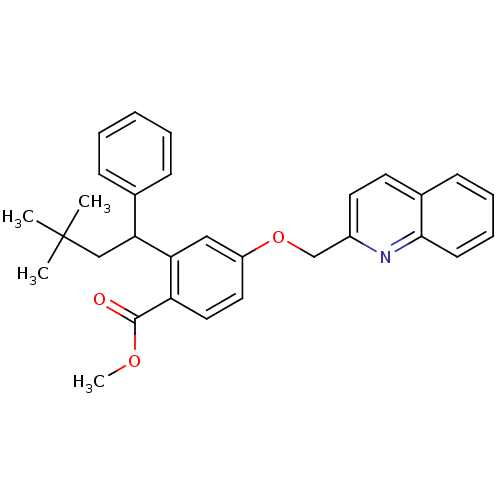 (CHEMBL2031448) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of [125I]L-691,831 from 5-lipoxygenase-activating protein in human polymorphonuclear cells | Bioorg Med Chem Lett 22: 4133-8 (2012) Article DOI: 10.1016/j.bmcl.2012.04.064 BindingDB Entry DOI: 10.7270/Q2348MCB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Arachidonate 5-lipoxygenase-activating protein (Homo sapiens (Human)) | BDBM50383899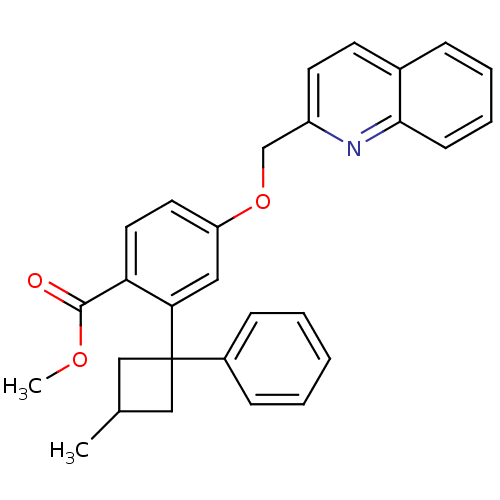 (CHEMBL2031453) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of [125I]L-691,831 from 5-lipoxygenase-activating protein in human polymorphonuclear cells | Bioorg Med Chem Lett 22: 4133-8 (2012) Article DOI: 10.1016/j.bmcl.2012.04.064 BindingDB Entry DOI: 10.7270/Q2348MCB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 311 total ) | Next | Last >> |