 Found 605 hits with Last Name = 'sparrow' and Initial = 'cp'
Found 605 hits with Last Name = 'sparrow' and Initial = 'cp' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Oxysterols receptor LXR-beta
(Homo sapiens (Human)) | BDBM50167697

((4aR,9S)-6-Hydroxy-1,4a-dimethyl-1,2,3,4,4a,9,10,1...)Show SMILES CC1(CCC[C@@]2(C)[C@H]1CCc1ccc(O)cc21)C(=O)NC(=O)[C@@]1(C)CCC[C@@]2(C)[C@H]1CCc1ccc(O)cc21 Show InChI InChI=1S/C34H43NO4/c1-31-15-5-17-33(3,27(31)13-9-21-7-11-23(36)19-25(21)31)29(38)35-30(39)34(4)18-6-16-32(2)26-20-24(37)12-8-22(26)10-14-28(32)34/h7-8,11-12,19-20,27-28,36-37H,5-6,9-10,13-18H2,1-4H3,(H,35,38,39)/t27-,28-,31-,32-,33+,34?/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory concentration in LXRSPA beta binding assay |
Bioorg Med Chem Lett 15: 4574-8 (2005)
Article DOI: 10.1016/j.bmcl.2005.06.100
BindingDB Entry DOI: 10.7270/Q2154GKK |
More data for this
Ligand-Target Pair | |
Oxysterols receptor LXR-beta
(Homo sapiens (Human)) | BDBM50167698
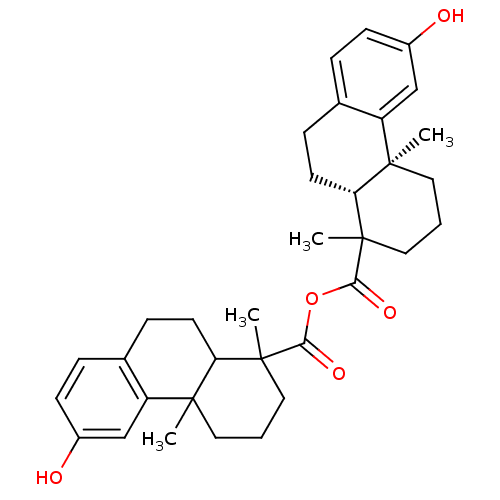
(13-hydroxy-2,6-dimethyl-(2S,7R)-tricyclo[8.4.0.02,...)Show SMILES CC1(CCCC2(C)C1CCc1ccc(O)cc21)C(=O)OC(=O)C1(C)CCC[C@@]2(C)[C@H]1CCc1ccc(O)cc21 Show InChI InChI=1S/C34H42O5/c1-31-15-5-17-33(3,27(31)13-9-21-7-11-23(35)19-25(21)31)29(37)39-30(38)34(4)18-6-16-32(2)26-20-24(36)12-8-22(26)10-14-28(32)34/h7-8,11-12,19-20,27-28,35-36H,5-6,9-10,13-18H2,1-4H3/t27-,28?,31-,32?,33?,34?/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [3H2]-F3-methyl AA (1) from liver X receptor-beta in SPA assay |
Bioorg Med Chem Lett 15: 2824-8 (2005)
Article DOI: 10.1016/j.bmcl.2005.03.100
BindingDB Entry DOI: 10.7270/Q29G5NM2 |
More data for this
Ligand-Target Pair | |
Oxysterols receptor LXR-alpha
(Homo sapiens (Human)) | BDBM50167697

((4aR,9S)-6-Hydroxy-1,4a-dimethyl-1,2,3,4,4a,9,10,1...)Show SMILES CC1(CCC[C@@]2(C)[C@H]1CCc1ccc(O)cc21)C(=O)NC(=O)[C@@]1(C)CCC[C@@]2(C)[C@H]1CCc1ccc(O)cc21 Show InChI InChI=1S/C34H43NO4/c1-31-15-5-17-33(3,27(31)13-9-21-7-11-23(36)19-25(21)31)29(38)35-30(39)34(4)18-6-16-32(2)26-20-24(37)12-8-22(26)10-14-28(32)34/h7-8,11-12,19-20,27-28,36-37H,5-6,9-10,13-18H2,1-4H3,(H,35,38,39)/t27-,28-,31-,32-,33+,34?/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory concentration in LXRSPA alpha binding assay |
Bioorg Med Chem Lett 15: 4574-8 (2005)
Article DOI: 10.1016/j.bmcl.2005.06.100
BindingDB Entry DOI: 10.7270/Q2154GKK |
More data for this
Ligand-Target Pair | |
Oxysterols receptor LXR-alpha
(Homo sapiens (Human)) | BDBM50167697

((4aR,9S)-6-Hydroxy-1,4a-dimethyl-1,2,3,4,4a,9,10,1...)Show SMILES CC1(CCC[C@@]2(C)[C@H]1CCc1ccc(O)cc21)C(=O)NC(=O)[C@@]1(C)CCC[C@@]2(C)[C@H]1CCc1ccc(O)cc21 Show InChI InChI=1S/C34H43NO4/c1-31-15-5-17-33(3,27(31)13-9-21-7-11-23(36)19-25(21)31)29(38)35-30(39)34(4)18-6-16-32(2)26-20-24(37)12-8-22(26)10-14-28(32)34/h7-8,11-12,19-20,27-28,36-37H,5-6,9-10,13-18H2,1-4H3,(H,35,38,39)/t27-,28-,31-,32-,33+,34?/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [3H2]-F3-methyl AA (1) from liver X receptor-alpha in SPA assay |
Bioorg Med Chem Lett 15: 2824-8 (2005)
Article DOI: 10.1016/j.bmcl.2005.03.100
BindingDB Entry DOI: 10.7270/Q29G5NM2 |
More data for this
Ligand-Target Pair | |
Oxysterols receptor LXR-beta
(Homo sapiens (Human)) | BDBM50167697

((4aR,9S)-6-Hydroxy-1,4a-dimethyl-1,2,3,4,4a,9,10,1...)Show SMILES CC1(CCC[C@@]2(C)[C@H]1CCc1ccc(O)cc21)C(=O)NC(=O)[C@@]1(C)CCC[C@@]2(C)[C@H]1CCc1ccc(O)cc21 Show InChI InChI=1S/C34H43NO4/c1-31-15-5-17-33(3,27(31)13-9-21-7-11-23(36)19-25(21)31)29(38)35-30(39)34(4)18-6-16-32(2)26-20-24(37)12-8-22(26)10-14-28(32)34/h7-8,11-12,19-20,27-28,36-37H,5-6,9-10,13-18H2,1-4H3,(H,35,38,39)/t27-,28-,31-,32-,33+,34?/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [3H2]-F3-methyl AA (1) from liver X receptor-beta in SPA assay |
Bioorg Med Chem Lett 15: 2824-8 (2005)
Article DOI: 10.1016/j.bmcl.2005.03.100
BindingDB Entry DOI: 10.7270/Q29G5NM2 |
More data for this
Ligand-Target Pair | |
Oxysterols receptor LXR-alpha
(Homo sapiens (Human)) | BDBM50167694

(2,6-dimethyl-13-methylcarbonyloxy-(2S)-tricyclo[8....)Show SMILES CC(=O)Oc1ccc2CC[C@H]3C(C)(CCC[C@]3(C)c2c1)C(=O)OC(=O)[C@@]1(C)CCC[C@@]2(C)[C@H]1CCc1ccc(OC(C)=O)cc21 Show InChI InChI=1S/C38H46O7/c1-23(39)43-27-13-9-25-11-15-31-35(3,29(25)21-27)17-7-19-37(31,5)33(41)45-34(42)38(6)20-8-18-36(4)30-22-28(44-24(2)40)14-10-26(30)12-16-32(36)38/h9-10,13-14,21-22,31-32H,7-8,11-12,15-20H2,1-6H3/t31-,32-,35-,36-,37+,38?/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory concentration in LXRSPA alpha binding assay |
Bioorg Med Chem Lett 15: 4574-8 (2005)
Article DOI: 10.1016/j.bmcl.2005.06.100
BindingDB Entry DOI: 10.7270/Q2154GKK |
More data for this
Ligand-Target Pair | |
Oxysterols receptor LXR-beta
(Homo sapiens (Human)) | BDBM50167694

(2,6-dimethyl-13-methylcarbonyloxy-(2S)-tricyclo[8....)Show SMILES CC(=O)Oc1ccc2CC[C@H]3C(C)(CCC[C@]3(C)c2c1)C(=O)OC(=O)[C@@]1(C)CCC[C@@]2(C)[C@H]1CCc1ccc(OC(C)=O)cc21 Show InChI InChI=1S/C38H46O7/c1-23(39)43-27-13-9-25-11-15-31-35(3,29(25)21-27)17-7-19-37(31,5)33(41)45-34(42)38(6)20-8-18-36(4)30-22-28(44-24(2)40)14-10-26(30)12-16-32(36)38/h9-10,13-14,21-22,31-32H,7-8,11-12,15-20H2,1-6H3/t31-,32-,35-,36-,37+,38?/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory concentration in LXRSPA beta binding assay |
Bioorg Med Chem Lett 15: 4574-8 (2005)
Article DOI: 10.1016/j.bmcl.2005.06.100
BindingDB Entry DOI: 10.7270/Q2154GKK |
More data for this
Ligand-Target Pair | |
Oxysterols receptor LXR-alpha
(Homo sapiens (Human)) | BDBM50167698

(13-hydroxy-2,6-dimethyl-(2S,7R)-tricyclo[8.4.0.02,...)Show SMILES CC1(CCCC2(C)C1CCc1ccc(O)cc21)C(=O)OC(=O)C1(C)CCC[C@@]2(C)[C@H]1CCc1ccc(O)cc21 Show InChI InChI=1S/C34H42O5/c1-31-15-5-17-33(3,27(31)13-9-21-7-11-23(35)19-25(21)31)29(37)39-30(38)34(4)18-6-16-32(2)26-20-24(36)12-8-22(26)10-14-28(32)34/h7-8,11-12,19-20,27-28,35-36H,5-6,9-10,13-18H2,1-4H3/t27-,28?,31-,32?,33?,34?/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [3H2]-F3-methyl AA (1) from liver X receptor-alpha in SPA assay |
Bioorg Med Chem Lett 15: 2824-8 (2005)
Article DOI: 10.1016/j.bmcl.2005.03.100
BindingDB Entry DOI: 10.7270/Q29G5NM2 |
More data for this
Ligand-Target Pair | |
Oxysterols receptor LXR-alpha
(Homo sapiens (Human)) | BDBM50167694

(2,6-dimethyl-13-methylcarbonyloxy-(2S)-tricyclo[8....)Show SMILES CC(=O)Oc1ccc2CC[C@H]3C(C)(CCC[C@]3(C)c2c1)C(=O)OC(=O)[C@@]1(C)CCC[C@@]2(C)[C@H]1CCc1ccc(OC(C)=O)cc21 Show InChI InChI=1S/C38H46O7/c1-23(39)43-27-13-9-25-11-15-31-35(3,29(25)21-27)17-7-19-37(31,5)33(41)45-34(42)38(6)20-8-18-36(4)30-22-28(44-24(2)40)14-10-26(30)12-16-32(36)38/h9-10,13-14,21-22,31-32H,7-8,11-12,15-20H2,1-6H3/t31-,32-,35-,36-,37+,38?/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [3H2]-F3-methyl AA (1) from liver X receptor-alpha in SPA assay |
Bioorg Med Chem Lett 15: 2824-8 (2005)
Article DOI: 10.1016/j.bmcl.2005.03.100
BindingDB Entry DOI: 10.7270/Q29G5NM2 |
More data for this
Ligand-Target Pair | |
Oxysterols receptor LXR-beta
(Homo sapiens (Human)) | BDBM50167694

(2,6-dimethyl-13-methylcarbonyloxy-(2S)-tricyclo[8....)Show SMILES CC(=O)Oc1ccc2CC[C@H]3C(C)(CCC[C@]3(C)c2c1)C(=O)OC(=O)[C@@]1(C)CCC[C@@]2(C)[C@H]1CCc1ccc(OC(C)=O)cc21 Show InChI InChI=1S/C38H46O7/c1-23(39)43-27-13-9-25-11-15-31-35(3,29(25)21-27)17-7-19-37(31,5)33(41)45-34(42)38(6)20-8-18-36(4)30-22-28(44-24(2)40)14-10-26(30)12-16-32(36)38/h9-10,13-14,21-22,31-32H,7-8,11-12,15-20H2,1-6H3/t31-,32-,35-,36-,37+,38?/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [3H2]-F3-methyl AA (1) from liver X receptor-beta in SPA assay |
Bioorg Med Chem Lett 15: 2824-8 (2005)
Article DOI: 10.1016/j.bmcl.2005.03.100
BindingDB Entry DOI: 10.7270/Q29G5NM2 |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor delta
(Homo sapiens (Human)) | BDBM50126018
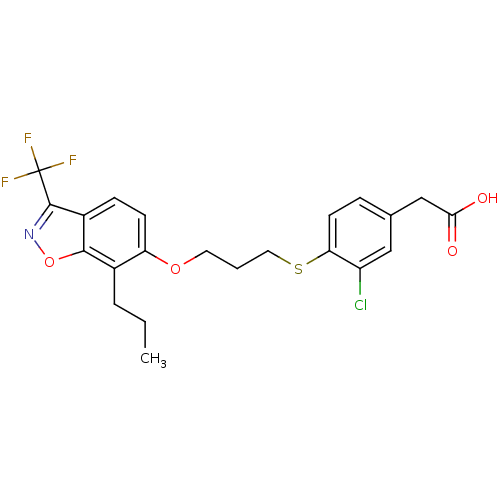
(2-(3-chloro-4-(3-(7-propyl-3-(trifluoromethyl)benz...)Show SMILES CCCc1c(OCCCSc2ccc(CC(O)=O)cc2Cl)ccc2c(noc12)C(F)(F)F Show InChI InChI=1S/C22H21ClF3NO4S/c1-2-4-14-17(7-6-15-20(14)31-27-21(15)22(24,25)26)30-9-3-10-32-18-8-5-13(11-16(18)23)12-19(28)29/h5-8,11H,2-4,9-10,12H2,1H3,(H,28,29) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of PPAR delta |
Bioorg Med Chem Lett 16: 3055-60 (2006)
Article DOI: 10.1016/j.bmcl.2006.02.050
BindingDB Entry DOI: 10.7270/Q20V8CD6 |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor delta
(Homo sapiens (Human)) | BDBM50184263
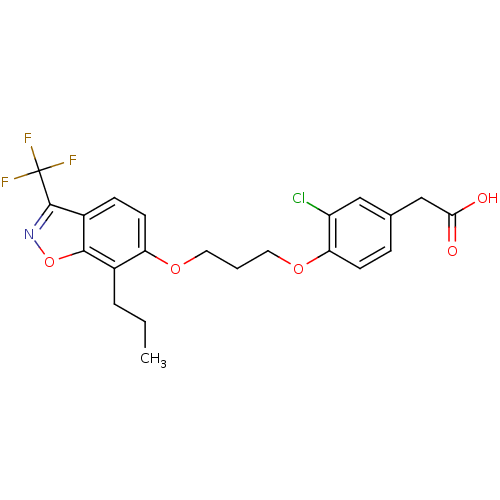
(2-(3-chloro-4-(3-(7-propyl-3-(trifluoromethyl)benz...)Show SMILES CCCc1c(OCCCOc2ccc(CC(O)=O)cc2Cl)ccc2c(noc12)C(F)(F)F Show InChI InChI=1S/C22H21ClF3NO5/c1-2-4-14-17(8-6-15-20(14)32-27-21(15)22(24,25)26)30-9-3-10-31-18-7-5-13(11-16(18)23)12-19(28)29/h5-8,11H,2-4,9-10,12H2,1H3,(H,28,29) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of PPAR delta |
Bioorg Med Chem Lett 16: 3055-60 (2006)
Article DOI: 10.1016/j.bmcl.2006.02.050
BindingDB Entry DOI: 10.7270/Q20V8CD6 |
More data for this
Ligand-Target Pair | |
Oxysterols receptor LXR-beta
(Homo sapiens (Human)) | BDBM50184249

(3-(1-(3-(7-propyl-3-(trifluoromethyl)benzo[d]isoxa...)Show SMILES CCCc1c(OCCCn2ccc3cc(CCC(O)=O)ccc23)ccc2c(noc12)C(F)(F)F Show InChI InChI=1S/C25H25F3N2O4/c1-2-4-18-21(9-7-19-23(18)34-29-24(19)25(26,27)28)33-14-3-12-30-13-11-17-15-16(5-8-20(17)30)6-10-22(31)32/h5,7-9,11,13,15H,2-4,6,10,12,14H2,1H3,(H,31,32) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of LXR beta |
Bioorg Med Chem Lett 16: 3055-60 (2006)
Article DOI: 10.1016/j.bmcl.2006.02.050
BindingDB Entry DOI: 10.7270/Q20V8CD6 |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor delta
(Homo sapiens (Human)) | BDBM50184261
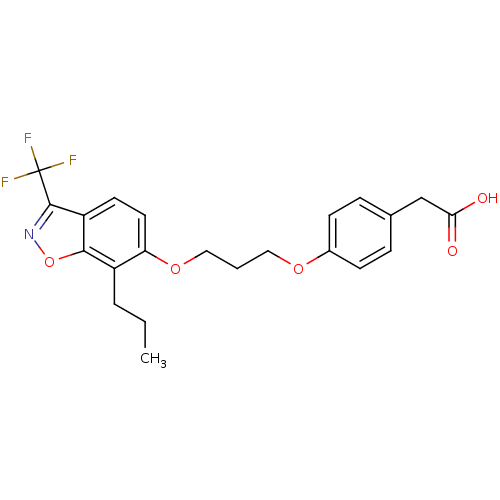
(2-(4-(3-(7-propyl-3-(trifluoromethyl)benzo[d]isoxa...)Show SMILES CCCc1c(OCCCOc2ccc(CC(O)=O)cc2)ccc2c(noc12)C(F)(F)F Show InChI InChI=1S/C22H22F3NO5/c1-2-4-16-18(10-9-17-20(16)31-26-21(17)22(23,24)25)30-12-3-11-29-15-7-5-14(6-8-15)13-19(27)28/h5-10H,2-4,11-13H2,1H3,(H,27,28) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of PPAR delta |
Bioorg Med Chem Lett 16: 3055-60 (2006)
Article DOI: 10.1016/j.bmcl.2006.02.050
BindingDB Entry DOI: 10.7270/Q20V8CD6 |
More data for this
Ligand-Target Pair | |
Oxysterols receptor LXR-beta
(Homo sapiens (Human)) | BDBM50184259

(2-(2-chloro-4-(methyl(3-(7-propyl-3-(trifluorometh...)Show SMILES CCCc1c(OCCCN(C)c2ccc(CC(O)=O)c(Cl)c2)ccc2c(noc12)C(F)(F)F Show InChI InChI=1S/C23H24ClF3N2O4/c1-3-5-16-19(9-8-17-21(16)33-28-22(17)23(25,26)27)32-11-4-10-29(2)15-7-6-14(12-20(30)31)18(24)13-15/h6-9,13H,3-5,10-12H2,1-2H3,(H,30,31) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of LXR beta |
Bioorg Med Chem Lett 16: 3055-60 (2006)
Article DOI: 10.1016/j.bmcl.2006.02.050
BindingDB Entry DOI: 10.7270/Q20V8CD6 |
More data for this
Ligand-Target Pair | |
Oxysterols receptor LXR-beta
(Homo sapiens (Human)) | BDBM50167700
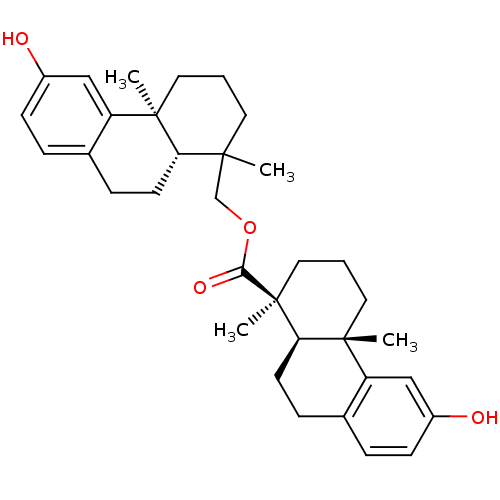
((1S,4aS,10aR)-6-Hydroxy-1,4a-dimethyl-1,2,3,4,4a,9...)Show SMILES CC1(COC(=O)[C@@]2(C)CCC[C@@]3(C)[C@H]2CCc2ccc(O)cc32)CCC[C@@]2(C)[C@H]1CCc1ccc(O)cc21 Show InChI InChI=1S/C34H44O4/c1-31(15-5-16-32(2)26-19-24(35)11-7-22(26)9-13-28(31)32)21-38-30(37)34(4)18-6-17-33(3)27-20-25(36)12-8-23(27)10-14-29(33)34/h7-8,11-12,19-20,28-29,35-36H,5-6,9-10,13-18,21H2,1-4H3/t28-,29+,31?,32+,33+,34-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [3H2]-F3-methyl AA (1) from liver X receptor-beta in SPA assay |
Bioorg Med Chem Lett 15: 2824-8 (2005)
Article DOI: 10.1016/j.bmcl.2005.03.100
BindingDB Entry DOI: 10.7270/Q29G5NM2 |
More data for this
Ligand-Target Pair | |
Oxysterols receptor LXR-alpha
(Homo sapiens (Human)) | BDBM50184259

(2-(2-chloro-4-(methyl(3-(7-propyl-3-(trifluorometh...)Show SMILES CCCc1c(OCCCN(C)c2ccc(CC(O)=O)c(Cl)c2)ccc2c(noc12)C(F)(F)F Show InChI InChI=1S/C23H24ClF3N2O4/c1-3-5-16-19(9-8-17-21(16)33-28-22(17)23(25,26)27)32-11-4-10-29(2)15-7-6-14(12-20(30)31)18(24)13-15/h6-9,13H,3-5,10-12H2,1-2H3,(H,30,31) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Activity at LXR alpha as beta-lactamase transactivation in CHO cells |
Bioorg Med Chem Lett 16: 3055-60 (2006)
Article DOI: 10.1016/j.bmcl.2006.02.050
BindingDB Entry DOI: 10.7270/Q20V8CD6 |
More data for this
Ligand-Target Pair | |
Cholesteryl ester transfer protein
(Homo sapiens (Human)) | BDBM50337153
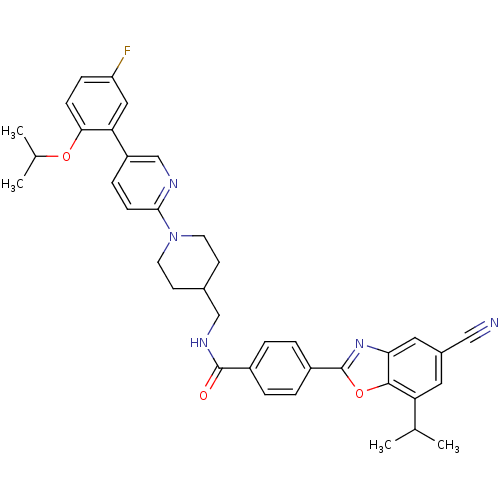
(4-(5-cyano-7-isopropylbenzo[d]oxazol-2-yl)-N-((1-(...)Show SMILES CC(C)Oc1ccc(F)cc1-c1ccc(nc1)N1CCC(CNC(=O)c2ccc(cc2)-c2nc3cc(cc(C(C)C)c3o2)C#N)CC1 Show InChI InChI=1S/C38H38FN5O3/c1-23(2)31-17-26(20-40)18-33-36(31)47-38(43-33)28-7-5-27(6-8-28)37(45)42-21-25-13-15-44(16-14-25)35-12-9-29(22-41-35)32-19-30(39)10-11-34(32)46-24(3)4/h5-12,17-19,22-25H,13-16,21H2,1-4H3,(H,42,45) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of CETP |
Bioorg Med Chem Lett 21: 1890-5 (2011)
Article DOI: 10.1016/j.bmcl.2010.11.090
BindingDB Entry DOI: 10.7270/Q2NG4QW6 |
More data for this
Ligand-Target Pair | |
Cholesteryl ester transfer protein
(Homo sapiens (Human)) | BDBM50312718
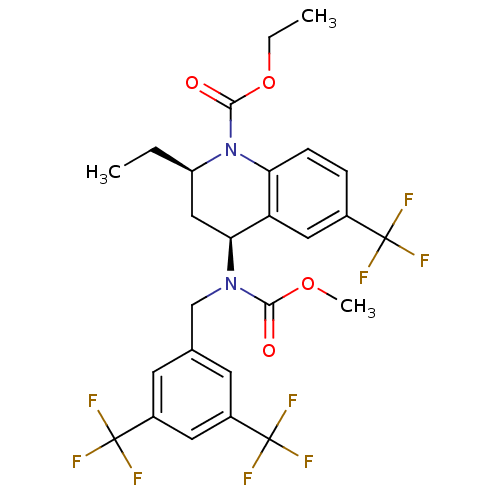
(CHEMBL479527 | torcetrapib)Show SMILES CCOC(=O)N1[C@H](CC)C[C@H](N(Cc2cc(cc(c2)C(F)(F)F)C(F)(F)F)C(=O)OC)c2cc(ccc12)C(F)(F)F |r| Show InChI InChI=1S/C26H25F9N2O4/c1-4-18-12-21(19-11-15(24(27,28)29)6-7-20(19)37(18)23(39)41-5-2)36(22(38)40-3)13-14-8-16(25(30,31)32)10-17(9-14)26(33,34)35/h6-11,18,21H,4-5,12-13H2,1-3H3/t18-,21+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of CETP-mediated BODIPY-labeled cholesteryl ester transfer after 45 mins by FRET analysis |
ACS Med Chem Lett 2: 424-427 (2011)
Article DOI: 10.1021/ml100309n
BindingDB Entry DOI: 10.7270/Q2125T47 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Oxysterols receptor LXR-beta
(Homo sapiens (Human)) | BDBM50184257
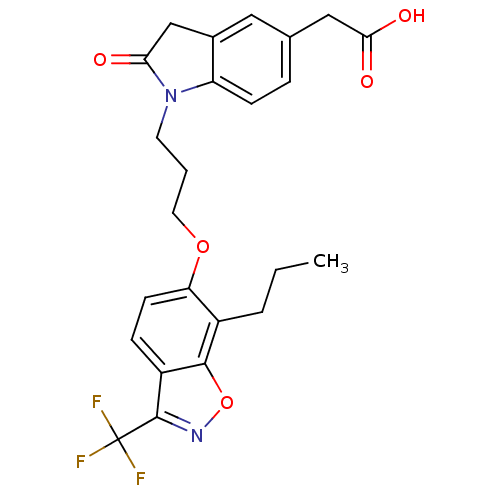
(2-(2-oxo-1-(3-(7-propyl-3-(trifluoromethyl)benzo[d...)Show SMILES CCCc1c(OCCCN2C(=O)Cc3cc(CC(O)=O)ccc23)ccc2c(noc12)C(F)(F)F Show InChI InChI=1S/C24H23F3N2O5/c1-2-4-16-19(8-6-17-22(16)34-28-23(17)24(25,26)27)33-10-3-9-29-18-7-5-14(12-21(31)32)11-15(18)13-20(29)30/h5-8,11H,2-4,9-10,12-13H2,1H3,(H,31,32) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of LXR beta |
Bioorg Med Chem Lett 16: 3055-60 (2006)
Article DOI: 10.1016/j.bmcl.2006.02.050
BindingDB Entry DOI: 10.7270/Q20V8CD6 |
More data for this
Ligand-Target Pair | |
Cholesteryl ester transfer protein
(Homo sapiens (Human)) | BDBM50337153

(4-(5-cyano-7-isopropylbenzo[d]oxazol-2-yl)-N-((1-(...)Show SMILES CC(C)Oc1ccc(F)cc1-c1ccc(nc1)N1CCC(CNC(=O)c2ccc(cc2)-c2nc3cc(cc(C(C)C)c3o2)C#N)CC1 Show InChI InChI=1S/C38H38FN5O3/c1-23(2)31-17-26(20-40)18-33-36(31)47-38(43-33)28-7-5-27(6-8-28)37(45)42-21-25-13-15-44(16-14-25)35-12-9-29(22-41-35)32-19-30(39)10-11-34(32)46-24(3)4/h5-12,17-19,22-25H,13-16,21H2,1-4H3,(H,42,45) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of CETP |
Bioorg Med Chem Lett 21: 2597-600 (2011)
Article DOI: 10.1016/j.bmcl.2011.02.049
BindingDB Entry DOI: 10.7270/Q2XG9RF5 |
More data for this
Ligand-Target Pair | |
Cholesteryl ester transfer protein
(Homo sapiens (Human)) | BDBM50334828
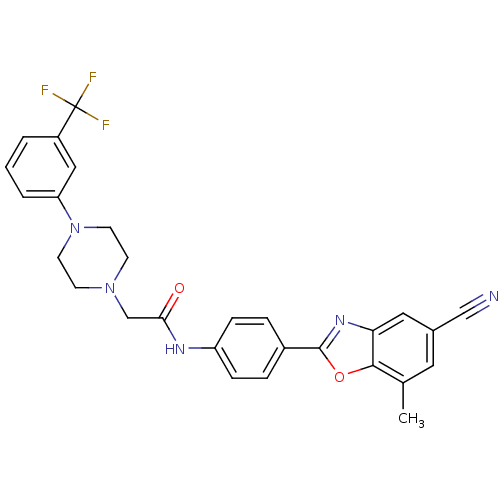
(CHEMBL1643160 | N-(4-(5-cyano-7-methylbenzo[d]oxaz...)Show SMILES Cc1cc(cc2nc(oc12)-c1ccc(NC(=O)CN2CCN(CC2)c2cccc(c2)C(F)(F)F)cc1)C#N Show InChI InChI=1S/C28H24F3N5O2/c1-18-13-19(16-32)14-24-26(18)38-27(34-24)20-5-7-22(8-6-20)33-25(37)17-35-9-11-36(12-10-35)23-4-2-3-21(15-23)28(29,30)31/h2-8,13-15H,9-12,17H2,1H3,(H,33,37) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant CETP-mediated cholesteryl ester transfer by fluorescence-based assay |
Bioorg Med Chem Lett 21: 558-61 (2010)
Article DOI: 10.1016/j.bmcl.2010.10.062
BindingDB Entry DOI: 10.7270/Q2XK8FT1 |
More data for this
Ligand-Target Pair | |
Cholesteryl ester transfer protein
(Homo sapiens (Human)) | BDBM50360871
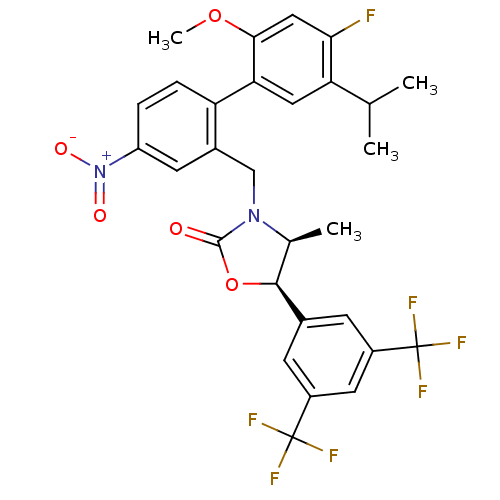
(CHEMBL1935001)Show SMILES COc1cc(F)c(cc1-c1ccc(cc1CN1[C@@H](C)[C@H](OC1=O)c1cc(cc(c1)C(F)(F)F)C(F)(F)F)[N+]([O-])=O)C(C)C |r| Show InChI InChI=1S/C29H25F7N2O5/c1-14(2)22-11-23(25(42-4)12-24(22)30)21-6-5-20(38(40)41)9-17(21)13-37-15(3)26(43-27(37)39)16-7-18(28(31,32)33)10-19(8-16)29(34,35)36/h5-12,14-15,26H,13H2,1-4H3/t15-,26-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 13.3 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp& Dohme Corp.
Curated by ChEMBL
| Assay Description
Inhibition of CETP-mediated neutral lipid transfer by fluorometric analysis |
Bioorg Med Chem Lett 22: 199-203 (2011)
Article DOI: 10.1016/j.bmcl.2011.11.039
BindingDB Entry DOI: 10.7270/Q29G5N74 |
More data for this
Ligand-Target Pair | |
Oxysterols receptor LXR-alpha
(Homo sapiens (Human)) | BDBM50167700

((1S,4aS,10aR)-6-Hydroxy-1,4a-dimethyl-1,2,3,4,4a,9...)Show SMILES CC1(COC(=O)[C@@]2(C)CCC[C@@]3(C)[C@H]2CCc2ccc(O)cc32)CCC[C@@]2(C)[C@H]1CCc1ccc(O)cc21 Show InChI InChI=1S/C34H44O4/c1-31(15-5-16-32(2)26-19-24(35)11-7-22(26)9-13-28(31)32)21-38-30(37)34(4)18-6-17-33(3)27-20-25(36)12-8-23(27)10-14-29(33)34/h7-8,11-12,19-20,28-29,35-36H,5-6,9-10,13-18,21H2,1-4H3/t28-,29+,31?,32+,33+,34-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Effective concentration against liver X receptor-alpha in HEK293 cell transactivation assay |
Bioorg Med Chem Lett 15: 2824-8 (2005)
Article DOI: 10.1016/j.bmcl.2005.03.100
BindingDB Entry DOI: 10.7270/Q29G5NM2 |
More data for this
Ligand-Target Pair | |
Cholesteryl ester transfer protein
(Homo sapiens (Human)) | BDBM50334828

(CHEMBL1643160 | N-(4-(5-cyano-7-methylbenzo[d]oxaz...)Show SMILES Cc1cc(cc2nc(oc12)-c1ccc(NC(=O)CN2CCN(CC2)c2cccc(c2)C(F)(F)F)cc1)C#N Show InChI InChI=1S/C28H24F3N5O2/c1-18-13-19(16-32)14-24-26(18)38-27(34-24)20-5-7-22(8-6-20)33-25(37)17-35-9-11-36(12-10-35)23-4-2-3-21(15-23)28(29,30)31/h2-8,13-15H,9-12,17H2,1H3,(H,33,37) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant CETP-mediated triglyceride transfer by fluorescence-based assay |
Bioorg Med Chem Lett 21: 558-61 (2010)
Article DOI: 10.1016/j.bmcl.2010.10.062
BindingDB Entry DOI: 10.7270/Q2XK8FT1 |
More data for this
Ligand-Target Pair | |
Cholesteryl ester transfer protein
(Homo sapiens (Human)) | BDBM50337152
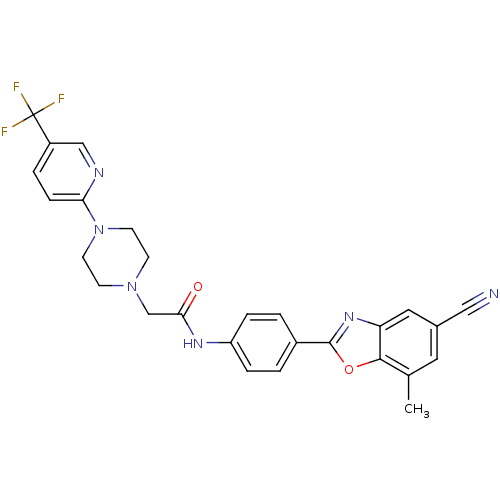
(CHEMBL1682747 | N-(4-(5-cyano-7-methylbenzo[d]oxaz...)Show SMILES Cc1cc(cc2nc(oc12)-c1ccc(NC(=O)CN2CCN(CC2)c2ccc(cn2)C(F)(F)F)cc1)C#N Show InChI InChI=1S/C27H23F3N6O2/c1-17-12-18(14-31)13-22-25(17)38-26(34-22)19-2-5-21(6-3-19)33-24(37)16-35-8-10-36(11-9-35)23-7-4-20(15-32-23)27(28,29)30/h2-7,12-13,15H,8-11,16H2,1H3,(H,33,37) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of CETP |
Bioorg Med Chem Lett 21: 1890-5 (2011)
Article DOI: 10.1016/j.bmcl.2010.11.090
BindingDB Entry DOI: 10.7270/Q2NG4QW6 |
More data for this
Ligand-Target Pair | |
Cholesteryl ester transfer protein
(Homo sapiens (Human)) | BDBM50348226
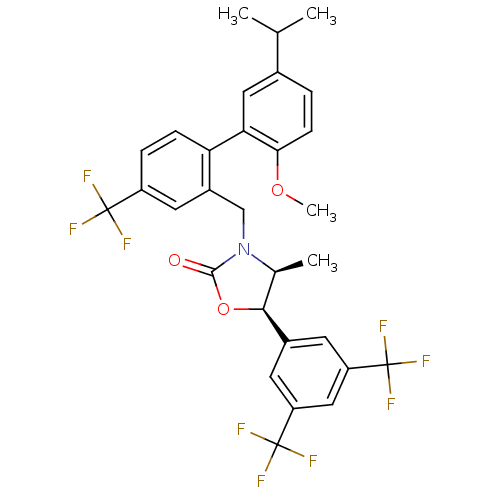
(CHEMBL1800622)Show SMILES COc1ccc(cc1-c1ccc(cc1CN1[C@@H](C)[C@H](OC1=O)c1cc(cc(c1)C(F)(F)F)C(F)(F)F)C(F)(F)F)C(C)C |r| Show InChI InChI=1S/C30H26F9NO3/c1-15(2)17-5-8-25(42-4)24(12-17)23-7-6-20(28(31,32)33)11-19(23)14-40-16(3)26(43-27(40)41)18-9-21(29(34,35)36)13-22(10-18)30(37,38)39/h5-13,15-16,26H,14H2,1-4H3/t16-,26-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 15.2 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant CETP-mediated cholesteryl ester transfer activity after 1 hr by fluorescent cholesteryl esters transfer assay |
J Med Chem 54: 4880-95 (2011)
Article DOI: 10.1021/jm200484c
BindingDB Entry DOI: 10.7270/Q2NG4QZ3 |
More data for this
Ligand-Target Pair | |
Oxysterols receptor LXR-alpha
(Homo sapiens (Human)) | BDBM50184255

(2-(1-(3-(7-propyl-3-(trifluoromethyl)benzo[d]isoxa...)Show SMILES CCCc1c(OCCCn2ccc3cc(CC(O)=O)ccc23)ccc2c(noc12)C(F)(F)F Show InChI InChI=1S/C24H23F3N2O4/c1-2-4-17-20(8-6-18-22(17)33-28-23(18)24(25,26)27)32-12-3-10-29-11-9-16-13-15(14-21(30)31)5-7-19(16)29/h5-9,11,13H,2-4,10,12,14H2,1H3,(H,30,31) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Activity at LXR alpha as beta-lactamase transactivation in CHO cells |
Bioorg Med Chem Lett 16: 3055-60 (2006)
Article DOI: 10.1016/j.bmcl.2006.02.050
BindingDB Entry DOI: 10.7270/Q20V8CD6 |
More data for this
Ligand-Target Pair | |
Oxysterols receptor LXR-beta
(Homo sapiens (Human)) | BDBM50184255

(2-(1-(3-(7-propyl-3-(trifluoromethyl)benzo[d]isoxa...)Show SMILES CCCc1c(OCCCn2ccc3cc(CC(O)=O)ccc23)ccc2c(noc12)C(F)(F)F Show InChI InChI=1S/C24H23F3N2O4/c1-2-4-17-20(8-6-18-22(17)33-28-23(18)24(25,26)27)32-12-3-10-29-11-9-16-13-15(14-21(30)31)5-7-19(16)29/h5-9,11,13H,2-4,10,12,14H2,1H3,(H,30,31) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of LXR beta |
Bioorg Med Chem Lett 16: 3055-60 (2006)
Article DOI: 10.1016/j.bmcl.2006.02.050
BindingDB Entry DOI: 10.7270/Q20V8CD6 |
More data for this
Ligand-Target Pair | |
Cholesteryl ester transfer protein
(Homo sapiens (Human)) | BDBM50334829
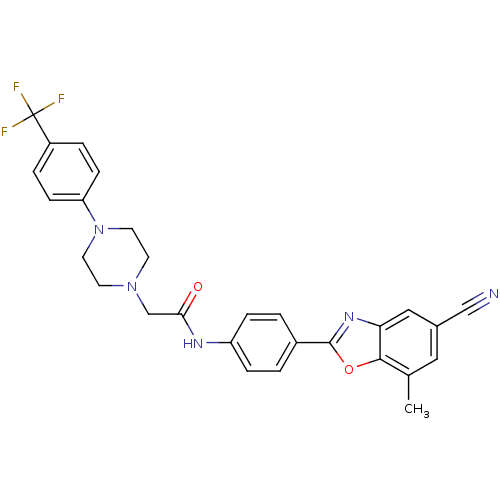
(CHEMBL1643161 | N-(4-(5-cyano-7-methylbenzo[d]oxaz...)Show SMILES Cc1cc(cc2nc(oc12)-c1ccc(NC(=O)CN2CCN(CC2)c2ccc(cc2)C(F)(F)F)cc1)C#N Show InChI InChI=1S/C28H24F3N5O2/c1-18-14-19(16-32)15-24-26(18)38-27(34-24)20-2-6-22(7-3-20)33-25(37)17-35-10-12-36(13-11-35)23-8-4-21(5-9-23)28(29,30)31/h2-9,14-15H,10-13,17H2,1H3,(H,33,37) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant CETP-mediated cholesteryl ester transfer by fluorescence-based assay |
Bioorg Med Chem Lett 21: 558-61 (2010)
Article DOI: 10.1016/j.bmcl.2010.10.062
BindingDB Entry DOI: 10.7270/Q2XK8FT1 |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor alpha
(Homo sapiens (Human)) | BDBM50184274
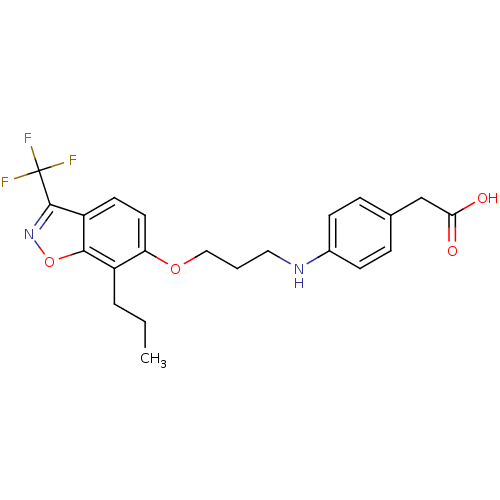
(2-(4-(3-(7-propyl-3-(trifluoromethyl)benzo[d]isoxa...)Show SMILES CCCc1c(OCCCNc2ccc(CC(O)=O)cc2)ccc2c(noc12)C(F)(F)F Show InChI InChI=1S/C22H23F3N2O4/c1-2-4-16-18(10-9-17-20(16)31-27-21(17)22(23,24)25)30-12-3-11-26-15-7-5-14(6-8-15)13-19(28)29/h5-10,26H,2-4,11-13H2,1H3,(H,28,29) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of PPAR alpha |
Bioorg Med Chem Lett 16: 3055-60 (2006)
Article DOI: 10.1016/j.bmcl.2006.02.050
BindingDB Entry DOI: 10.7270/Q20V8CD6 |
More data for this
Ligand-Target Pair | |
Cholesteryl ester transfer protein
(Homo sapiens (Human)) | BDBM50334829

(CHEMBL1643161 | N-(4-(5-cyano-7-methylbenzo[d]oxaz...)Show SMILES Cc1cc(cc2nc(oc12)-c1ccc(NC(=O)CN2CCN(CC2)c2ccc(cc2)C(F)(F)F)cc1)C#N Show InChI InChI=1S/C28H24F3N5O2/c1-18-14-19(16-32)15-24-26(18)38-27(34-24)20-2-6-22(7-3-20)33-25(37)17-35-10-12-36(13-11-35)23-8-4-21(5-9-23)28(29,30)31/h2-9,14-15H,10-13,17H2,1H3,(H,33,37) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of CETP |
Bioorg Med Chem Lett 21: 1890-5 (2011)
Article DOI: 10.1016/j.bmcl.2010.11.090
BindingDB Entry DOI: 10.7270/Q2NG4QW6 |
More data for this
Ligand-Target Pair | |
Cholesteryl ester transfer protein
(Homo sapiens (Human)) | BDBM50360876

(CHEMBL1935006)Show SMILES COc1cc(F)c(cc1-c1ccc(SC)cc1CN1[C@@H](C)[C@H](OC1=O)c1cc(cc(c1)C(F)(F)F)C(F)(F)F)C(C)C |r| Show InChI InChI=1S/C30H28F7NO3S/c1-15(2)23-12-24(26(40-4)13-25(23)31)22-7-6-21(42-5)10-18(22)14-38-16(3)27(41-28(38)39)17-8-19(29(32,33)34)11-20(9-17)30(35,36)37/h6-13,15-16,27H,14H2,1-5H3/t16-,27-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 16.2 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp& Dohme Corp.
Curated by ChEMBL
| Assay Description
Inhibition of CETP-mediated neutral lipid transfer by fluorometric analysis |
Bioorg Med Chem Lett 22: 199-203 (2011)
Article DOI: 10.1016/j.bmcl.2011.11.039
BindingDB Entry DOI: 10.7270/Q29G5N74 |
More data for this
Ligand-Target Pair | |
Cholesteryl ester transfer protein
(Homo sapiens (Human)) | BDBM50360867

(CHEMBL1934997)Show SMILES COc1cc(F)c(cc1-c1ccc(Cl)cc1CN1[C@@H](C)[C@H](OC1=O)c1cc(cc(c1)C(F)(F)F)C(F)(F)F)C(C)C |r| Show InChI InChI=1S/C29H25ClF7NO3/c1-14(2)22-11-23(25(40-4)12-24(22)31)21-6-5-20(30)9-17(21)13-38-15(3)26(41-27(38)39)16-7-18(28(32,33)34)10-19(8-16)29(35,36)37/h5-12,14-15,26H,13H2,1-4H3/t15-,26-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 16.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp& Dohme Corp.
Curated by ChEMBL
| Assay Description
Inhibition of CETP-mediated neutral lipid transfer by fluorometric analysis |
Bioorg Med Chem Lett 22: 199-203 (2011)
Article DOI: 10.1016/j.bmcl.2011.11.039
BindingDB Entry DOI: 10.7270/Q29G5N74 |
More data for this
Ligand-Target Pair | |
Cholesteryl ester transfer protein
(Homo sapiens (Human)) | BDBM50360873
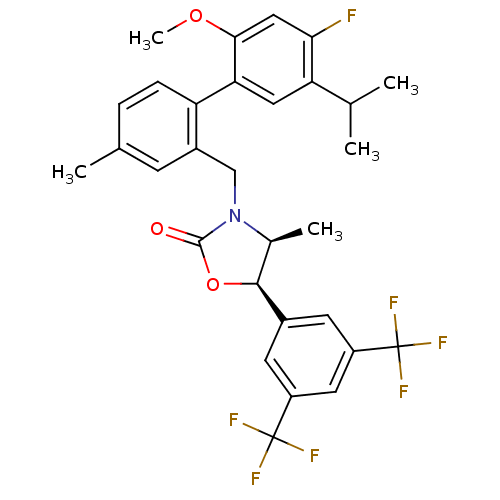
(CHEMBL1935003)Show SMILES COc1cc(F)c(cc1-c1ccc(C)cc1CN1[C@@H](C)[C@H](OC1=O)c1cc(cc(c1)C(F)(F)F)C(F)(F)F)C(C)C |r| Show InChI InChI=1S/C30H28F7NO3/c1-15(2)23-12-24(26(40-5)13-25(23)31)22-7-6-16(3)8-19(22)14-38-17(4)27(41-28(38)39)18-9-20(29(32,33)34)11-21(10-18)30(35,36)37/h6-13,15,17,27H,14H2,1-5H3/t17-,27-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 16.7 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp& Dohme Corp.
Curated by ChEMBL
| Assay Description
Inhibition of CETP-mediated neutral lipid transfer by fluorometric analysis |
Bioorg Med Chem Lett 22: 199-203 (2011)
Article DOI: 10.1016/j.bmcl.2011.11.039
BindingDB Entry DOI: 10.7270/Q29G5N74 |
More data for this
Ligand-Target Pair | |
Cholesteryl ester transfer protein
(Homo sapiens (Human)) | BDBM50348228
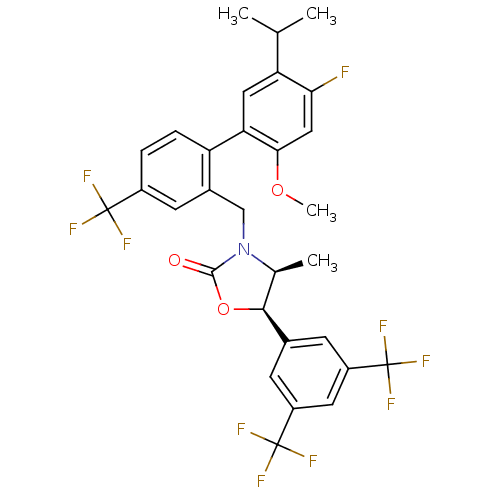
(CHEMBL1800807)Show SMILES COc1cc(F)c(cc1-c1ccc(cc1CN1[C@@H](C)[C@H](OC1=O)c1cc(cc(c1)C(F)(F)F)C(F)(F)F)C(F)(F)F)C(C)C |r| Show InChI InChI=1S/C30H25F10NO3/c1-14(2)22-11-23(25(43-4)12-24(22)31)21-6-5-18(28(32,33)34)9-17(21)13-41-15(3)26(44-27(41)42)16-7-19(29(35,36)37)10-20(8-16)30(38,39)40/h5-12,14-15,26H,13H2,1-4H3/t15-,26-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 17.2 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp& Dohme Corp.
Curated by ChEMBL
| Assay Description
Inhibition of CETP-mediated neutral lipid transfer by fluorometric analysis |
Bioorg Med Chem Lett 22: 199-203 (2011)
Article DOI: 10.1016/j.bmcl.2011.11.039
BindingDB Entry DOI: 10.7270/Q29G5N74 |
More data for this
Ligand-Target Pair | |
Cholesteryl ester transfer protein
(Homo sapiens (Human)) | BDBM50348228

(CHEMBL1800807)Show SMILES COc1cc(F)c(cc1-c1ccc(cc1CN1[C@@H](C)[C@H](OC1=O)c1cc(cc(c1)C(F)(F)F)C(F)(F)F)C(F)(F)F)C(C)C |r| Show InChI InChI=1S/C30H25F10NO3/c1-14(2)22-11-23(25(43-4)12-24(22)31)21-6-5-18(28(32,33)34)9-17(21)13-41-15(3)26(44-27(41)42)16-7-19(29(35,36)37)10-20(8-16)30(38,39)40/h5-12,14-15,26H,13H2,1-4H3/t15-,26-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 17.2 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant CETP-mediated cholesteryl ester transfer activity after 1 hr by fluorescent cholesteryl esters transfer assay |
J Med Chem 54: 4880-95 (2011)
Article DOI: 10.1021/jm200484c
BindingDB Entry DOI: 10.7270/Q2NG4QZ3 |
More data for this
Ligand-Target Pair | |
Cholesteryl ester transfer protein
(Homo sapiens (Human)) | BDBM50348230

(CHEMBL1800810)Show SMILES COc1cc(F)c(cc1-c1ccc(cc1CN1C(=O)O[C@H](c2cc(cc(c2)C(F)(F)F)C(F)(F)F)C1(C)C)C(F)(F)F)C(C)C |r| Show InChI InChI=1S/C31H27F10NO3/c1-15(2)22-12-23(25(44-5)13-24(22)32)21-7-6-18(29(33,34)35)10-17(21)14-42-27(43)45-26(28(42,3)4)16-8-19(30(36,37)38)11-20(9-16)31(39,40)41/h6-13,15,26H,14H2,1-5H3/t26-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 17.8 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant CETP-mediated cholesteryl ester transfer activity after 1 hr by fluorescent cholesteryl esters transfer assay |
J Med Chem 54: 4880-95 (2011)
Article DOI: 10.1021/jm200484c
BindingDB Entry DOI: 10.7270/Q2NG4QZ3 |
More data for this
Ligand-Target Pair | |
Cholesteryl ester transfer protein
(Homo sapiens (Human)) | BDBM50360881
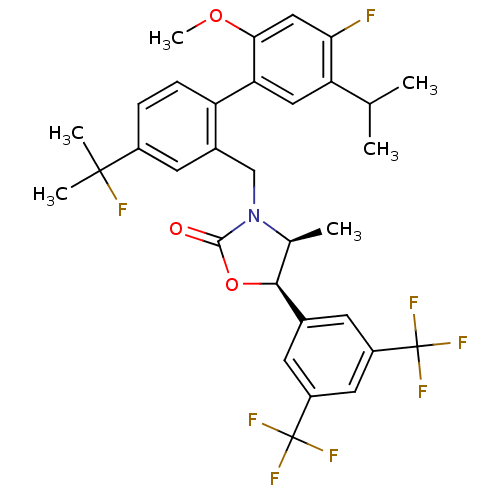
(CHEMBL1935011)Show SMILES COc1cc(F)c(cc1-c1ccc(cc1CN1[C@@H](C)[C@H](OC1=O)c1cc(cc(c1)C(F)(F)F)C(F)(F)F)C(C)(C)F)C(C)C |r| Show InChI InChI=1S/C32H31F8NO3/c1-16(2)24-13-25(27(43-6)14-26(24)33)23-8-7-20(30(4,5)34)11-19(23)15-41-17(3)28(44-29(41)42)18-9-21(31(35,36)37)12-22(10-18)32(38,39)40/h7-14,16-17,28H,15H2,1-6H3/t17-,28-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 18 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp& Dohme Corp.
Curated by ChEMBL
| Assay Description
Inhibition of CETP-mediated neutral lipid transfer by fluorometric analysis |
Bioorg Med Chem Lett 22: 199-203 (2011)
Article DOI: 10.1016/j.bmcl.2011.11.039
BindingDB Entry DOI: 10.7270/Q29G5N74 |
More data for this
Ligand-Target Pair | |
Cholesteryl ester transfer protein
(Homo sapiens (Human)) | BDBM50334829

(CHEMBL1643161 | N-(4-(5-cyano-7-methylbenzo[d]oxaz...)Show SMILES Cc1cc(cc2nc(oc12)-c1ccc(NC(=O)CN2CCN(CC2)c2ccc(cc2)C(F)(F)F)cc1)C#N Show InChI InChI=1S/C28H24F3N5O2/c1-18-14-19(16-32)15-24-26(18)38-27(34-24)20-2-6-22(7-3-20)33-25(37)17-35-10-12-36(13-11-35)23-8-4-21(5-9-23)28(29,30)31/h2-9,14-15H,10-13,17H2,1H3,(H,33,37) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 18 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant CETP-mediated triglyceride transfer by fluorescence-based assay |
Bioorg Med Chem Lett 21: 558-61 (2010)
Article DOI: 10.1016/j.bmcl.2010.10.062
BindingDB Entry DOI: 10.7270/Q2XK8FT1 |
More data for this
Ligand-Target Pair | |
Oxysterols receptor LXR-alpha
(Homo sapiens (Human)) | BDBM50184249

(3-(1-(3-(7-propyl-3-(trifluoromethyl)benzo[d]isoxa...)Show SMILES CCCc1c(OCCCn2ccc3cc(CCC(O)=O)ccc23)ccc2c(noc12)C(F)(F)F Show InChI InChI=1S/C25H25F3N2O4/c1-2-4-18-21(9-7-19-23(18)34-29-24(19)25(26,27)28)33-14-3-12-30-13-11-17-15-16(5-8-20(17)30)6-10-22(31)32/h5,7-9,11,13,15H,2-4,6,10,12,14H2,1H3,(H,31,32) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 18 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Activity at LXR alpha as beta-lactamase transactivation in CHO cells |
Bioorg Med Chem Lett 16: 3055-60 (2006)
Article DOI: 10.1016/j.bmcl.2006.02.050
BindingDB Entry DOI: 10.7270/Q20V8CD6 |
More data for this
Ligand-Target Pair | |
Oxysterols receptor LXR-beta
(Homo sapiens (Human)) | BDBM50184250
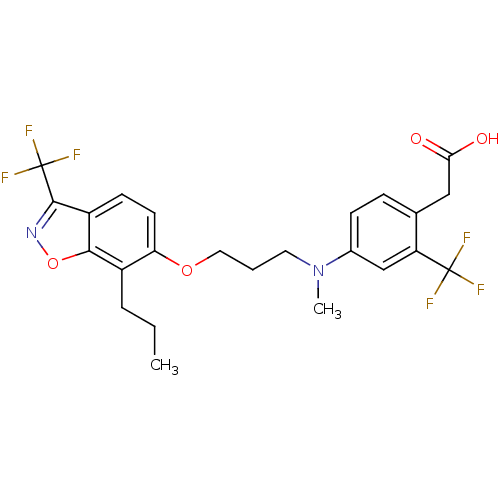
(2-(4-(methyl(3-(7-propyl-3-(trifluoromethyl)benzo[...)Show SMILES CCCc1c(OCCCN(C)c2ccc(CC(O)=O)c(c2)C(F)(F)F)ccc2c(noc12)C(F)(F)F Show InChI InChI=1S/C24H24F6N2O4/c1-3-5-16-19(9-8-17-21(16)36-31-22(17)24(28,29)30)35-11-4-10-32(2)15-7-6-14(12-20(33)34)18(13-15)23(25,26)27/h6-9,13H,3-5,10-12H2,1-2H3,(H,33,34) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 18 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of LXR beta |
Bioorg Med Chem Lett 16: 3055-60 (2006)
Article DOI: 10.1016/j.bmcl.2006.02.050
BindingDB Entry DOI: 10.7270/Q20V8CD6 |
More data for this
Ligand-Target Pair | |
Oxysterols receptor LXR-alpha
(Homo sapiens (Human)) | BDBM50184254

(2-(4-(methyl(3-(7-propyl-3-(trifluoromethyl)benzo[...)Show SMILES CCCc1c(OCCCN(C)c2ccc(CC(O)=O)cc2)ccc2c(noc12)C(F)(F)F Show InChI InChI=1S/C23H25F3N2O4/c1-3-5-17-19(11-10-18-21(17)32-27-22(18)23(24,25)26)31-13-4-12-28(2)16-8-6-15(7-9-16)14-20(29)30/h6-11H,3-5,12-14H2,1-2H3,(H,29,30) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 18 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Activity at LXR alpha as beta-lactamase transactivation in CHO cells |
Bioorg Med Chem Lett 16: 3055-60 (2006)
Article DOI: 10.1016/j.bmcl.2006.02.050
BindingDB Entry DOI: 10.7270/Q20V8CD6 |
More data for this
Ligand-Target Pair | |
Cholesteryl ester transfer protein
(Homo sapiens (Human)) | BDBM50342850

((S)-4-(5-cyano-7-isopropylbenzo[d]oxazol-2-yl)-N-(...)Show SMILES CC(C)c1cc(cc2nc(oc12)-c1ccc(cc1)C(=O)NC[C@@]1(C)CN(C(=O)O1)c1ccc(cn1)-c1ccccc1OC(F)(F)F)C#N |r| Show InChI InChI=1S/C35H28F3N5O5/c1-20(2)26-14-21(16-39)15-27-30(26)46-32(42-27)23-10-8-22(9-11-23)31(44)41-18-34(3)19-43(33(45)48-34)29-13-12-24(17-40-29)25-6-4-5-7-28(25)47-35(36,37)38/h4-15,17,20H,18-19H2,1-3H3,(H,41,44)/t34-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 18 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of CETP |
Bioorg Med Chem Lett 21: 2597-600 (2011)
Article DOI: 10.1016/j.bmcl.2011.02.049
BindingDB Entry DOI: 10.7270/Q2XG9RF5 |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor alpha
(Homo sapiens (Human)) | BDBM50184261

(2-(4-(3-(7-propyl-3-(trifluoromethyl)benzo[d]isoxa...)Show SMILES CCCc1c(OCCCOc2ccc(CC(O)=O)cc2)ccc2c(noc12)C(F)(F)F Show InChI InChI=1S/C22H22F3NO5/c1-2-4-16-18(10-9-17-20(16)31-26-21(17)22(23,24)25)30-12-3-11-29-15-7-5-14(6-8-15)13-19(27)28/h5-10H,2-4,11-13H2,1H3,(H,27,28) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 19 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of PPAR alpha |
Bioorg Med Chem Lett 16: 3055-60 (2006)
Article DOI: 10.1016/j.bmcl.2006.02.050
BindingDB Entry DOI: 10.7270/Q20V8CD6 |
More data for this
Ligand-Target Pair | |
Cholesteryl ester transfer protein
(Homo sapiens (Human)) | BDBM50334825
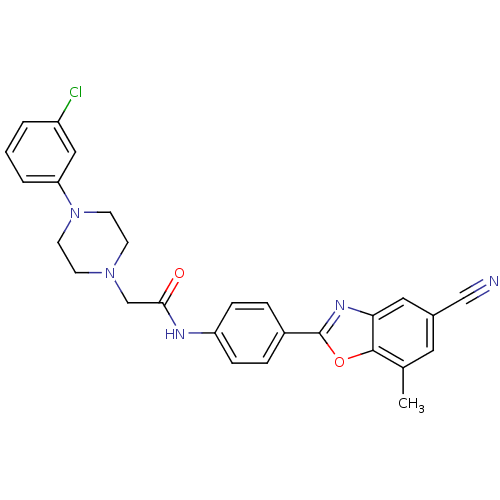
(2-(4-(3-chlorophenyl)piperazin-1-yl)-N-(4-(5-cyano...)Show SMILES Cc1cc(cc2nc(oc12)-c1ccc(NC(=O)CN2CCN(CC2)c2cccc(Cl)c2)cc1)C#N Show InChI InChI=1S/C27H24ClN5O2/c1-18-13-19(16-29)14-24-26(18)35-27(31-24)20-5-7-22(8-6-20)30-25(34)17-32-9-11-33(12-10-32)23-4-2-3-21(28)15-23/h2-8,13-15H,9-12,17H2,1H3,(H,30,34) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 19 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant CETP-mediated cholesteryl ester transfer by fluorescence-based assay |
Bioorg Med Chem Lett 21: 558-61 (2010)
Article DOI: 10.1016/j.bmcl.2010.10.062
BindingDB Entry DOI: 10.7270/Q2XK8FT1 |
More data for this
Ligand-Target Pair | |
Oxysterols receptor LXR-alpha
(Homo sapiens (Human)) | BDBM50184257

(2-(2-oxo-1-(3-(7-propyl-3-(trifluoromethyl)benzo[d...)Show SMILES CCCc1c(OCCCN2C(=O)Cc3cc(CC(O)=O)ccc23)ccc2c(noc12)C(F)(F)F Show InChI InChI=1S/C24H23F3N2O5/c1-2-4-16-19(8-6-17-22(16)34-28-23(17)24(25,26)27)33-10-3-9-29-18-7-5-14(12-21(31)32)11-15(18)13-20(29)30/h5-8,11H,2-4,9-10,12-13H2,1H3,(H,31,32) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 19 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Activity at LXR alpha as beta-lactamase transactivation in CHO cells |
Bioorg Med Chem Lett 16: 3055-60 (2006)
Article DOI: 10.1016/j.bmcl.2006.02.050
BindingDB Entry DOI: 10.7270/Q20V8CD6 |
More data for this
Ligand-Target Pair | |
Cholesteryl ester transfer protein
(Homo sapiens (Human)) | BDBM50337194
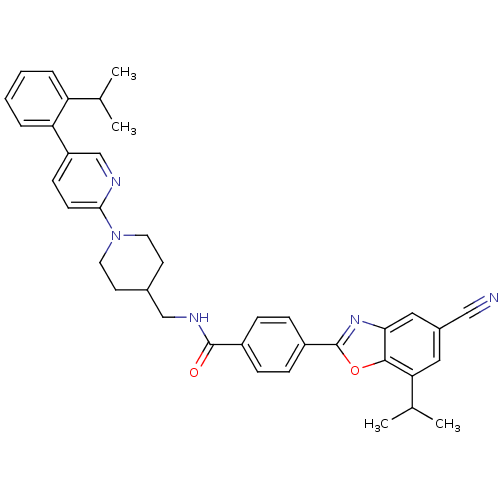
(4-(5-cyano-7-isopropylbenzo[d]oxazol-2-yl)-N-((1-(...)Show SMILES CC(C)c1ccccc1-c1ccc(nc1)N1CCC(CNC(=O)c2ccc(cc2)-c2nc3cc(cc(C(C)C)c3o2)C#N)CC1 Show InChI InChI=1S/C38H39N5O2/c1-24(2)31-7-5-6-8-32(31)30-13-14-35(40-23-30)43-17-15-26(16-18-43)22-41-37(44)28-9-11-29(12-10-28)38-42-34-20-27(21-39)19-33(25(3)4)36(34)45-38/h5-14,19-20,23-26H,15-18,22H2,1-4H3,(H,41,44) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 19 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of CETP |
Bioorg Med Chem Lett 21: 1890-5 (2011)
Article DOI: 10.1016/j.bmcl.2010.11.090
BindingDB Entry DOI: 10.7270/Q2NG4QW6 |
More data for this
Ligand-Target Pair | |
Cholesteryl ester transfer protein
(Homo sapiens (Human)) | BDBM50360879
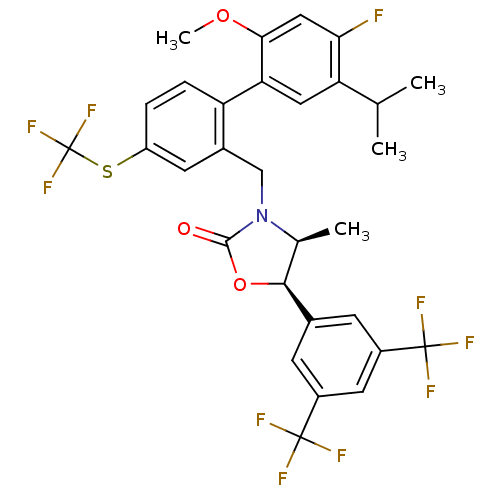
(CHEMBL1935009)Show SMILES COc1cc(F)c(cc1-c1ccc(SC(F)(F)F)cc1CN1[C@@H](C)[C@H](OC1=O)c1cc(cc(c1)C(F)(F)F)C(F)(F)F)C(C)C |r| Show InChI InChI=1S/C30H25F10NO3S/c1-14(2)22-11-23(25(43-4)12-24(22)31)21-6-5-20(45-30(38,39)40)9-17(21)13-41-15(3)26(44-27(41)42)16-7-18(28(32,33)34)10-19(8-16)29(35,36)37/h5-12,14-15,26H,13H2,1-4H3/t15-,26-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 19.6 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp& Dohme Corp.
Curated by ChEMBL
| Assay Description
Inhibition of CETP-mediated neutral lipid transfer by fluorometric analysis |
Bioorg Med Chem Lett 22: 199-203 (2011)
Article DOI: 10.1016/j.bmcl.2011.11.039
BindingDB Entry DOI: 10.7270/Q29G5N74 |
More data for this
Ligand-Target Pair | |
Cholesteryl ester transfer protein
(Homo sapiens (Human)) | BDBM50360868
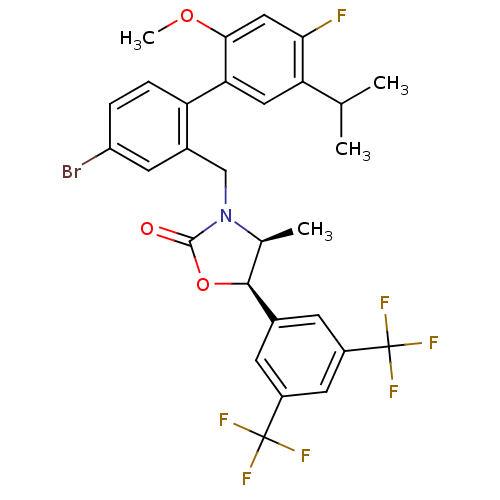
(CHEMBL1934998)Show SMILES COc1cc(F)c(cc1-c1ccc(Br)cc1CN1[C@@H](C)[C@H](OC1=O)c1cc(cc(c1)C(F)(F)F)C(F)(F)F)C(C)C |r| Show InChI InChI=1S/C29H25BrF7NO3/c1-14(2)22-11-23(25(40-4)12-24(22)31)21-6-5-20(30)9-17(21)13-38-15(3)26(41-27(38)39)16-7-18(28(32,33)34)10-19(8-16)29(35,36)37/h5-12,14-15,26H,13H2,1-4H3/t15-,26-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 19.8 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp& Dohme Corp.
Curated by ChEMBL
| Assay Description
Inhibition of CETP-mediated neutral lipid transfer by fluorometric analysis |
Bioorg Med Chem Lett 22: 199-203 (2011)
Article DOI: 10.1016/j.bmcl.2011.11.039
BindingDB Entry DOI: 10.7270/Q29G5N74 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data