 Found 87 hits of Enzyme Inhibition Constant Data
Found 87 hits of Enzyme Inhibition Constant Data Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Collagenase 3
(Homo sapiens (Human)) | BDBM50173235

((S)-2-{4'-[(5-Bromo-4-methoxy-benzofuran-2-carbony...)Show SMILES COc1c(Br)ccc2oc(cc12)C(=O)Nc1ccc(cc1)-c1ccc(cc1)S(=O)(=O)N[C@@H](C(C)C)C(O)=O Show InChI InChI=1S/C27H25BrN2O7S/c1-15(2)24(27(32)33)30-38(34,35)19-10-6-17(7-11-19)16-4-8-18(9-5-16)29-26(31)23-14-20-22(37-23)13-12-21(28)25(20)36-3/h4-15,24,30H,1-3H3,(H,29,31)(H,32,33)/t24-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Inhibitory concentration against MMP-13 |
Bioorg Med Chem Lett 15: 4961-6 (2005)
Article DOI: 10.1016/j.bmcl.2005.08.001
BindingDB Entry DOI: 10.7270/Q20Z72TH |
More data for this
Ligand-Target Pair | |
Collagenase 3
(Homo sapiens (Human)) | BDBM50173236

((S)-2-{4'-[(5-Bromo-benzofuran-2-carbonyl)-amino]-...)Show SMILES CC(C)[C@H](NS(=O)(=O)c1ccc(cc1)-c1ccc(NC(=O)c2cc3cc(Br)ccc3o2)cc1)C(O)=O Show InChI InChI=1S/C26H23BrN2O6S/c1-15(2)24(26(31)32)29-36(33,34)21-10-5-17(6-11-21)16-3-8-20(9-4-16)28-25(30)23-14-18-13-19(27)7-12-22(18)35-23/h3-15,24,29H,1-2H3,(H,28,30)(H,31,32)/t24-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.610 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Inhibitory concentration against MMP-13 |
Bioorg Med Chem Lett 15: 4961-6 (2005)
Article DOI: 10.1016/j.bmcl.2005.08.001
BindingDB Entry DOI: 10.7270/Q20Z72TH |
More data for this
Ligand-Target Pair | |
72 kDa type IV collagenase
(Homo sapiens (Human)) | BDBM50173236

((S)-2-{4'-[(5-Bromo-benzofuran-2-carbonyl)-amino]-...)Show SMILES CC(C)[C@H](NS(=O)(=O)c1ccc(cc1)-c1ccc(NC(=O)c2cc3cc(Br)ccc3o2)cc1)C(O)=O Show InChI InChI=1S/C26H23BrN2O6S/c1-15(2)24(26(31)32)29-36(33,34)21-10-5-17(6-11-21)16-3-8-20(9-4-16)28-25(30)23-14-18-13-19(27)7-12-22(18)35-23/h3-15,24,29H,1-2H3,(H,28,30)(H,31,32)/t24-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.910 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Inhibitory concentration against MMP-2 |
Bioorg Med Chem Lett 15: 4961-6 (2005)
Article DOI: 10.1016/j.bmcl.2005.08.001
BindingDB Entry DOI: 10.7270/Q20Z72TH |
More data for this
Ligand-Target Pair | |
Collagenase 3
(Homo sapiens (Human)) | BDBM50173220

((S)-2-{4'-[(5-Chloro-4-methoxy-3-methyl-benzofuran...)Show SMILES COc1c(Cl)ccc2oc(C(=O)Nc3ccc(cc3)-c3ccc(cc3)S(=O)(=O)N[C@@H](C(C)C)C(O)=O)c(C)c12 Show InChI InChI=1S/C28H27ClN2O7S/c1-15(2)24(28(33)34)31-39(35,36)20-11-7-18(8-12-20)17-5-9-19(10-6-17)30-27(32)25-16(3)23-22(38-25)14-13-21(29)26(23)37-4/h5-15,24,31H,1-4H3,(H,30,32)(H,33,34)/t24-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Inhibitory concentration against MMP-13 |
Bioorg Med Chem Lett 15: 4961-6 (2005)
Article DOI: 10.1016/j.bmcl.2005.08.001
BindingDB Entry DOI: 10.7270/Q20Z72TH |
More data for this
Ligand-Target Pair | |
Collagenase 3
(Homo sapiens (Human)) | BDBM50173218

((S)-2-{4'-[(5-Iodo-4-methoxy-3-methyl-benzofuran-2...)Show SMILES COc1c(I)ccc2oc(C(=O)Nc3ccc(cc3)-c3ccc(cc3)S(=O)(=O)N[C@@H](C(C)C)C(O)=O)c(C)c12 Show InChI InChI=1S/C28H27IN2O7S/c1-15(2)24(28(33)34)31-39(35,36)20-11-7-18(8-12-20)17-5-9-19(10-6-17)30-27(32)25-16(3)23-22(38-25)14-13-21(29)26(23)37-4/h5-15,24,31H,1-4H3,(H,30,32)(H,33,34)/t24-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Inhibitory concentration against MMP-13 |
Bioorg Med Chem Lett 15: 4961-6 (2005)
Article DOI: 10.1016/j.bmcl.2005.08.001
BindingDB Entry DOI: 10.7270/Q20Z72TH |
More data for this
Ligand-Target Pair | |
Collagenase 3
(Homo sapiens (Human)) | BDBM50170298
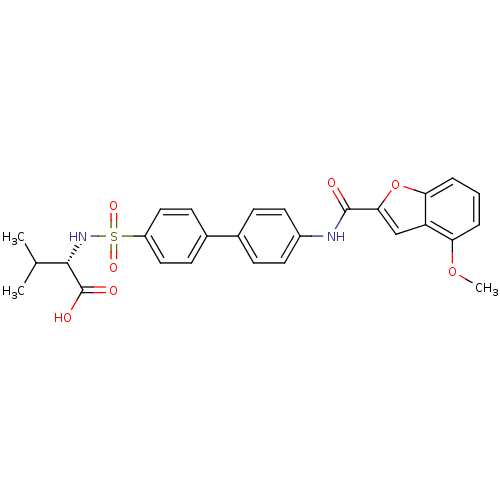
((S)-2-{4'-[(4-Methoxy-benzofuran-2-carbonyl)-amino...)Show SMILES COc1cccc2oc(cc12)C(=O)Nc1ccc(cc1)-c1ccc(cc1)S(=O)(=O)N[C@@H](C(C)C)C(O)=O Show InChI InChI=1S/C27H26N2O7S/c1-16(2)25(27(31)32)29-37(33,34)20-13-9-18(10-14-20)17-7-11-19(12-8-17)28-26(30)24-15-21-22(35-3)5-4-6-23(21)36-24/h4-16,25,29H,1-3H3,(H,28,30)(H,31,32)/t25-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Inhibitory concentration against MMP-13 |
Bioorg Med Chem Lett 15: 4961-6 (2005)
Article DOI: 10.1016/j.bmcl.2005.08.001
BindingDB Entry DOI: 10.7270/Q20Z72TH |
More data for this
Ligand-Target Pair | |
Collagenase 3
(Homo sapiens (Human)) | BDBM50173223

((S)-2-(4'-(5-bromo-4-methoxy-3-methylbenzofuran-2-...)Show SMILES COc1c(Br)ccc2oc(C(=O)Nc3ccc(cc3)-c3ccc(cc3)S(=O)(=O)N[C@@H](C(C)C)C(O)=O)c(C)c12 Show InChI InChI=1S/C28H27BrN2O7S/c1-15(2)24(28(33)34)31-39(35,36)20-11-7-18(8-12-20)17-5-9-19(10-6-17)30-27(32)25-16(3)23-22(38-25)14-13-21(29)26(23)37-4/h5-15,24,31H,1-4H3,(H,30,32)(H,33,34)/t24-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Inhibitory concentration against MMP-13 |
Bioorg Med Chem Lett 15: 4961-6 (2005)
Article DOI: 10.1016/j.bmcl.2005.08.001
BindingDB Entry DOI: 10.7270/Q20Z72TH |
More data for this
Ligand-Target Pair | |
Collagenase 3
(Homo sapiens (Human)) | BDBM28485
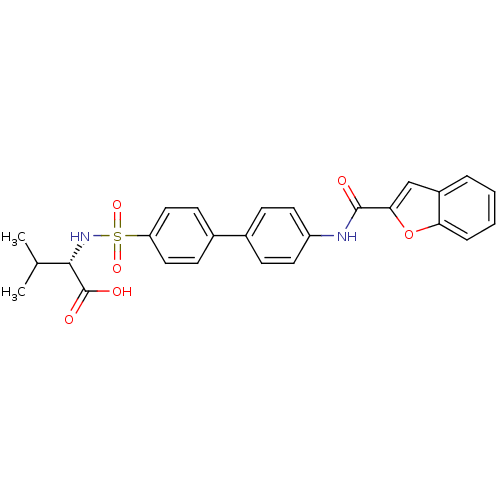
((2S)-2-({4-[4-(1-benzofuran-2-amido)phenyl]benzene...)Show SMILES CC(C)[C@H](NS(=O)(=O)c1ccc(cc1)-c1ccc(NC(=O)c2cc3ccccc3o2)cc1)C(O)=O |r| Show InChI InChI=1S/C26H24N2O6S/c1-16(2)24(26(30)31)28-35(32,33)21-13-9-18(10-14-21)17-7-11-20(12-8-17)27-25(29)23-15-19-5-3-4-6-22(19)34-23/h3-16,24,28H,1-2H3,(H,27,29)(H,30,31)/t24-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| MMDB
PDB
Article
PubMed
| n/a | n/a | 2.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Inhibitory concentration against MMP-13 |
Bioorg Med Chem Lett 15: 4961-6 (2005)
Article DOI: 10.1016/j.bmcl.2005.08.001
BindingDB Entry DOI: 10.7270/Q20Z72TH |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Collagenase 3
(Homo sapiens (Human)) | BDBM24043
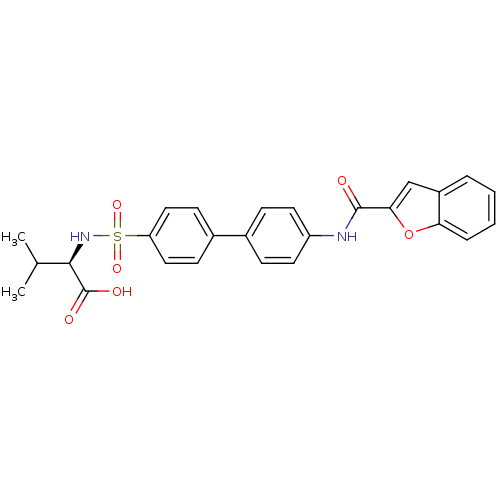
((2R)-2-({4-[4-(1-benzofuran-2-amido)phenyl]benzene...)Show SMILES CC(C)[C@@H](NS(=O)(=O)c1ccc(cc1)-c1ccc(NC(=O)c2cc3ccccc3o2)cc1)C(O)=O |r| Show InChI InChI=1S/C26H24N2O6S/c1-16(2)24(26(30)31)28-35(32,33)21-13-9-18(10-14-21)17-7-11-20(12-8-17)27-25(29)23-15-19-5-3-4-6-22(19)34-23/h3-16,24,28H,1-2H3,(H,27,29)(H,30,31)/t24-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Inhibitory concentration against MMP-13 |
Bioorg Med Chem Lett 15: 4961-6 (2005)
Article DOI: 10.1016/j.bmcl.2005.08.001
BindingDB Entry DOI: 10.7270/Q20Z72TH |
More data for this
Ligand-Target Pair | |
Collagenase 3
(Homo sapiens (Human)) | BDBM50173229
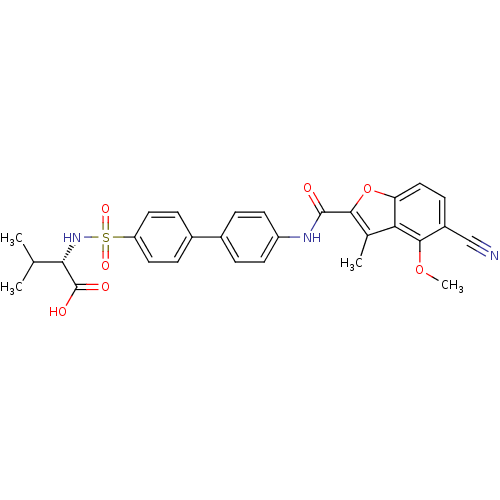
((S)-2-{4'-[(5-Cyano-4-methoxy-3-methyl-benzofuran-...)Show SMILES COc1c(ccc2oc(C(=O)Nc3ccc(cc3)-c3ccc(cc3)S(=O)(=O)N[C@@H](C(C)C)C(O)=O)c(C)c12)C#N Show InChI InChI=1S/C29H27N3O7S/c1-16(2)25(29(34)35)32-40(36,37)22-12-7-19(8-13-22)18-5-10-21(11-6-18)31-28(33)26-17(3)24-23(39-26)14-9-20(15-30)27(24)38-4/h5-14,16,25,32H,1-4H3,(H,31,33)(H,34,35)/t25-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Inhibitory concentration against MMP-13 |
Bioorg Med Chem Lett 15: 4961-6 (2005)
Article DOI: 10.1016/j.bmcl.2005.08.001
BindingDB Entry DOI: 10.7270/Q20Z72TH |
More data for this
Ligand-Target Pair | |
Collagenase 3
(Homo sapiens (Human)) | BDBM50173224
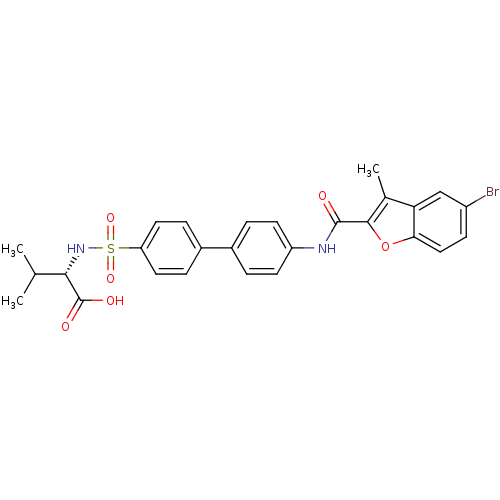
((S)-2-{4'-[(5-Bromo-3-methyl-benzofuran-2-carbonyl...)Show SMILES CC(C)[C@H](NS(=O)(=O)c1ccc(cc1)-c1ccc(NC(=O)c2oc3ccc(Br)cc3c2C)cc1)C(O)=O Show InChI InChI=1S/C27H25BrN2O6S/c1-15(2)24(27(32)33)30-37(34,35)21-11-6-18(7-12-21)17-4-9-20(10-5-17)29-26(31)25-16(3)22-14-19(28)8-13-23(22)36-25/h4-15,24,30H,1-3H3,(H,29,31)(H,32,33)/t24-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Inhibitory concentration against MMP-13 |
Bioorg Med Chem Lett 15: 4961-6 (2005)
Article DOI: 10.1016/j.bmcl.2005.08.001
BindingDB Entry DOI: 10.7270/Q20Z72TH |
More data for this
Ligand-Target Pair | |
Collagenase 3
(Homo sapiens (Human)) | BDBM50173237

((S)-2-{4'-[(5-Acetyl-4-hydroxy-3-methyl-benzofuran...)Show SMILES CC(C)[C@H](NS(=O)(=O)c1ccc(cc1)-c1ccc(NC(=O)c2oc3ccc(C(C)=O)c(O)c3c2C)cc1)C(O)=O Show InChI InChI=1S/C29H28N2O8S/c1-15(2)25(29(35)36)31-40(37,38)21-11-7-19(8-12-21)18-5-9-20(10-6-18)30-28(34)27-16(3)24-23(39-27)14-13-22(17(4)32)26(24)33/h5-15,25,31,33H,1-4H3,(H,30,34)(H,35,36)/t25-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 3.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Inhibitory concentration against MMP-13 |
Bioorg Med Chem Lett 15: 4961-6 (2005)
Article DOI: 10.1016/j.bmcl.2005.08.001
BindingDB Entry DOI: 10.7270/Q20Z72TH |
More data for this
Ligand-Target Pair | |
Collagenase 3
(Homo sapiens (Human)) | BDBM50173221
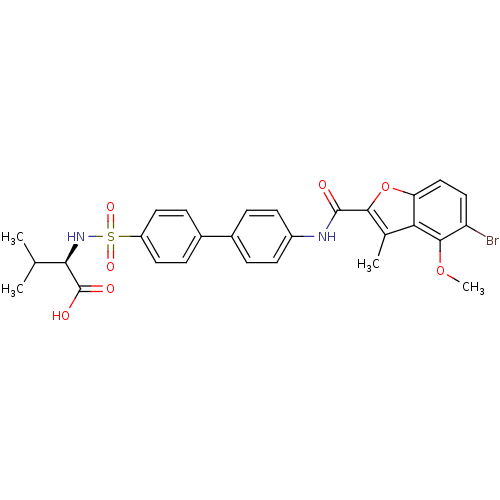
((R)-2-{4'-[(5-Bromo-4-methoxy-3-methyl-benzofuran-...)Show SMILES COc1c(Br)ccc2oc(C(=O)Nc3ccc(cc3)-c3ccc(cc3)S(=O)(=O)N[C@H](C(C)C)C(O)=O)c(C)c12 Show InChI InChI=1S/C28H27BrN2O7S/c1-15(2)24(28(33)34)31-39(35,36)20-11-7-18(8-12-20)17-5-9-19(10-6-17)30-27(32)25-16(3)23-22(38-25)14-13-21(29)26(23)37-4/h5-15,24,31H,1-4H3,(H,30,32)(H,33,34)/t24-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Inhibitory concentration against MMP-13 |
Bioorg Med Chem Lett 15: 4961-6 (2005)
Article DOI: 10.1016/j.bmcl.2005.08.001
BindingDB Entry DOI: 10.7270/Q20Z72TH |
More data for this
Ligand-Target Pair | |
Collagenase 3
(Homo sapiens (Human)) | BDBM50173228

((S)-2-{4'-[(5-Ethyl-4-methoxy-3-methyl-benzofuran-...)Show SMILES CCc1ccc2oc(C(=O)Nc3ccc(cc3)-c3ccc(cc3)S(=O)(=O)N[C@@H](C(C)C)C(O)=O)c(C)c2c1OC Show InChI InChI=1S/C30H32N2O7S/c1-6-19-11-16-24-25(28(19)38-5)18(4)27(39-24)29(33)31-22-12-7-20(8-13-22)21-9-14-23(15-10-21)40(36,37)32-26(17(2)3)30(34)35/h7-17,26,32H,6H2,1-5H3,(H,31,33)(H,34,35)/t26-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 3.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Inhibitory concentration against MMP-13 |
Bioorg Med Chem Lett 15: 4961-6 (2005)
Article DOI: 10.1016/j.bmcl.2005.08.001
BindingDB Entry DOI: 10.7270/Q20Z72TH |
More data for this
Ligand-Target Pair | |
72 kDa type IV collagenase
(Homo sapiens (Human)) | BDBM24043

((2R)-2-({4-[4-(1-benzofuran-2-amido)phenyl]benzene...)Show SMILES CC(C)[C@@H](NS(=O)(=O)c1ccc(cc1)-c1ccc(NC(=O)c2cc3ccccc3o2)cc1)C(O)=O |r| Show InChI InChI=1S/C26H24N2O6S/c1-16(2)24(26(30)31)28-35(32,33)21-13-9-18(10-14-21)17-7-11-20(12-8-17)27-25(29)23-15-19-5-3-4-6-22(19)34-23/h3-16,24,28H,1-2H3,(H,27,29)(H,30,31)/t24-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Inhibitory concentration against MMP-2 |
Bioorg Med Chem Lett 15: 4961-6 (2005)
Article DOI: 10.1016/j.bmcl.2005.08.001
BindingDB Entry DOI: 10.7270/Q20Z72TH |
More data for this
Ligand-Target Pair | |
Collagenase 3
(Homo sapiens (Human)) | BDBM50173222
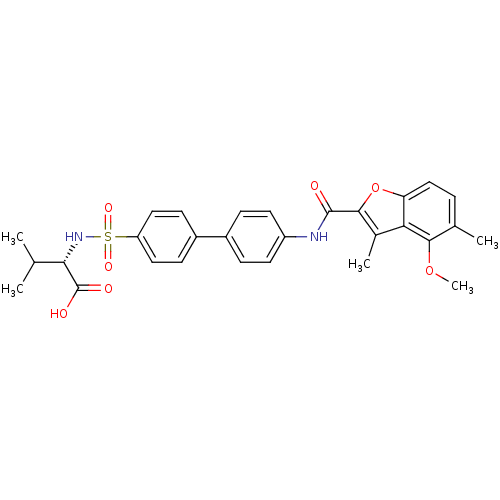
((S)-2-{4'-[(4-methoxy-3,5-dimethyl-benzofuran-2-ca...)Show SMILES COc1c(C)ccc2oc(C(=O)Nc3ccc(cc3)-c3ccc(cc3)S(=O)(=O)N[C@@H](C(C)C)C(O)=O)c(C)c12 Show InChI InChI=1S/C29H30N2O7S/c1-16(2)25(29(33)34)31-39(35,36)22-13-9-20(10-14-22)19-7-11-21(12-8-19)30-28(32)27-18(4)24-23(38-27)15-6-17(3)26(24)37-5/h6-16,25,31H,1-5H3,(H,30,32)(H,33,34)/t25-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 4.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Inhibitory concentration against MMP-13 |
Bioorg Med Chem Lett 15: 4961-6 (2005)
Article DOI: 10.1016/j.bmcl.2005.08.001
BindingDB Entry DOI: 10.7270/Q20Z72TH |
More data for this
Ligand-Target Pair | |
72 kDa type IV collagenase
(Homo sapiens (Human)) | BDBM28485

((2S)-2-({4-[4-(1-benzofuran-2-amido)phenyl]benzene...)Show SMILES CC(C)[C@H](NS(=O)(=O)c1ccc(cc1)-c1ccc(NC(=O)c2cc3ccccc3o2)cc1)C(O)=O |r| Show InChI InChI=1S/C26H24N2O6S/c1-16(2)24(26(30)31)28-35(32,33)21-13-9-18(10-14-21)17-7-11-20(12-8-17)27-25(29)23-15-19-5-3-4-6-22(19)34-23/h3-16,24,28H,1-2H3,(H,27,29)(H,30,31)/t24-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Inhibitory concentration against MMP-2 |
Bioorg Med Chem Lett 15: 4961-6 (2005)
Article DOI: 10.1016/j.bmcl.2005.08.001
BindingDB Entry DOI: 10.7270/Q20Z72TH |
More data for this
Ligand-Target Pair | |
Collagenase 3
(Homo sapiens (Human)) | BDBM50173226
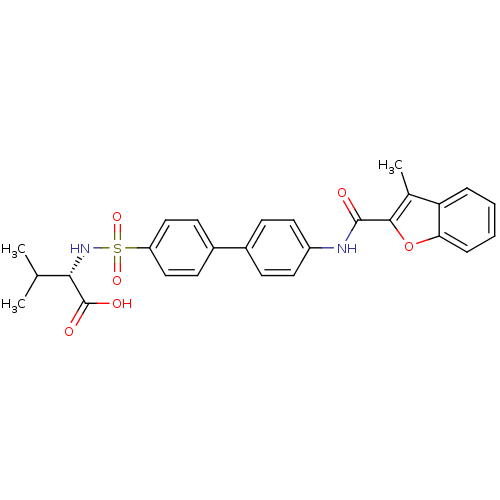
((S)-3-Methyl-2-{4'-[(3-methyl-benzofuran-2-carbony...)Show SMILES CC(C)[C@H](NS(=O)(=O)c1ccc(cc1)-c1ccc(NC(=O)c2oc3ccccc3c2C)cc1)C(O)=O Show InChI InChI=1S/C27H26N2O6S/c1-16(2)24(27(31)32)29-36(33,34)21-14-10-19(11-15-21)18-8-12-20(13-9-18)28-26(30)25-17(3)22-6-4-5-7-23(22)35-25/h4-16,24,29H,1-3H3,(H,28,30)(H,31,32)/t24-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 5.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Inhibitory concentration against MMP-13 |
Bioorg Med Chem Lett 15: 4961-6 (2005)
Article DOI: 10.1016/j.bmcl.2005.08.001
BindingDB Entry DOI: 10.7270/Q20Z72TH |
More data for this
Ligand-Target Pair | |
72 kDa type IV collagenase
(Homo sapiens (Human)) | BDBM50173235

((S)-2-{4'-[(5-Bromo-4-methoxy-benzofuran-2-carbony...)Show SMILES COc1c(Br)ccc2oc(cc12)C(=O)Nc1ccc(cc1)-c1ccc(cc1)S(=O)(=O)N[C@@H](C(C)C)C(O)=O Show InChI InChI=1S/C27H25BrN2O7S/c1-15(2)24(27(32)33)30-38(34,35)19-10-6-17(7-11-19)16-4-8-18(9-5-16)29-26(31)23-14-20-22(37-23)13-12-21(28)25(20)36-3/h4-15,24,30H,1-3H3,(H,29,31)(H,32,33)/t24-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 7.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Inhibitory concentration against MMP-2 |
Bioorg Med Chem Lett 15: 4961-6 (2005)
Article DOI: 10.1016/j.bmcl.2005.08.001
BindingDB Entry DOI: 10.7270/Q20Z72TH |
More data for this
Ligand-Target Pair | |
Collagenase 3
(Homo sapiens (Human)) | BDBM50173225

((S)-2-{4'-[(5-ethyl-4-hydroxy-3-methyl-benzofuran-...)Show SMILES CCc1ccc2oc(C(=O)Nc3ccc(cc3)-c3ccc(cc3)S(=O)(=O)N[C@@H](C(C)C)C(O)=O)c(C)c2c1O Show InChI InChI=1S/C29H30N2O7S/c1-5-18-10-15-23-24(26(18)32)17(4)27(38-23)28(33)30-21-11-6-19(7-12-21)20-8-13-22(14-9-20)39(36,37)31-25(16(2)3)29(34)35/h6-16,25,31-32H,5H2,1-4H3,(H,30,33)(H,34,35)/t25-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 7.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Inhibitory concentration against MMP-13 |
Bioorg Med Chem Lett 15: 4961-6 (2005)
Article DOI: 10.1016/j.bmcl.2005.08.001
BindingDB Entry DOI: 10.7270/Q20Z72TH |
More data for this
Ligand-Target Pair | |
Collagenase 3
(Homo sapiens (Human)) | BDBM50173233
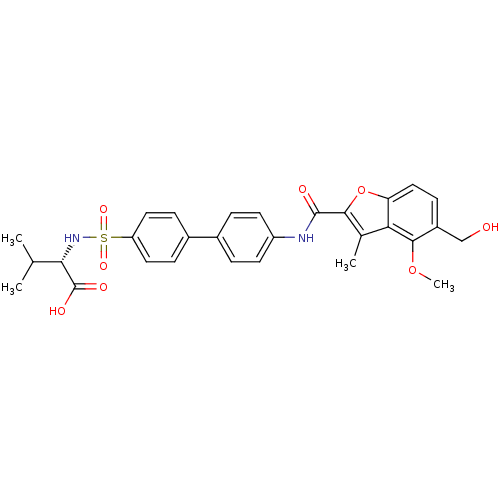
((S)-2-{4'-[(5-Hydroxymethyl-4-methoxy-3-methyl-ben...)Show SMILES COc1c(CO)ccc2oc(C(=O)Nc3ccc(cc3)-c3ccc(cc3)S(=O)(=O)N[C@@H](C(C)C)C(O)=O)c(C)c12 Show InChI InChI=1S/C29H30N2O8S/c1-16(2)25(29(34)35)31-40(36,37)22-12-7-19(8-13-22)18-5-10-21(11-6-18)30-28(33)26-17(3)24-23(39-26)14-9-20(15-32)27(24)38-4/h5-14,16,25,31-32H,15H2,1-4H3,(H,30,33)(H,34,35)/t25-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 7.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Inhibitory concentration against MMP-13 |
Bioorg Med Chem Lett 15: 4961-6 (2005)
Article DOI: 10.1016/j.bmcl.2005.08.001
BindingDB Entry DOI: 10.7270/Q20Z72TH |
More data for this
Ligand-Target Pair | |
Collagenase 3
(Homo sapiens (Human)) | BDBM50173227
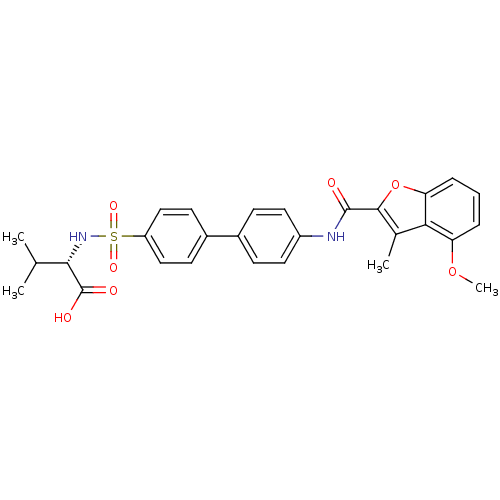
((S)-2-(4'-(4-methoxy-3-methylbenzofuran-2-carboxam...)Show SMILES COc1cccc2oc(C(=O)Nc3ccc(cc3)-c3ccc(cc3)S(=O)(=O)N[C@@H](C(C)C)C(O)=O)c(C)c12 Show InChI InChI=1S/C28H28N2O7S/c1-16(2)25(28(32)33)30-38(34,35)21-14-10-19(11-15-21)18-8-12-20(13-9-18)29-27(31)26-17(3)24-22(36-4)6-5-7-23(24)37-26/h5-16,25,30H,1-4H3,(H,29,31)(H,32,33)/t25-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 9.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Inhibitory concentration against MMP-13 |
Bioorg Med Chem Lett 15: 4961-6 (2005)
Article DOI: 10.1016/j.bmcl.2005.08.001
BindingDB Entry DOI: 10.7270/Q20Z72TH |
More data for this
Ligand-Target Pair | |
Collagenase 3
(Homo sapiens (Human)) | BDBM50173231
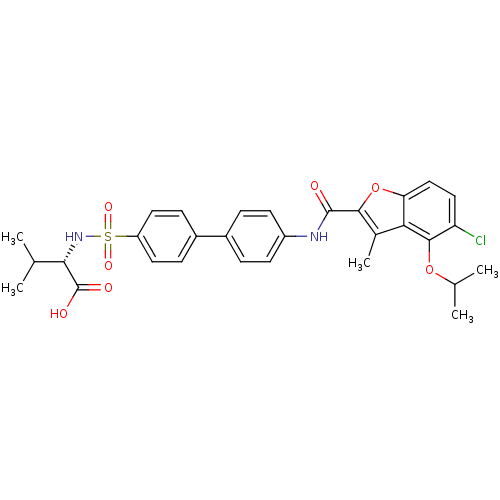
((S)-2-{4'-[(5-Chloro-4-isopropoxy-3-methyl-benzofu...)Show SMILES CC(C)Oc1c(Cl)ccc2oc(C(=O)Nc3ccc(cc3)-c3ccc(cc3)S(=O)(=O)N[C@@H](C(C)C)C(O)=O)c(C)c12 Show InChI InChI=1S/C30H31ClN2O7S/c1-16(2)26(30(35)36)33-41(37,38)22-12-8-20(9-13-22)19-6-10-21(11-7-19)32-29(34)27-18(5)25-24(40-27)15-14-23(31)28(25)39-17(3)4/h6-17,26,33H,1-5H3,(H,32,34)(H,35,36)/t26-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Inhibitory concentration against MMP-13 |
Bioorg Med Chem Lett 15: 4961-6 (2005)
Article DOI: 10.1016/j.bmcl.2005.08.001
BindingDB Entry DOI: 10.7270/Q20Z72TH |
More data for this
Ligand-Target Pair | |
72 kDa type IV collagenase
(Homo sapiens (Human)) | BDBM50170298

((S)-2-{4'-[(4-Methoxy-benzofuran-2-carbonyl)-amino...)Show SMILES COc1cccc2oc(cc12)C(=O)Nc1ccc(cc1)-c1ccc(cc1)S(=O)(=O)N[C@@H](C(C)C)C(O)=O Show InChI InChI=1S/C27H26N2O7S/c1-16(2)25(27(31)32)29-37(33,34)20-13-9-18(10-14-20)17-7-11-19(12-8-17)28-26(30)24-15-21-22(35-3)5-4-6-23(21)36-24/h4-16,25,29H,1-3H3,(H,28,30)(H,31,32)/t25-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Inhibitory concentration against MMP-2 |
Bioorg Med Chem Lett 15: 4961-6 (2005)
Article DOI: 10.1016/j.bmcl.2005.08.001
BindingDB Entry DOI: 10.7270/Q20Z72TH |
More data for this
Ligand-Target Pair | |
Collagenase 3
(Homo sapiens (Human)) | BDBM50173230
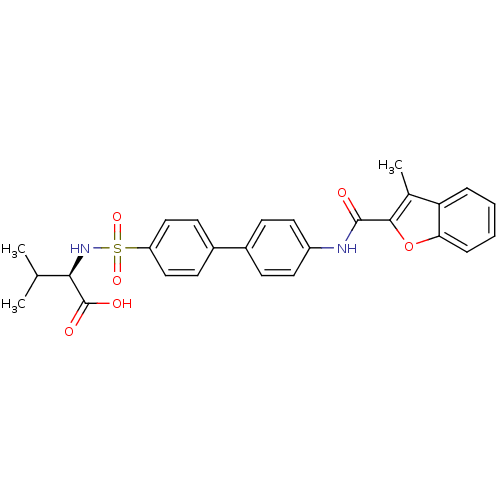
((R)-3-Methyl-2-{4'-[(3-methyl-benzofuran-2-carbony...)Show SMILES CC(C)[C@@H](NS(=O)(=O)c1ccc(cc1)-c1ccc(NC(=O)c2oc3ccccc3c2C)cc1)C(O)=O Show InChI InChI=1S/C27H26N2O6S/c1-16(2)24(27(31)32)29-36(33,34)21-14-10-19(11-15-21)18-8-12-20(13-9-18)28-26(30)25-17(3)22-6-4-5-7-23(22)35-25/h4-16,24,29H,1-3H3,(H,28,30)(H,31,32)/t24-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 17 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Inhibitory concentration against MMP-13 |
Bioorg Med Chem Lett 15: 4961-6 (2005)
Article DOI: 10.1016/j.bmcl.2005.08.001
BindingDB Entry DOI: 10.7270/Q20Z72TH |
More data for this
Ligand-Target Pair | |
Collagenase 3
(Homo sapiens (Human)) | BDBM50173232
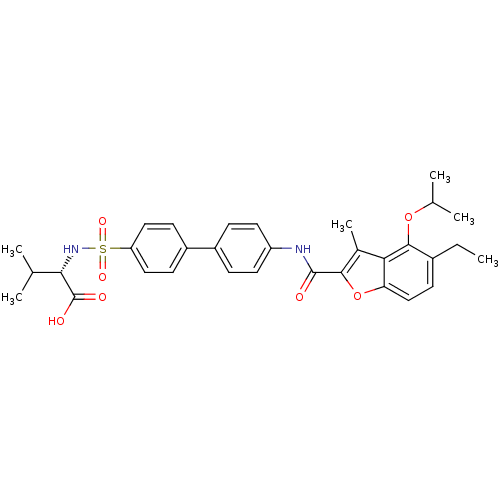
((S)-2-{4'-[(5-Ethyl-4-isopropoxy-3-methyl-benzofur...)Show SMILES CCc1ccc2oc(C(=O)Nc3ccc(cc3)-c3ccc(cc3)S(=O)(=O)N[C@@H](C(C)C)C(O)=O)c(C)c2c1OC(C)C Show InChI InChI=1S/C32H36N2O7S/c1-7-21-12-17-26-27(30(21)40-19(4)5)20(6)29(41-26)31(35)33-24-13-8-22(9-14-24)23-10-15-25(16-11-23)42(38,39)34-28(18(2)3)32(36)37/h8-19,28,34H,7H2,1-6H3,(H,33,35)(H,36,37)/t28-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 27 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Inhibitory concentration against MMP-13 |
Bioorg Med Chem Lett 15: 4961-6 (2005)
Article DOI: 10.1016/j.bmcl.2005.08.001
BindingDB Entry DOI: 10.7270/Q20Z72TH |
More data for this
Ligand-Target Pair | |
Neutrophil collagenase
(Homo sapiens (Human)) | BDBM50173222

((S)-2-{4'-[(4-methoxy-3,5-dimethyl-benzofuran-2-ca...)Show SMILES COc1c(C)ccc2oc(C(=O)Nc3ccc(cc3)-c3ccc(cc3)S(=O)(=O)N[C@@H](C(C)C)C(O)=O)c(C)c12 Show InChI InChI=1S/C29H30N2O7S/c1-16(2)25(29(33)34)31-39(35,36)22-13-9-20(10-14-22)19-7-11-21(12-8-19)30-28(32)27-18(4)24-23(38-27)15-6-17(3)26(24)37-5/h6-16,25,31H,1-5H3,(H,30,32)(H,33,34)/t25-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 52 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Inhibitory concentration against MMP-8 |
Bioorg Med Chem Lett 15: 4961-6 (2005)
Article DOI: 10.1016/j.bmcl.2005.08.001
BindingDB Entry DOI: 10.7270/Q20Z72TH |
More data for this
Ligand-Target Pair | |
Collagenase 3
(Homo sapiens (Human)) | BDBM50173234

((S)-2-{4'-[(3-Ethyl-benzofuran-2-carbonyl)-amino]-...)Show SMILES CCc1c(oc2ccccc12)C(=O)Nc1ccc(cc1)-c1ccc(cc1)S(=O)(=O)N[C@@H](C(C)C)C(O)=O Show InChI InChI=1S/C28H28N2O6S/c1-4-22-23-7-5-6-8-24(23)36-26(22)27(31)29-20-13-9-18(10-14-20)19-11-15-21(16-12-19)37(34,35)30-25(17(2)3)28(32)33/h5-17,25,30H,4H2,1-3H3,(H,29,31)(H,32,33)/t25-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 55 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Inhibitory concentration against MMP-13 |
Bioorg Med Chem Lett 15: 4961-6 (2005)
Article DOI: 10.1016/j.bmcl.2005.08.001
BindingDB Entry DOI: 10.7270/Q20Z72TH |
More data for this
Ligand-Target Pair | |
72 kDa type IV collagenase
(Homo sapiens (Human)) | BDBM50173237

((S)-2-{4'-[(5-Acetyl-4-hydroxy-3-methyl-benzofuran...)Show SMILES CC(C)[C@H](NS(=O)(=O)c1ccc(cc1)-c1ccc(NC(=O)c2oc3ccc(C(C)=O)c(O)c3c2C)cc1)C(O)=O Show InChI InChI=1S/C29H28N2O8S/c1-15(2)25(29(35)36)31-40(37,38)21-11-7-19(8-12-21)18-5-9-20(10-6-18)30-28(34)27-16(3)24-23(39-27)14-13-22(17(4)32)26(24)33/h5-15,25,31,33H,1-4H3,(H,30,34)(H,35,36)/t25-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 63 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Inhibitory concentration against MMP-2 |
Bioorg Med Chem Lett 15: 4961-6 (2005)
Article DOI: 10.1016/j.bmcl.2005.08.001
BindingDB Entry DOI: 10.7270/Q20Z72TH |
More data for this
Ligand-Target Pair | |
Collagenase 3
(Homo sapiens (Human)) | BDBM50173238

((S)-2-{4'-[(4-Benzyloxy-5-ethyl-3-methyl-benzofura...)Show SMILES CCc1ccc2oc(C(=O)Nc3ccc(cc3)-c3ccc(cc3)S(=O)(=O)N[C@@H](C(C)C)C(O)=O)c(C)c2c1OCc1ccccc1 Show InChI InChI=1S/C36H36N2O7S/c1-5-25-15-20-30-31(34(25)44-21-24-9-7-6-8-10-24)23(4)33(45-30)35(39)37-28-16-11-26(12-17-28)27-13-18-29(19-14-27)46(42,43)38-32(22(2)3)36(40)41/h6-20,22,32,38H,5,21H2,1-4H3,(H,37,39)(H,40,41)/t32-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 67 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Inhibitory concentration against MMP-13 |
Bioorg Med Chem Lett 15: 4961-6 (2005)
Article DOI: 10.1016/j.bmcl.2005.08.001
BindingDB Entry DOI: 10.7270/Q20Z72TH |
More data for this
Ligand-Target Pair | |
Neutrophil collagenase
(Homo sapiens (Human)) | BDBM50173223

((S)-2-(4'-(5-bromo-4-methoxy-3-methylbenzofuran-2-...)Show SMILES COc1c(Br)ccc2oc(C(=O)Nc3ccc(cc3)-c3ccc(cc3)S(=O)(=O)N[C@@H](C(C)C)C(O)=O)c(C)c12 Show InChI InChI=1S/C28H27BrN2O7S/c1-15(2)24(28(33)34)31-39(35,36)20-11-7-18(8-12-20)17-5-9-19(10-6-17)30-27(32)25-16(3)23-22(38-25)14-13-21(29)26(23)37-4/h5-15,24,31H,1-4H3,(H,30,32)(H,33,34)/t24-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 73 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Inhibitory concentration against MMP-8 |
Bioorg Med Chem Lett 15: 4961-6 (2005)
Article DOI: 10.1016/j.bmcl.2005.08.001
BindingDB Entry DOI: 10.7270/Q20Z72TH |
More data for this
Ligand-Target Pair | |
72 kDa type IV collagenase
(Homo sapiens (Human)) | BDBM50173224

((S)-2-{4'-[(5-Bromo-3-methyl-benzofuran-2-carbonyl...)Show SMILES CC(C)[C@H](NS(=O)(=O)c1ccc(cc1)-c1ccc(NC(=O)c2oc3ccc(Br)cc3c2C)cc1)C(O)=O Show InChI InChI=1S/C27H25BrN2O6S/c1-15(2)24(27(32)33)30-37(34,35)21-11-6-18(7-12-21)17-4-9-20(10-5-17)29-26(31)25-16(3)22-14-19(28)8-13-23(22)36-25/h4-15,24,30H,1-3H3,(H,29,31)(H,32,33)/t24-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 81 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Inhibitory concentration against MMP-2 |
Bioorg Med Chem Lett 15: 4961-6 (2005)
Article DOI: 10.1016/j.bmcl.2005.08.001
BindingDB Entry DOI: 10.7270/Q20Z72TH |
More data for this
Ligand-Target Pair | |
Neutrophil collagenase
(Homo sapiens (Human)) | BDBM50173225

((S)-2-{4'-[(5-ethyl-4-hydroxy-3-methyl-benzofuran-...)Show SMILES CCc1ccc2oc(C(=O)Nc3ccc(cc3)-c3ccc(cc3)S(=O)(=O)N[C@@H](C(C)C)C(O)=O)c(C)c2c1O Show InChI InChI=1S/C29H30N2O7S/c1-5-18-10-15-23-24(26(18)32)17(4)27(38-23)28(33)30-21-11-6-19(7-12-21)20-8-13-22(14-9-20)39(36,37)31-25(16(2)3)29(34)35/h6-16,25,31-32H,5H2,1-4H3,(H,30,33)(H,34,35)/t25-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 94 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Inhibitory concentration against MMP-8 |
Bioorg Med Chem Lett 15: 4961-6 (2005)
Article DOI: 10.1016/j.bmcl.2005.08.001
BindingDB Entry DOI: 10.7270/Q20Z72TH |
More data for this
Ligand-Target Pair | |
Neutrophil collagenase
(Homo sapiens (Human)) | BDBM50173228

((S)-2-{4'-[(5-Ethyl-4-methoxy-3-methyl-benzofuran-...)Show SMILES CCc1ccc2oc(C(=O)Nc3ccc(cc3)-c3ccc(cc3)S(=O)(=O)N[C@@H](C(C)C)C(O)=O)c(C)c2c1OC Show InChI InChI=1S/C30H32N2O7S/c1-6-19-11-16-24-25(28(19)38-5)18(4)27(39-24)29(33)31-22-12-7-20(8-13-22)21-9-14-23(15-10-21)40(36,37)32-26(17(2)3)30(34)35/h7-17,26,32H,6H2,1-5H3,(H,31,33)(H,34,35)/t26-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 101 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Inhibitory concentration against MMP-8 |
Bioorg Med Chem Lett 15: 4961-6 (2005)
Article DOI: 10.1016/j.bmcl.2005.08.001
BindingDB Entry DOI: 10.7270/Q20Z72TH |
More data for this
Ligand-Target Pair | |
Neutrophil collagenase
(Homo sapiens (Human)) | BDBM50173231

((S)-2-{4'-[(5-Chloro-4-isopropoxy-3-methyl-benzofu...)Show SMILES CC(C)Oc1c(Cl)ccc2oc(C(=O)Nc3ccc(cc3)-c3ccc(cc3)S(=O)(=O)N[C@@H](C(C)C)C(O)=O)c(C)c12 Show InChI InChI=1S/C30H31ClN2O7S/c1-16(2)26(30(35)36)33-41(37,38)22-12-8-20(9-13-22)19-6-10-21(11-7-19)32-29(34)27-18(5)25-24(40-27)15-14-23(31)28(25)39-17(3)4/h6-17,26,33H,1-5H3,(H,32,34)(H,35,36)/t26-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 110 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Inhibitory concentration against MMP-8 |
Bioorg Med Chem Lett 15: 4961-6 (2005)
Article DOI: 10.1016/j.bmcl.2005.08.001
BindingDB Entry DOI: 10.7270/Q20Z72TH |
More data for this
Ligand-Target Pair | |
Stromelysin-1
(Homo sapiens (Human)) | BDBM50173223

((S)-2-(4'-(5-bromo-4-methoxy-3-methylbenzofuran-2-...)Show SMILES COc1c(Br)ccc2oc(C(=O)Nc3ccc(cc3)-c3ccc(cc3)S(=O)(=O)N[C@@H](C(C)C)C(O)=O)c(C)c12 Show InChI InChI=1S/C28H27BrN2O7S/c1-15(2)24(28(33)34)31-39(35,36)20-11-7-18(8-12-20)17-5-9-19(10-6-17)30-27(32)25-16(3)23-22(38-25)14-13-21(29)26(23)37-4/h5-15,24,31H,1-4H3,(H,30,32)(H,33,34)/t24-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 144 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Inhibitory concentration against MMP-3 |
Bioorg Med Chem Lett 15: 4961-6 (2005)
Article DOI: 10.1016/j.bmcl.2005.08.001
BindingDB Entry DOI: 10.7270/Q20Z72TH |
More data for this
Ligand-Target Pair | |
72 kDa type IV collagenase
(Homo sapiens (Human)) | BDBM50173230

((R)-3-Methyl-2-{4'-[(3-methyl-benzofuran-2-carbony...)Show SMILES CC(C)[C@@H](NS(=O)(=O)c1ccc(cc1)-c1ccc(NC(=O)c2oc3ccccc3c2C)cc1)C(O)=O Show InChI InChI=1S/C27H26N2O6S/c1-16(2)24(27(31)32)29-36(33,34)21-14-10-19(11-15-21)18-8-12-20(13-9-18)28-26(30)25-17(3)22-6-4-5-7-23(22)35-25/h4-16,24,29H,1-3H3,(H,28,30)(H,31,32)/t24-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 164 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Inhibitory concentration against MMP-2 |
Bioorg Med Chem Lett 15: 4961-6 (2005)
Article DOI: 10.1016/j.bmcl.2005.08.001
BindingDB Entry DOI: 10.7270/Q20Z72TH |
More data for this
Ligand-Target Pair | |
Collagenase 3
(Homo sapiens (Human)) | BDBM50173219
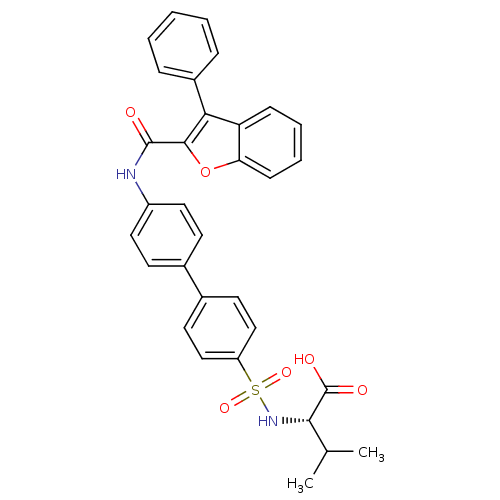
((S)-3-Methyl-2-{4'-[(3-phenyl-benzofuran-2-carbony...)Show SMILES CC(C)[C@H](NS(=O)(=O)c1ccc(cc1)-c1ccc(NC(=O)c2oc3ccccc3c2-c2ccccc2)cc1)C(O)=O Show InChI InChI=1S/C32H28N2O6S/c1-20(2)29(32(36)37)34-41(38,39)25-18-14-22(15-19-25)21-12-16-24(17-13-21)33-31(35)30-28(23-8-4-3-5-9-23)26-10-6-7-11-27(26)40-30/h3-20,29,34H,1-2H3,(H,33,35)(H,36,37)/t29-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 220 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Inhibitory concentration against MMP-13 |
Bioorg Med Chem Lett 15: 4961-6 (2005)
Article DOI: 10.1016/j.bmcl.2005.08.001
BindingDB Entry DOI: 10.7270/Q20Z72TH |
More data for this
Ligand-Target Pair | |
72 kDa type IV collagenase
(Homo sapiens (Human)) | BDBM50173226

((S)-3-Methyl-2-{4'-[(3-methyl-benzofuran-2-carbony...)Show SMILES CC(C)[C@H](NS(=O)(=O)c1ccc(cc1)-c1ccc(NC(=O)c2oc3ccccc3c2C)cc1)C(O)=O Show InChI InChI=1S/C27H26N2O6S/c1-16(2)24(27(31)32)29-36(33,34)21-14-10-19(11-15-21)18-8-12-20(13-9-18)28-26(30)25-17(3)22-6-4-5-7-23(22)35-25/h4-16,24,29H,1-3H3,(H,28,30)(H,31,32)/t24-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 259 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Inhibitory concentration against MMP-2 |
Bioorg Med Chem Lett 15: 4961-6 (2005)
Article DOI: 10.1016/j.bmcl.2005.08.001
BindingDB Entry DOI: 10.7270/Q20Z72TH |
More data for this
Ligand-Target Pair | |
Neutrophil collagenase
(Homo sapiens (Human)) | BDBM50173218

((S)-2-{4'-[(5-Iodo-4-methoxy-3-methyl-benzofuran-2...)Show SMILES COc1c(I)ccc2oc(C(=O)Nc3ccc(cc3)-c3ccc(cc3)S(=O)(=O)N[C@@H](C(C)C)C(O)=O)c(C)c12 Show InChI InChI=1S/C28H27IN2O7S/c1-15(2)24(28(33)34)31-39(35,36)20-11-7-18(8-12-20)17-5-9-19(10-6-17)30-27(32)25-16(3)23-22(38-25)14-13-21(29)26(23)37-4/h5-15,24,31H,1-4H3,(H,30,32)(H,33,34)/t24-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 404 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Inhibitory concentration against MMP-8 |
Bioorg Med Chem Lett 15: 4961-6 (2005)
Article DOI: 10.1016/j.bmcl.2005.08.001
BindingDB Entry DOI: 10.7270/Q20Z72TH |
More data for this
Ligand-Target Pair | |
Stromelysin-1
(Homo sapiens (Human)) | BDBM50173222

((S)-2-{4'-[(4-methoxy-3,5-dimethyl-benzofuran-2-ca...)Show SMILES COc1c(C)ccc2oc(C(=O)Nc3ccc(cc3)-c3ccc(cc3)S(=O)(=O)N[C@@H](C(C)C)C(O)=O)c(C)c12 Show InChI InChI=1S/C29H30N2O7S/c1-16(2)25(29(33)34)31-39(35,36)22-13-9-20(10-14-22)19-7-11-21(12-8-19)30-28(32)27-18(4)24-23(38-27)15-6-17(3)26(24)37-5/h6-16,25,31H,1-5H3,(H,30,32)(H,33,34)/t25-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 442 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Inhibitory concentration against MMP-3 |
Bioorg Med Chem Lett 15: 4961-6 (2005)
Article DOI: 10.1016/j.bmcl.2005.08.001
BindingDB Entry DOI: 10.7270/Q20Z72TH |
More data for this
Ligand-Target Pair | |
Matrilysin
(Homo sapiens (Human)) | BDBM50173225

((S)-2-{4'-[(5-ethyl-4-hydroxy-3-methyl-benzofuran-...)Show SMILES CCc1ccc2oc(C(=O)Nc3ccc(cc3)-c3ccc(cc3)S(=O)(=O)N[C@@H](C(C)C)C(O)=O)c(C)c2c1O Show InChI InChI=1S/C29H30N2O7S/c1-5-18-10-15-23-24(26(18)32)17(4)27(38-23)28(33)30-21-11-6-19(7-12-21)20-8-13-22(14-9-20)39(36,37)31-25(16(2)3)29(34)35/h6-16,25,31-32H,5H2,1-4H3,(H,30,33)(H,34,35)/t25-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 458 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Inhibitory concentration against MMP-7 |
Bioorg Med Chem Lett 15: 4961-6 (2005)
Article DOI: 10.1016/j.bmcl.2005.08.001
BindingDB Entry DOI: 10.7270/Q20Z72TH |
More data for this
Ligand-Target Pair | |
Stromelysin-1
(Homo sapiens (Human)) | BDBM50173225

((S)-2-{4'-[(5-ethyl-4-hydroxy-3-methyl-benzofuran-...)Show SMILES CCc1ccc2oc(C(=O)Nc3ccc(cc3)-c3ccc(cc3)S(=O)(=O)N[C@@H](C(C)C)C(O)=O)c(C)c2c1O Show InChI InChI=1S/C29H30N2O7S/c1-5-18-10-15-23-24(26(18)32)17(4)27(38-23)28(33)30-21-11-6-19(7-12-21)20-8-13-22(14-9-20)39(36,37)31-25(16(2)3)29(34)35/h6-16,25,31-32H,5H2,1-4H3,(H,30,33)(H,34,35)/t25-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 492 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Inhibitory concentration against MMP-3 |
Bioorg Med Chem Lett 15: 4961-6 (2005)
Article DOI: 10.1016/j.bmcl.2005.08.001
BindingDB Entry DOI: 10.7270/Q20Z72TH |
More data for this
Ligand-Target Pair | |
72 kDa type IV collagenase
(Homo sapiens (Human)) | BDBM50173229

((S)-2-{4'-[(5-Cyano-4-methoxy-3-methyl-benzofuran-...)Show SMILES COc1c(ccc2oc(C(=O)Nc3ccc(cc3)-c3ccc(cc3)S(=O)(=O)N[C@@H](C(C)C)C(O)=O)c(C)c12)C#N Show InChI InChI=1S/C29H27N3O7S/c1-16(2)25(29(34)35)32-40(36,37)22-12-7-19(8-13-22)18-5-10-21(11-6-18)31-28(33)26-17(3)24-23(39-26)14-9-20(15-30)27(24)38-4/h5-14,16,25,32H,1-4H3,(H,31,33)(H,34,35)/t25-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 522 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Inhibitory concentration against MMP-2 |
Bioorg Med Chem Lett 15: 4961-6 (2005)
Article DOI: 10.1016/j.bmcl.2005.08.001
BindingDB Entry DOI: 10.7270/Q20Z72TH |
More data for this
Ligand-Target Pair | |
Matrilysin
(Homo sapiens (Human)) | BDBM50173222

((S)-2-{4'-[(4-methoxy-3,5-dimethyl-benzofuran-2-ca...)Show SMILES COc1c(C)ccc2oc(C(=O)Nc3ccc(cc3)-c3ccc(cc3)S(=O)(=O)N[C@@H](C(C)C)C(O)=O)c(C)c12 Show InChI InChI=1S/C29H30N2O7S/c1-16(2)25(29(33)34)31-39(35,36)22-13-9-20(10-14-22)19-7-11-21(12-8-19)30-28(32)27-18(4)24-23(38-27)15-6-17(3)26(24)37-5/h6-16,25,31H,1-5H3,(H,30,32)(H,33,34)/t25-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 534 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Inhibitory concentration against MMP-7 |
Bioorg Med Chem Lett 15: 4961-6 (2005)
Article DOI: 10.1016/j.bmcl.2005.08.001
BindingDB Entry DOI: 10.7270/Q20Z72TH |
More data for this
Ligand-Target Pair | |
Stromelysin-1
(Homo sapiens (Human)) | BDBM50173228

((S)-2-{4'-[(5-Ethyl-4-methoxy-3-methyl-benzofuran-...)Show SMILES CCc1ccc2oc(C(=O)Nc3ccc(cc3)-c3ccc(cc3)S(=O)(=O)N[C@@H](C(C)C)C(O)=O)c(C)c2c1OC Show InChI InChI=1S/C30H32N2O7S/c1-6-19-11-16-24-25(28(19)38-5)18(4)27(39-24)29(33)31-22-12-7-20(8-13-22)21-9-14-23(15-10-21)40(36,37)32-26(17(2)3)30(34)35/h7-17,26,32H,6H2,1-5H3,(H,31,33)(H,34,35)/t26-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 561 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Inhibitory concentration against MMP-3 |
Bioorg Med Chem Lett 15: 4961-6 (2005)
Article DOI: 10.1016/j.bmcl.2005.08.001
BindingDB Entry DOI: 10.7270/Q20Z72TH |
More data for this
Ligand-Target Pair | |
72 kDa type IV collagenase
(Homo sapiens (Human)) | BDBM50173221

((R)-2-{4'-[(5-Bromo-4-methoxy-3-methyl-benzofuran-...)Show SMILES COc1c(Br)ccc2oc(C(=O)Nc3ccc(cc3)-c3ccc(cc3)S(=O)(=O)N[C@H](C(C)C)C(O)=O)c(C)c12 Show InChI InChI=1S/C28H27BrN2O7S/c1-15(2)24(28(33)34)31-39(35,36)20-11-7-18(8-12-20)17-5-9-19(10-6-17)30-27(32)25-16(3)23-22(38-25)14-13-21(29)26(23)37-4/h5-15,24,31H,1-4H3,(H,30,32)(H,33,34)/t24-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 688 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Inhibitory concentration against MMP-2 |
Bioorg Med Chem Lett 15: 4961-6 (2005)
Article DOI: 10.1016/j.bmcl.2005.08.001
BindingDB Entry DOI: 10.7270/Q20Z72TH |
More data for this
Ligand-Target Pair | |
Matrilysin
(Homo sapiens (Human)) | BDBM50173223

((S)-2-(4'-(5-bromo-4-methoxy-3-methylbenzofuran-2-...)Show SMILES COc1c(Br)ccc2oc(C(=O)Nc3ccc(cc3)-c3ccc(cc3)S(=O)(=O)N[C@@H](C(C)C)C(O)=O)c(C)c12 Show InChI InChI=1S/C28H27BrN2O7S/c1-15(2)24(28(33)34)31-39(35,36)20-11-7-18(8-12-20)17-5-9-19(10-6-17)30-27(32)25-16(3)23-22(38-25)14-13-21(29)26(23)37-4/h5-15,24,31H,1-4H3,(H,30,32)(H,33,34)/t24-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 866 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Inhibitory concentration against MMP-7 |
Bioorg Med Chem Lett 15: 4961-6 (2005)
Article DOI: 10.1016/j.bmcl.2005.08.001
BindingDB Entry DOI: 10.7270/Q20Z72TH |
More data for this
Ligand-Target Pair | |
72 kDa type IV collagenase
(Homo sapiens (Human)) | BDBM50173227

((S)-2-(4'-(4-methoxy-3-methylbenzofuran-2-carboxam...)Show SMILES COc1cccc2oc(C(=O)Nc3ccc(cc3)-c3ccc(cc3)S(=O)(=O)N[C@@H](C(C)C)C(O)=O)c(C)c12 Show InChI InChI=1S/C28H28N2O7S/c1-16(2)25(28(32)33)30-38(34,35)21-14-10-19(11-15-21)18-8-12-20(13-9-18)29-27(31)26-17(3)24-22(36-4)6-5-7-23(24)37-26/h5-16,25,30H,1-4H3,(H,29,31)(H,32,33)/t25-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.05E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Inhibitory concentration against MMP-2 |
Bioorg Med Chem Lett 15: 4961-6 (2005)
Article DOI: 10.1016/j.bmcl.2005.08.001
BindingDB Entry DOI: 10.7270/Q20Z72TH |
More data for this
Ligand-Target Pair | |
Stromelysin-1
(Homo sapiens (Human)) | BDBM50173218

((S)-2-{4'-[(5-Iodo-4-methoxy-3-methyl-benzofuran-2...)Show SMILES COc1c(I)ccc2oc(C(=O)Nc3ccc(cc3)-c3ccc(cc3)S(=O)(=O)N[C@@H](C(C)C)C(O)=O)c(C)c12 Show InChI InChI=1S/C28H27IN2O7S/c1-15(2)24(28(33)34)31-39(35,36)20-11-7-18(8-12-20)17-5-9-19(10-6-17)30-27(32)25-16(3)23-22(38-25)14-13-21(29)26(23)37-4/h5-15,24,31H,1-4H3,(H,30,32)(H,33,34)/t24-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.06E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Inhibitory concentration against MMP-3 |
Bioorg Med Chem Lett 15: 4961-6 (2005)
Article DOI: 10.1016/j.bmcl.2005.08.001
BindingDB Entry DOI: 10.7270/Q20Z72TH |
More data for this
Ligand-Target Pair | |
Stromelysin-1
(Homo sapiens (Human)) | BDBM50173231

((S)-2-{4'-[(5-Chloro-4-isopropoxy-3-methyl-benzofu...)Show SMILES CC(C)Oc1c(Cl)ccc2oc(C(=O)Nc3ccc(cc3)-c3ccc(cc3)S(=O)(=O)N[C@@H](C(C)C)C(O)=O)c(C)c12 Show InChI InChI=1S/C30H31ClN2O7S/c1-16(2)26(30(35)36)33-41(37,38)22-12-8-20(9-13-22)19-6-10-21(11-7-19)32-29(34)27-18(5)25-24(40-27)15-14-23(31)28(25)39-17(3)4/h6-17,26,33H,1-5H3,(H,32,34)(H,35,36)/t26-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.09E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Inhibitory concentration against MMP-3 |
Bioorg Med Chem Lett 15: 4961-6 (2005)
Article DOI: 10.1016/j.bmcl.2005.08.001
BindingDB Entry DOI: 10.7270/Q20Z72TH |
More data for this
Ligand-Target Pair | |
72 kDa type IV collagenase
(Homo sapiens (Human)) | BDBM50173219

((S)-3-Methyl-2-{4'-[(3-phenyl-benzofuran-2-carbony...)Show SMILES CC(C)[C@H](NS(=O)(=O)c1ccc(cc1)-c1ccc(NC(=O)c2oc3ccccc3c2-c2ccccc2)cc1)C(O)=O Show InChI InChI=1S/C32H28N2O6S/c1-20(2)29(32(36)37)34-41(38,39)25-18-14-22(15-19-25)21-12-16-24(17-13-21)33-31(35)30-28(23-8-4-3-5-9-23)26-10-6-7-11-27(26)40-30/h3-20,29,34H,1-2H3,(H,33,35)(H,36,37)/t29-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Inhibitory concentration against MMP-2 |
Bioorg Med Chem Lett 15: 4961-6 (2005)
Article DOI: 10.1016/j.bmcl.2005.08.001
BindingDB Entry DOI: 10.7270/Q20Z72TH |
More data for this
Ligand-Target Pair | |
Matrilysin
(Homo sapiens (Human)) | BDBM50173228

((S)-2-{4'-[(5-Ethyl-4-methoxy-3-methyl-benzofuran-...)Show SMILES CCc1ccc2oc(C(=O)Nc3ccc(cc3)-c3ccc(cc3)S(=O)(=O)N[C@@H](C(C)C)C(O)=O)c(C)c2c1OC Show InChI InChI=1S/C30H32N2O7S/c1-6-19-11-16-24-25(28(19)38-5)18(4)27(39-24)29(33)31-22-12-7-20(8-13-22)21-9-14-23(15-10-21)40(36,37)32-26(17(2)3)30(34)35/h7-17,26,32H,6H2,1-5H3,(H,31,33)(H,34,35)/t26-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.26E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Inhibitory concentration against MMP-7 |
Bioorg Med Chem Lett 15: 4961-6 (2005)
Article DOI: 10.1016/j.bmcl.2005.08.001
BindingDB Entry DOI: 10.7270/Q20Z72TH |
More data for this
Ligand-Target Pair | |
72 kDa type IV collagenase
(Homo sapiens (Human)) | BDBM50173218

((S)-2-{4'-[(5-Iodo-4-methoxy-3-methyl-benzofuran-2...)Show SMILES COc1c(I)ccc2oc(C(=O)Nc3ccc(cc3)-c3ccc(cc3)S(=O)(=O)N[C@@H](C(C)C)C(O)=O)c(C)c12 Show InChI InChI=1S/C28H27IN2O7S/c1-15(2)24(28(33)34)31-39(35,36)20-11-7-18(8-12-20)17-5-9-19(10-6-17)30-27(32)25-16(3)23-22(38-25)14-13-21(29)26(23)37-4/h5-15,24,31H,1-4H3,(H,30,32)(H,33,34)/t24-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.37E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Inhibitory concentration against MMP-2 |
Bioorg Med Chem Lett 15: 4961-6 (2005)
Article DOI: 10.1016/j.bmcl.2005.08.001
BindingDB Entry DOI: 10.7270/Q20Z72TH |
More data for this
Ligand-Target Pair | |
72 kDa type IV collagenase
(Homo sapiens (Human)) | BDBM50173233

((S)-2-{4'-[(5-Hydroxymethyl-4-methoxy-3-methyl-ben...)Show SMILES COc1c(CO)ccc2oc(C(=O)Nc3ccc(cc3)-c3ccc(cc3)S(=O)(=O)N[C@@H](C(C)C)C(O)=O)c(C)c12 Show InChI InChI=1S/C29H30N2O8S/c1-16(2)25(29(34)35)31-40(36,37)22-12-7-19(8-13-22)18-5-10-21(11-6-18)30-28(33)26-17(3)24-23(39-26)14-9-20(15-32)27(24)38-4/h5-14,16,25,31-32H,15H2,1-4H3,(H,30,33)(H,34,35)/t25-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.41E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Inhibitory concentration against MMP-2 |
Bioorg Med Chem Lett 15: 4961-6 (2005)
Article DOI: 10.1016/j.bmcl.2005.08.001
BindingDB Entry DOI: 10.7270/Q20Z72TH |
More data for this
Ligand-Target Pair | |
72 kDa type IV collagenase
(Homo sapiens (Human)) | BDBM50173220

((S)-2-{4'-[(5-Chloro-4-methoxy-3-methyl-benzofuran...)Show SMILES COc1c(Cl)ccc2oc(C(=O)Nc3ccc(cc3)-c3ccc(cc3)S(=O)(=O)N[C@@H](C(C)C)C(O)=O)c(C)c12 Show InChI InChI=1S/C28H27ClN2O7S/c1-15(2)24(28(33)34)31-39(35,36)20-11-7-18(8-12-20)17-5-9-19(10-6-17)30-27(32)25-16(3)23-22(38-25)14-13-21(29)26(23)37-4/h5-15,24,31H,1-4H3,(H,30,32)(H,33,34)/t24-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.49E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Inhibitory concentration against MMP-2 |
Bioorg Med Chem Lett 15: 4961-6 (2005)
Article DOI: 10.1016/j.bmcl.2005.08.001
BindingDB Entry DOI: 10.7270/Q20Z72TH |
More data for this
Ligand-Target Pair | |
72 kDa type IV collagenase
(Homo sapiens (Human)) | BDBM50173222

((S)-2-{4'-[(4-methoxy-3,5-dimethyl-benzofuran-2-ca...)Show SMILES COc1c(C)ccc2oc(C(=O)Nc3ccc(cc3)-c3ccc(cc3)S(=O)(=O)N[C@@H](C(C)C)C(O)=O)c(C)c12 Show InChI InChI=1S/C29H30N2O7S/c1-16(2)25(29(33)34)31-39(35,36)22-13-9-20(10-14-22)19-7-11-21(12-8-19)30-28(32)27-18(4)24-23(38-27)15-6-17(3)26(24)37-5/h6-16,25,31H,1-5H3,(H,30,32)(H,33,34)/t25-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.61E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Inhibitory concentration against MMP-2 |
Bioorg Med Chem Lett 15: 4961-6 (2005)
Article DOI: 10.1016/j.bmcl.2005.08.001
BindingDB Entry DOI: 10.7270/Q20Z72TH |
More data for this
Ligand-Target Pair | |
Matrilysin
(Homo sapiens (Human)) | BDBM50173231

((S)-2-{4'-[(5-Chloro-4-isopropoxy-3-methyl-benzofu...)Show SMILES CC(C)Oc1c(Cl)ccc2oc(C(=O)Nc3ccc(cc3)-c3ccc(cc3)S(=O)(=O)N[C@@H](C(C)C)C(O)=O)c(C)c12 Show InChI InChI=1S/C30H31ClN2O7S/c1-16(2)26(30(35)36)33-41(37,38)22-12-8-20(9-13-22)19-6-10-21(11-7-19)32-29(34)27-18(5)25-24(40-27)15-14-23(31)28(25)39-17(3)4/h6-17,26,33H,1-5H3,(H,32,34)(H,35,36)/t26-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.64E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Inhibitory concentration against MMP-7 |
Bioorg Med Chem Lett 15: 4961-6 (2005)
Article DOI: 10.1016/j.bmcl.2005.08.001
BindingDB Entry DOI: 10.7270/Q20Z72TH |
More data for this
Ligand-Target Pair | |
72 kDa type IV collagenase
(Homo sapiens (Human)) | BDBM50173223

((S)-2-(4'-(5-bromo-4-methoxy-3-methylbenzofuran-2-...)Show SMILES COc1c(Br)ccc2oc(C(=O)Nc3ccc(cc3)-c3ccc(cc3)S(=O)(=O)N[C@@H](C(C)C)C(O)=O)c(C)c12 Show InChI InChI=1S/C28H27BrN2O7S/c1-15(2)24(28(33)34)31-39(35,36)20-11-7-18(8-12-20)17-5-9-19(10-6-17)30-27(32)25-16(3)23-22(38-25)14-13-21(29)26(23)37-4/h5-15,24,31H,1-4H3,(H,30,32)(H,33,34)/t24-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Inhibitory concentration against MMP-2 |
Bioorg Med Chem Lett 15: 4961-6 (2005)
Article DOI: 10.1016/j.bmcl.2005.08.001
BindingDB Entry DOI: 10.7270/Q20Z72TH |
More data for this
Ligand-Target Pair | |
72 kDa type IV collagenase
(Homo sapiens (Human)) | BDBM50173225

((S)-2-{4'-[(5-ethyl-4-hydroxy-3-methyl-benzofuran-...)Show SMILES CCc1ccc2oc(C(=O)Nc3ccc(cc3)-c3ccc(cc3)S(=O)(=O)N[C@@H](C(C)C)C(O)=O)c(C)c2c1O Show InChI InChI=1S/C29H30N2O7S/c1-5-18-10-15-23-24(26(18)32)17(4)27(38-23)28(33)30-21-11-6-19(7-12-21)20-8-13-22(14-9-20)39(36,37)31-25(16(2)3)29(34)35/h6-16,25,31-32H,5H2,1-4H3,(H,30,33)(H,34,35)/t25-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.76E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Inhibitory concentration against MMP-2 |
Bioorg Med Chem Lett 15: 4961-6 (2005)
Article DOI: 10.1016/j.bmcl.2005.08.001
BindingDB Entry DOI: 10.7270/Q20Z72TH |
More data for this
Ligand-Target Pair | |
72 kDa type IV collagenase
(Homo sapiens (Human)) | BDBM50173234

((S)-2-{4'-[(3-Ethyl-benzofuran-2-carbonyl)-amino]-...)Show SMILES CCc1c(oc2ccccc12)C(=O)Nc1ccc(cc1)-c1ccc(cc1)S(=O)(=O)N[C@@H](C(C)C)C(O)=O Show InChI InChI=1S/C28H28N2O6S/c1-4-22-23-7-5-6-8-24(23)36-26(22)27(31)29-20-13-9-18(10-14-20)19-11-15-21(16-12-19)37(34,35)30-25(17(2)3)28(32)33/h5-17,25,30H,4H2,1-3H3,(H,29,31)(H,32,33)/t25-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.77E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Inhibitory concentration against MMP-2 |
Bioorg Med Chem Lett 15: 4961-6 (2005)
Article DOI: 10.1016/j.bmcl.2005.08.001
BindingDB Entry DOI: 10.7270/Q20Z72TH |
More data for this
Ligand-Target Pair | |
72 kDa type IV collagenase
(Homo sapiens (Human)) | BDBM50173228

((S)-2-{4'-[(5-Ethyl-4-methoxy-3-methyl-benzofuran-...)Show SMILES CCc1ccc2oc(C(=O)Nc3ccc(cc3)-c3ccc(cc3)S(=O)(=O)N[C@@H](C(C)C)C(O)=O)c(C)c2c1OC Show InChI InChI=1S/C30H32N2O7S/c1-6-19-11-16-24-25(28(19)38-5)18(4)27(39-24)29(33)31-22-12-7-20(8-13-22)21-9-14-23(15-10-21)40(36,37)32-26(17(2)3)30(34)35/h7-17,26,32H,6H2,1-5H3,(H,31,33)(H,34,35)/t26-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Inhibitory concentration against MMP-2 |
Bioorg Med Chem Lett 15: 4961-6 (2005)
Article DOI: 10.1016/j.bmcl.2005.08.001
BindingDB Entry DOI: 10.7270/Q20Z72TH |
More data for this
Ligand-Target Pair | |
72 kDa type IV collagenase
(Homo sapiens (Human)) | BDBM50173231

((S)-2-{4'-[(5-Chloro-4-isopropoxy-3-methyl-benzofu...)Show SMILES CC(C)Oc1c(Cl)ccc2oc(C(=O)Nc3ccc(cc3)-c3ccc(cc3)S(=O)(=O)N[C@@H](C(C)C)C(O)=O)c(C)c12 Show InChI InChI=1S/C30H31ClN2O7S/c1-16(2)26(30(35)36)33-41(37,38)22-12-8-20(9-13-22)19-6-10-21(11-7-19)32-29(34)27-18(5)25-24(40-27)15-14-23(31)28(25)39-17(3)4/h6-17,26,33H,1-5H3,(H,32,34)(H,35,36)/t26-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 3.01E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Inhibitory concentration against MMP-2 |
Bioorg Med Chem Lett 15: 4961-6 (2005)
Article DOI: 10.1016/j.bmcl.2005.08.001
BindingDB Entry DOI: 10.7270/Q20Z72TH |
More data for this
Ligand-Target Pair | |
Matrilysin
(Homo sapiens (Human)) | BDBM50173218

((S)-2-{4'-[(5-Iodo-4-methoxy-3-methyl-benzofuran-2...)Show SMILES COc1c(I)ccc2oc(C(=O)Nc3ccc(cc3)-c3ccc(cc3)S(=O)(=O)N[C@@H](C(C)C)C(O)=O)c(C)c12 Show InChI InChI=1S/C28H27IN2O7S/c1-15(2)24(28(33)34)31-39(35,36)20-11-7-18(8-12-20)17-5-9-19(10-6-17)30-27(32)25-16(3)23-22(38-25)14-13-21(29)26(23)37-4/h5-15,24,31H,1-4H3,(H,30,32)(H,33,34)/t24-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 3.01E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Inhibitory concentration against MMP-7 |
Bioorg Med Chem Lett 15: 4961-6 (2005)
Article DOI: 10.1016/j.bmcl.2005.08.001
BindingDB Entry DOI: 10.7270/Q20Z72TH |
More data for this
Ligand-Target Pair | |
72 kDa type IV collagenase
(Homo sapiens (Human)) | BDBM50173238

((S)-2-{4'-[(4-Benzyloxy-5-ethyl-3-methyl-benzofura...)Show SMILES CCc1ccc2oc(C(=O)Nc3ccc(cc3)-c3ccc(cc3)S(=O)(=O)N[C@@H](C(C)C)C(O)=O)c(C)c2c1OCc1ccccc1 Show InChI InChI=1S/C36H36N2O7S/c1-5-25-15-20-30-31(34(25)44-21-24-9-7-6-8-10-24)23(4)33(45-30)35(39)37-28-16-11-26(12-17-28)27-13-18-29(19-14-27)46(42,43)38-32(22(2)3)36(40)41/h6-20,22,32,38H,5,21H2,1-4H3,(H,37,39)(H,40,41)/t32-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 4.34E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Inhibitory concentration against MMP-2 |
Bioorg Med Chem Lett 15: 4961-6 (2005)
Article DOI: 10.1016/j.bmcl.2005.08.001
BindingDB Entry DOI: 10.7270/Q20Z72TH |
More data for this
Ligand-Target Pair | |
A disintegrin and metalloproteinase with thrombospondin motifs 4
(Homo sapiens (Human)) | BDBM50173225

((S)-2-{4'-[(5-ethyl-4-hydroxy-3-methyl-benzofuran-...)Show SMILES CCc1ccc2oc(C(=O)Nc3ccc(cc3)-c3ccc(cc3)S(=O)(=O)N[C@@H](C(C)C)C(O)=O)c(C)c2c1O Show InChI InChI=1S/C29H30N2O7S/c1-5-18-10-15-23-24(26(18)32)17(4)27(38-23)28(33)30-21-11-6-19(7-12-21)20-8-13-22(14-9-20)39(36,37)31-25(16(2)3)29(34)35/h6-16,25,31-32H,5H2,1-4H3,(H,30,33)(H,34,35)/t25-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 4.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Inhibitory concentration against Aggrecanase 1 |
Bioorg Med Chem Lett 15: 4961-6 (2005)
Article DOI: 10.1016/j.bmcl.2005.08.001
BindingDB Entry DOI: 10.7270/Q20Z72TH |
More data for this
Ligand-Target Pair | |
A disintegrin and metalloproteinase with thrombospondin motifs 4
(Homo sapiens (Human)) | BDBM50173223

((S)-2-(4'-(5-bromo-4-methoxy-3-methylbenzofuran-2-...)Show SMILES COc1c(Br)ccc2oc(C(=O)Nc3ccc(cc3)-c3ccc(cc3)S(=O)(=O)N[C@@H](C(C)C)C(O)=O)c(C)c12 Show InChI InChI=1S/C28H27BrN2O7S/c1-15(2)24(28(33)34)31-39(35,36)20-11-7-18(8-12-20)17-5-9-19(10-6-17)30-27(32)25-16(3)23-22(38-25)14-13-21(29)26(23)37-4/h5-15,24,31H,1-4H3,(H,30,32)(H,33,34)/t24-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 5.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Inhibitory concentration against Aggrecanase 1 |
Bioorg Med Chem Lett 15: 4961-6 (2005)
Article DOI: 10.1016/j.bmcl.2005.08.001
BindingDB Entry DOI: 10.7270/Q20Z72TH |
More data for this
Ligand-Target Pair | |
72 kDa type IV collagenase
(Homo sapiens (Human)) | BDBM50173232

((S)-2-{4'-[(5-Ethyl-4-isopropoxy-3-methyl-benzofur...)Show SMILES CCc1ccc2oc(C(=O)Nc3ccc(cc3)-c3ccc(cc3)S(=O)(=O)N[C@@H](C(C)C)C(O)=O)c(C)c2c1OC(C)C Show InChI InChI=1S/C32H36N2O7S/c1-7-21-12-17-26-27(30(21)40-19(4)5)20(6)29(41-26)31(35)33-24-13-8-22(9-14-24)23-10-15-25(16-11-23)42(38,39)34-28(18(2)3)32(36)37/h8-19,28,34H,7H2,1-6H3,(H,33,35)(H,36,37)/t28-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 7.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Inhibitory concentration against MMP-2 |
Bioorg Med Chem Lett 15: 4961-6 (2005)
Article DOI: 10.1016/j.bmcl.2005.08.001
BindingDB Entry DOI: 10.7270/Q20Z72TH |
More data for this
Ligand-Target Pair | |
A disintegrin and metalloproteinase with thrombospondin motifs 4
(Homo sapiens (Human)) | BDBM50173222

((S)-2-{4'-[(4-methoxy-3,5-dimethyl-benzofuran-2-ca...)Show SMILES COc1c(C)ccc2oc(C(=O)Nc3ccc(cc3)-c3ccc(cc3)S(=O)(=O)N[C@@H](C(C)C)C(O)=O)c(C)c12 Show InChI InChI=1S/C29H30N2O7S/c1-16(2)25(29(33)34)31-39(35,36)22-13-9-20(10-14-22)19-7-11-21(12-8-19)30-28(32)27-18(4)24-23(38-27)15-6-17(3)26(24)37-5/h6-16,25,31H,1-5H3,(H,30,32)(H,33,34)/t25-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 8.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Inhibitory concentration against Aggrecanase 1 |
Bioorg Med Chem Lett 15: 4961-6 (2005)
Article DOI: 10.1016/j.bmcl.2005.08.001
BindingDB Entry DOI: 10.7270/Q20Z72TH |
More data for this
Ligand-Target Pair | |
Matrix metalloproteinase-14
(Homo sapiens (Human)) | BDBM50173220

((S)-2-{4'-[(5-Chloro-4-methoxy-3-methyl-benzofuran...)Show SMILES COc1c(Cl)ccc2oc(C(=O)Nc3ccc(cc3)-c3ccc(cc3)S(=O)(=O)N[C@@H](C(C)C)C(O)=O)c(C)c12 Show InChI InChI=1S/C28H27ClN2O7S/c1-15(2)24(28(33)34)31-39(35,36)20-11-7-18(8-12-20)17-5-9-19(10-6-17)30-27(32)25-16(3)23-22(38-25)14-13-21(29)26(23)37-4/h5-15,24,31H,1-4H3,(H,30,32)(H,33,34)/t24-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.24E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Inhibitory concentration against MMP-14 |
Bioorg Med Chem Lett 15: 4961-6 (2005)
Article DOI: 10.1016/j.bmcl.2005.08.001
BindingDB Entry DOI: 10.7270/Q20Z72TH |
More data for this
Ligand-Target Pair | |
A disintegrin and metalloproteinase with thrombospondin motifs 4
(Homo sapiens (Human)) | BDBM50173231

((S)-2-{4'-[(5-Chloro-4-isopropoxy-3-methyl-benzofu...)Show SMILES CC(C)Oc1c(Cl)ccc2oc(C(=O)Nc3ccc(cc3)-c3ccc(cc3)S(=O)(=O)N[C@@H](C(C)C)C(O)=O)c(C)c12 Show InChI InChI=1S/C30H31ClN2O7S/c1-16(2)26(30(35)36)33-41(37,38)22-12-8-20(9-13-22)19-6-10-21(11-7-19)32-29(34)27-18(5)25-24(40-27)15-14-23(31)28(25)39-17(3)4/h6-17,26,33H,1-5H3,(H,32,34)(H,35,36)/t26-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.43E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Inhibitory concentration against Aggrecanase 1 |
Bioorg Med Chem Lett 15: 4961-6 (2005)
Article DOI: 10.1016/j.bmcl.2005.08.001
BindingDB Entry DOI: 10.7270/Q20Z72TH |
More data for this
Ligand-Target Pair | |
A disintegrin and metalloproteinase with thrombospondin motifs 4
(Homo sapiens (Human)) | BDBM50173218

((S)-2-{4'-[(5-Iodo-4-methoxy-3-methyl-benzofuran-2...)Show SMILES COc1c(I)ccc2oc(C(=O)Nc3ccc(cc3)-c3ccc(cc3)S(=O)(=O)N[C@@H](C(C)C)C(O)=O)c(C)c12 Show InChI InChI=1S/C28H27IN2O7S/c1-15(2)24(28(33)34)31-39(35,36)20-11-7-18(8-12-20)17-5-9-19(10-6-17)30-27(32)25-16(3)23-22(38-25)14-13-21(29)26(23)37-4/h5-15,24,31H,1-4H3,(H,30,32)(H,33,34)/t24-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Inhibitory concentration against Aggrecanase 1 |
Bioorg Med Chem Lett 15: 4961-6 (2005)
Article DOI: 10.1016/j.bmcl.2005.08.001
BindingDB Entry DOI: 10.7270/Q20Z72TH |
More data for this
Ligand-Target Pair | |
Matrix metalloproteinase-14
(Homo sapiens (Human)) | BDBM50173222

((S)-2-{4'-[(4-methoxy-3,5-dimethyl-benzofuran-2-ca...)Show SMILES COc1c(C)ccc2oc(C(=O)Nc3ccc(cc3)-c3ccc(cc3)S(=O)(=O)N[C@@H](C(C)C)C(O)=O)c(C)c12 Show InChI InChI=1S/C29H30N2O7S/c1-16(2)25(29(33)34)31-39(35,36)22-13-9-20(10-14-22)19-7-11-21(12-8-19)30-28(32)27-18(4)24-23(38-27)15-6-17(3)26(24)37-5/h6-16,25,31H,1-5H3,(H,30,32)(H,33,34)/t25-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Inhibitory concentration against MMP-14 |
Bioorg Med Chem Lett 15: 4961-6 (2005)
Article DOI: 10.1016/j.bmcl.2005.08.001
BindingDB Entry DOI: 10.7270/Q20Z72TH |
More data for this
Ligand-Target Pair | |
Matrix metalloproteinase-14
(Homo sapiens (Human)) | BDBM50173223

((S)-2-(4'-(5-bromo-4-methoxy-3-methylbenzofuran-2-...)Show SMILES COc1c(Br)ccc2oc(C(=O)Nc3ccc(cc3)-c3ccc(cc3)S(=O)(=O)N[C@@H](C(C)C)C(O)=O)c(C)c12 Show InChI InChI=1S/C28H27BrN2O7S/c1-15(2)24(28(33)34)31-39(35,36)20-11-7-18(8-12-20)17-5-9-19(10-6-17)30-27(32)25-16(3)23-22(38-25)14-13-21(29)26(23)37-4/h5-15,24,31H,1-4H3,(H,30,32)(H,33,34)/t24-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Inhibitory concentration against MMP-14 |
Bioorg Med Chem Lett 15: 4961-6 (2005)
Article DOI: 10.1016/j.bmcl.2005.08.001
BindingDB Entry DOI: 10.7270/Q20Z72TH |
More data for this
Ligand-Target Pair | |
A disintegrin and metalloproteinase with thrombospondin motifs 4
(Homo sapiens (Human)) | BDBM50173228

((S)-2-{4'-[(5-Ethyl-4-methoxy-3-methyl-benzofuran-...)Show SMILES CCc1ccc2oc(C(=O)Nc3ccc(cc3)-c3ccc(cc3)S(=O)(=O)N[C@@H](C(C)C)C(O)=O)c(C)c2c1OC Show InChI InChI=1S/C30H32N2O7S/c1-6-19-11-16-24-25(28(19)38-5)18(4)27(39-24)29(33)31-22-12-7-20(8-13-22)21-9-14-23(15-10-21)40(36,37)32-26(17(2)3)30(34)35/h7-17,26,32H,6H2,1-5H3,(H,31,33)(H,34,35)/t26-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >1.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Inhibitory concentration against Aggrecanase 1 |
Bioorg Med Chem Lett 15: 4961-6 (2005)
Article DOI: 10.1016/j.bmcl.2005.08.001
BindingDB Entry DOI: 10.7270/Q20Z72TH |
More data for this
Ligand-Target Pair | |
Matrix metalloproteinase-14
(Homo sapiens (Human)) | BDBM50173225

((S)-2-{4'-[(5-ethyl-4-hydroxy-3-methyl-benzofuran-...)Show SMILES CCc1ccc2oc(C(=O)Nc3ccc(cc3)-c3ccc(cc3)S(=O)(=O)N[C@@H](C(C)C)C(O)=O)c(C)c2c1O Show InChI InChI=1S/C29H30N2O7S/c1-5-18-10-15-23-24(26(18)32)17(4)27(38-23)28(33)30-21-11-6-19(7-12-21)20-8-13-22(14-9-20)39(36,37)31-25(16(2)3)29(34)35/h6-16,25,31-32H,5H2,1-4H3,(H,30,33)(H,34,35)/t25-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.58E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Inhibitory concentration against MMP-14 |
Bioorg Med Chem Lett 15: 4961-6 (2005)
Article DOI: 10.1016/j.bmcl.2005.08.001
BindingDB Entry DOI: 10.7270/Q20Z72TH |
More data for this
Ligand-Target Pair | |
Interstitial collagenase
(Homo sapiens (Human)) | BDBM50173225

((S)-2-{4'-[(5-ethyl-4-hydroxy-3-methyl-benzofuran-...)Show SMILES CCc1ccc2oc(C(=O)Nc3ccc(cc3)-c3ccc(cc3)S(=O)(=O)N[C@@H](C(C)C)C(O)=O)c(C)c2c1O Show InChI InChI=1S/C29H30N2O7S/c1-5-18-10-15-23-24(26(18)32)17(4)27(38-23)28(33)30-21-11-6-19(7-12-21)20-8-13-22(14-9-20)39(36,37)31-25(16(2)3)29(34)35/h6-16,25,31-32H,5H2,1-4H3,(H,30,33)(H,34,35)/t25-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2.34E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Inhibitory concentration against MMP-1 |
Bioorg Med Chem Lett 15: 4961-6 (2005)
Article DOI: 10.1016/j.bmcl.2005.08.001
BindingDB Entry DOI: 10.7270/Q20Z72TH |
More data for this
Ligand-Target Pair | |
Interstitial collagenase
(Homo sapiens (Human)) | BDBM50173228

((S)-2-{4'-[(5-Ethyl-4-methoxy-3-methyl-benzofuran-...)Show SMILES CCc1ccc2oc(C(=O)Nc3ccc(cc3)-c3ccc(cc3)S(=O)(=O)N[C@@H](C(C)C)C(O)=O)c(C)c2c1OC Show InChI InChI=1S/C30H32N2O7S/c1-6-19-11-16-24-25(28(19)38-5)18(4)27(39-24)29(33)31-22-12-7-20(8-13-22)21-9-14-23(15-10-21)40(36,37)32-26(17(2)3)30(34)35/h7-17,26,32H,6H2,1-5H3,(H,31,33)(H,34,35)/t26-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2.80E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Inhibitory concentration against MMP-1 |
Bioorg Med Chem Lett 15: 4961-6 (2005)
Article DOI: 10.1016/j.bmcl.2005.08.001
BindingDB Entry DOI: 10.7270/Q20Z72TH |
More data for this
Ligand-Target Pair | |
Matrix metalloproteinase-14
(Homo sapiens (Human)) | BDBM50173218

((S)-2-{4'-[(5-Iodo-4-methoxy-3-methyl-benzofuran-2...)Show SMILES COc1c(I)ccc2oc(C(=O)Nc3ccc(cc3)-c3ccc(cc3)S(=O)(=O)N[C@@H](C(C)C)C(O)=O)c(C)c12 Show InChI InChI=1S/C28H27IN2O7S/c1-15(2)24(28(33)34)31-39(35,36)20-11-7-18(8-12-20)17-5-9-19(10-6-17)30-27(32)25-16(3)23-22(38-25)14-13-21(29)26(23)37-4/h5-15,24,31H,1-4H3,(H,30,32)(H,33,34)/t24-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Inhibitory concentration against MMP-14 |
Bioorg Med Chem Lett 15: 4961-6 (2005)
Article DOI: 10.1016/j.bmcl.2005.08.001
BindingDB Entry DOI: 10.7270/Q20Z72TH |
More data for this
Ligand-Target Pair | |
Matrix metalloproteinase-14
(Homo sapiens (Human)) | BDBM50173228

((S)-2-{4'-[(5-Ethyl-4-methoxy-3-methyl-benzofuran-...)Show SMILES CCc1ccc2oc(C(=O)Nc3ccc(cc3)-c3ccc(cc3)S(=O)(=O)N[C@@H](C(C)C)C(O)=O)c(C)c2c1OC Show InChI InChI=1S/C30H32N2O7S/c1-6-19-11-16-24-25(28(19)38-5)18(4)27(39-24)29(33)31-22-12-7-20(8-13-22)21-9-14-23(15-10-21)40(36,37)32-26(17(2)3)30(34)35/h7-17,26,32H,6H2,1-5H3,(H,31,33)(H,34,35)/t26-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Inhibitory concentration against MMP-14 |
Bioorg Med Chem Lett 15: 4961-6 (2005)
Article DOI: 10.1016/j.bmcl.2005.08.001
BindingDB Entry DOI: 10.7270/Q20Z72TH |
More data for this
Ligand-Target Pair | |
Interstitial collagenase
(Homo sapiens (Human)) | BDBM50173223

((S)-2-(4'-(5-bromo-4-methoxy-3-methylbenzofuran-2-...)Show SMILES COc1c(Br)ccc2oc(C(=O)Nc3ccc(cc3)-c3ccc(cc3)S(=O)(=O)N[C@@H](C(C)C)C(O)=O)c(C)c12 Show InChI InChI=1S/C28H27BrN2O7S/c1-15(2)24(28(33)34)31-39(35,36)20-11-7-18(8-12-20)17-5-9-19(10-6-17)30-27(32)25-16(3)23-22(38-25)14-13-21(29)26(23)37-4/h5-15,24,31H,1-4H3,(H,30,32)(H,33,34)/t24-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Inhibitory concentration against MMP-1 |
Bioorg Med Chem Lett 15: 4961-6 (2005)
Article DOI: 10.1016/j.bmcl.2005.08.001
BindingDB Entry DOI: 10.7270/Q20Z72TH |
More data for this
Ligand-Target Pair | |
Interstitial collagenase
(Homo sapiens (Human)) | BDBM50173220

((S)-2-{4'-[(5-Chloro-4-methoxy-3-methyl-benzofuran...)Show SMILES COc1c(Cl)ccc2oc(C(=O)Nc3ccc(cc3)-c3ccc(cc3)S(=O)(=O)N[C@@H](C(C)C)C(O)=O)c(C)c12 Show InChI InChI=1S/C28H27ClN2O7S/c1-15(2)24(28(33)34)31-39(35,36)20-11-7-18(8-12-20)17-5-9-19(10-6-17)30-27(32)25-16(3)23-22(38-25)14-13-21(29)26(23)37-4/h5-15,24,31H,1-4H3,(H,30,32)(H,33,34)/t24-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 3.40E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Inhibitory concentration against MMP-1 |
Bioorg Med Chem Lett 15: 4961-6 (2005)
Article DOI: 10.1016/j.bmcl.2005.08.001
BindingDB Entry DOI: 10.7270/Q20Z72TH |
More data for this
Ligand-Target Pair | |
Disintegrin and metalloproteinase domain-containing protein 17
(Homo sapiens (Human)) | BDBM50173223

((S)-2-(4'-(5-bromo-4-methoxy-3-methylbenzofuran-2-...)Show SMILES COc1c(Br)ccc2oc(C(=O)Nc3ccc(cc3)-c3ccc(cc3)S(=O)(=O)N[C@@H](C(C)C)C(O)=O)c(C)c12 Show InChI InChI=1S/C28H27BrN2O7S/c1-15(2)24(28(33)34)31-39(35,36)20-11-7-18(8-12-20)17-5-9-19(10-6-17)30-27(32)25-16(3)23-22(38-25)14-13-21(29)26(23)37-4/h5-15,24,31H,1-4H3,(H,30,32)(H,33,34)/t24-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 3.40E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Inhibitory concentration against TACE |
Bioorg Med Chem Lett 15: 4961-6 (2005)
Article DOI: 10.1016/j.bmcl.2005.08.001
BindingDB Entry DOI: 10.7270/Q20Z72TH |
More data for this
Ligand-Target Pair | |
Interstitial collagenase
(Homo sapiens (Human)) | BDBM50173222

((S)-2-{4'-[(4-methoxy-3,5-dimethyl-benzofuran-2-ca...)Show SMILES COc1c(C)ccc2oc(C(=O)Nc3ccc(cc3)-c3ccc(cc3)S(=O)(=O)N[C@@H](C(C)C)C(O)=O)c(C)c12 Show InChI InChI=1S/C29H30N2O7S/c1-16(2)25(29(33)34)31-39(35,36)22-13-9-20(10-14-22)19-7-11-21(12-8-19)30-28(32)27-18(4)24-23(38-27)15-6-17(3)26(24)37-5/h6-16,25,31H,1-5H3,(H,30,32)(H,33,34)/t25-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 3.71E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Inhibitory concentration against MMP-1 |
Bioorg Med Chem Lett 15: 4961-6 (2005)
Article DOI: 10.1016/j.bmcl.2005.08.001
BindingDB Entry DOI: 10.7270/Q20Z72TH |
More data for this
Ligand-Target Pair | |
Interstitial collagenase
(Homo sapiens (Human)) | BDBM50173231

((S)-2-{4'-[(5-Chloro-4-isopropoxy-3-methyl-benzofu...)Show SMILES CC(C)Oc1c(Cl)ccc2oc(C(=O)Nc3ccc(cc3)-c3ccc(cc3)S(=O)(=O)N[C@@H](C(C)C)C(O)=O)c(C)c12 Show InChI InChI=1S/C30H31ClN2O7S/c1-16(2)26(30(35)36)33-41(37,38)22-12-8-20(9-13-22)19-6-10-21(11-7-19)32-29(34)27-18(5)25-24(40-27)15-14-23(31)28(25)39-17(3)4/h6-17,26,33H,1-5H3,(H,32,34)(H,35,36)/t26-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 3.71E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Inhibitory concentration against MMP-1 |
Bioorg Med Chem Lett 15: 4961-6 (2005)
Article DOI: 10.1016/j.bmcl.2005.08.001
BindingDB Entry DOI: 10.7270/Q20Z72TH |
More data for this
Ligand-Target Pair | |
Matrix metalloproteinase-14
(Homo sapiens (Human)) | BDBM50173231

((S)-2-{4'-[(5-Chloro-4-isopropoxy-3-methyl-benzofu...)Show SMILES CC(C)Oc1c(Cl)ccc2oc(C(=O)Nc3ccc(cc3)-c3ccc(cc3)S(=O)(=O)N[C@@H](C(C)C)C(O)=O)c(C)c12 Show InChI InChI=1S/C30H31ClN2O7S/c1-16(2)26(30(35)36)33-41(37,38)22-12-8-20(9-13-22)19-6-10-21(11-7-19)32-29(34)27-18(5)25-24(40-27)15-14-23(31)28(25)39-17(3)4/h6-17,26,33H,1-5H3,(H,32,34)(H,35,36)/t26-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Inhibitory concentration against MMP-14 |
Bioorg Med Chem Lett 15: 4961-6 (2005)
Article DOI: 10.1016/j.bmcl.2005.08.001
BindingDB Entry DOI: 10.7270/Q20Z72TH |
More data for this
Ligand-Target Pair | |
Interstitial collagenase
(Homo sapiens (Human)) | BDBM50173218

((S)-2-{4'-[(5-Iodo-4-methoxy-3-methyl-benzofuran-2...)Show SMILES COc1c(I)ccc2oc(C(=O)Nc3ccc(cc3)-c3ccc(cc3)S(=O)(=O)N[C@@H](C(C)C)C(O)=O)c(C)c12 Show InChI InChI=1S/C28H27IN2O7S/c1-15(2)24(28(33)34)31-39(35,36)20-11-7-18(8-12-20)17-5-9-19(10-6-17)30-27(32)25-16(3)23-22(38-25)14-13-21(29)26(23)37-4/h5-15,24,31H,1-4H3,(H,30,32)(H,33,34)/t24-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 6.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Inhibitory concentration against MMP-1 |
Bioorg Med Chem Lett 15: 4961-6 (2005)
Article DOI: 10.1016/j.bmcl.2005.08.001
BindingDB Entry DOI: 10.7270/Q20Z72TH |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data