

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Proto-oncogene tyrosine-protein kinase receptor Ret (Homo sapiens (Human)) | BDBM25198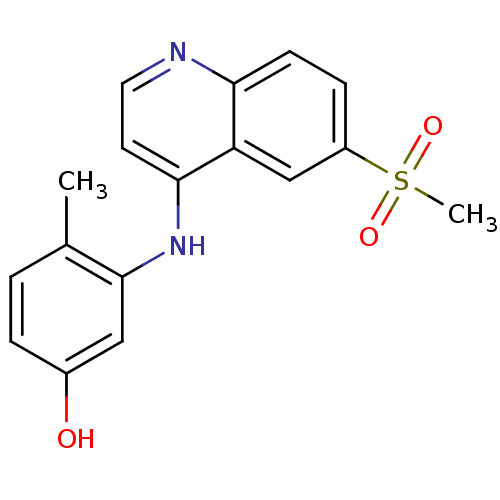 (3-[(6-methanesulfonylquinolin-4-yl)amino]-4-methyl...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem Patents | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | 7.4 | 22 |
GlaxoSmithKline | Assay Description The biochemical activity of compound was determined by incubation with Ret kinase and the substrate in the presence of ATP/ [gamma-33P] ATP. After in... | Bioorg Med Chem Lett 17: 5886-93 (2007) Article DOI: 10.1016/j.bmcl.2007.07.104 BindingDB Entry DOI: 10.7270/Q2H1309S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proto-oncogene tyrosine-protein kinase receptor Ret (Homo sapiens (Human)) | BDBM25194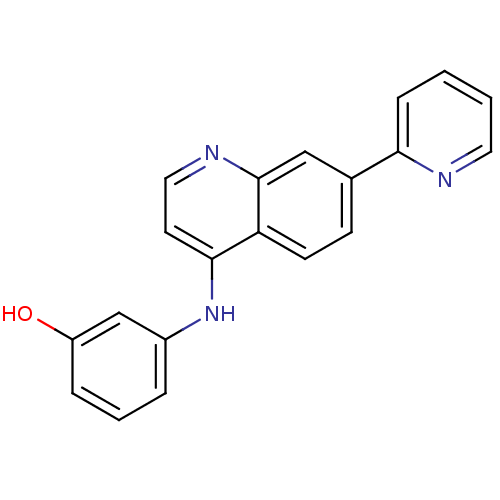 (3-{[7-(pyridin-2-yl)quinolin-4-yl]amino}phenol | 7...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 6 | n/a | n/a | n/a | n/a | 7.4 | 22 |
GlaxoSmithKline | Assay Description The biochemical activity of compound was determined by incubation with Ret kinase and the substrate in the presence of ATP/ [gamma-33P] ATP. After in... | Bioorg Med Chem Lett 17: 5886-93 (2007) Article DOI: 10.1016/j.bmcl.2007.07.104 BindingDB Entry DOI: 10.7270/Q2H1309S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proto-oncogene tyrosine-protein kinase receptor Ret (Homo sapiens (Human)) | BDBM25191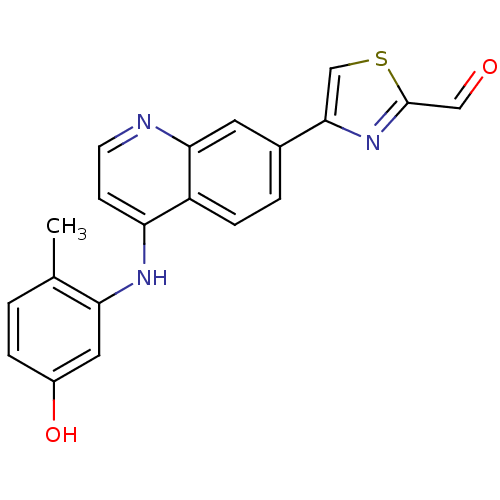 (4-{4-[(5-hydroxy-2-methylphenyl)amino]quinolin-7-y...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | DrugBank MMDB PC cid PC sid PDB UniChem Patents | Article PubMed | n/a | n/a | 25 | n/a | n/a | n/a | n/a | 7.4 | 22 |
GlaxoSmithKline | Assay Description The biochemical activity of compound was determined by incubation with Ret kinase and the substrate in the presence of ATP/ [gamma-33P] ATP. After in... | Bioorg Med Chem Lett 17: 5886-93 (2007) Article DOI: 10.1016/j.bmcl.2007.07.104 BindingDB Entry DOI: 10.7270/Q2H1309S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proto-oncogene tyrosine-protein kinase receptor Ret (Homo sapiens (Human)) | BDBM25192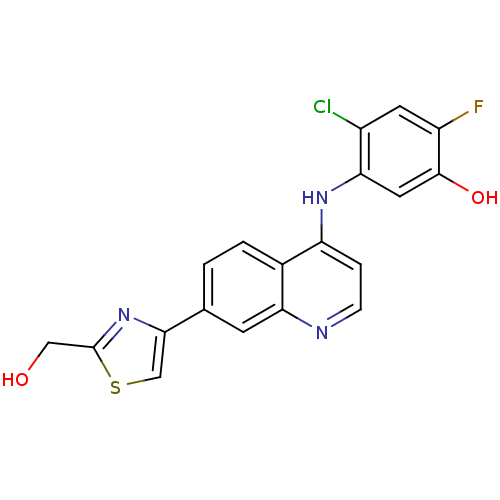 (4-chloro-2-fluoro-5-({7-[2-(hydroxymethyl)-1,3-thi...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | 25 | n/a | n/a | n/a | n/a | 7.4 | 22 |
GlaxoSmithKline | Assay Description The biochemical activity of compound was determined by incubation with Ret kinase and the substrate in the presence of ATP/ [gamma-33P] ATP. After in... | Bioorg Med Chem Lett 17: 5886-93 (2007) Article DOI: 10.1016/j.bmcl.2007.07.104 BindingDB Entry DOI: 10.7270/Q2H1309S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proto-oncogene tyrosine-protein kinase receptor Ret (Homo sapiens (Human)) | BDBM25200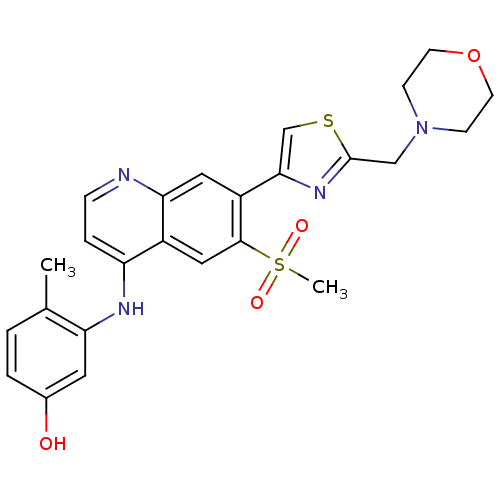 (3-({6-methanesulfonyl-7-[2-(morpholin-4-ylmethyl)-...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | 34 | n/a | n/a | n/a | n/a | 7.4 | 22 |
GlaxoSmithKline | Assay Description The biochemical activity of compound was determined by incubation with Ret kinase and the substrate in the presence of ATP/ [gamma-33P] ATP. After in... | Bioorg Med Chem Lett 17: 5886-93 (2007) Article DOI: 10.1016/j.bmcl.2007.07.104 BindingDB Entry DOI: 10.7270/Q2H1309S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proto-oncogene tyrosine-protein kinase receptor Ret (Homo sapiens (Human)) | BDBM25190 (2-chloro-4-fluoro-5-({7-[3-({[2-(morpholin-4-yl)et...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | 75 | n/a | n/a | n/a | n/a | 7.4 | 22 |
GlaxoSmithKline | Assay Description The biochemical activity of compound was determined by incubation with Ret kinase and the substrate in the presence of ATP/ [gamma-33P] ATP. After in... | Bioorg Med Chem Lett 17: 5886-93 (2007) Article DOI: 10.1016/j.bmcl.2007.07.104 BindingDB Entry DOI: 10.7270/Q2H1309S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proto-oncogene tyrosine-protein kinase receptor Ret (Homo sapiens (Human)) | BDBM25193 (4-methyl-3-{[7-(pyridin-2-yl)quinolin-4-yl]amino}p...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 75 | n/a | n/a | n/a | n/a | 7.4 | 22 |
GlaxoSmithKline | Assay Description The biochemical activity of compound was determined by incubation with Ret kinase and the substrate in the presence of ATP/ [gamma-33P] ATP. After in... | Bioorg Med Chem Lett 17: 5886-93 (2007) Article DOI: 10.1016/j.bmcl.2007.07.104 BindingDB Entry DOI: 10.7270/Q2H1309S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proto-oncogene tyrosine-protein kinase receptor Ret (Homo sapiens (Human)) | BDBM25195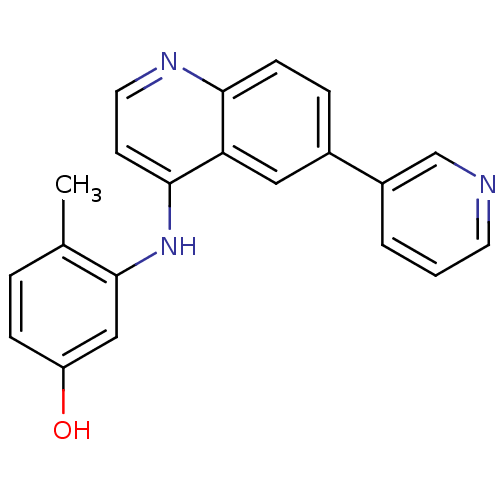 (4-methyl-3-{[6-(pyridin-3-yl)quinolin-4-yl]amino}p...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | 80 | n/a | n/a | n/a | n/a | 7.4 | 22 |
GlaxoSmithKline | Assay Description The biochemical activity of compound was determined by incubation with Ret kinase and the substrate in the presence of ATP/ [gamma-33P] ATP. After in... | Bioorg Med Chem Lett 17: 5886-93 (2007) Article DOI: 10.1016/j.bmcl.2007.07.104 BindingDB Entry DOI: 10.7270/Q2H1309S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proto-oncogene tyrosine-protein kinase receptor Ret (Homo sapiens (Human)) | BDBM25199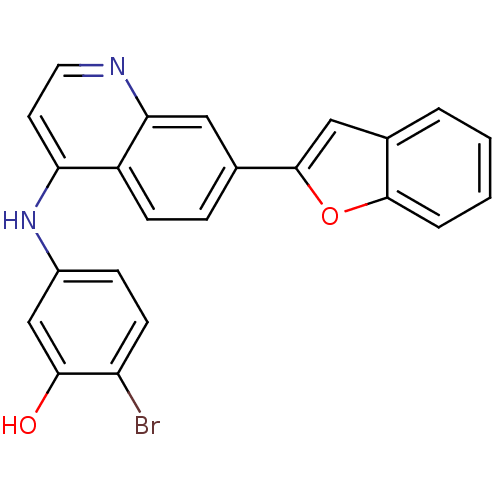 (5-{[7-(1-benzofuran-2-yl)quinolin-4-yl]amino}-2-br...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | 85 | n/a | n/a | n/a | n/a | 7.4 | 22 |
GlaxoSmithKline | Assay Description The biochemical activity of compound was determined by incubation with Ret kinase and the substrate in the presence of ATP/ [gamma-33P] ATP. After in... | Bioorg Med Chem Lett 17: 5886-93 (2007) Article DOI: 10.1016/j.bmcl.2007.07.104 BindingDB Entry DOI: 10.7270/Q2H1309S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proto-oncogene tyrosine-protein kinase receptor Ret (Homo sapiens (Human)) | BDBM25197 (6-substituted 4-anilinoquinoline, 37 | N-(cyclopro...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 90 | n/a | n/a | n/a | n/a | 7.4 | 22 |
GlaxoSmithKline | Assay Description The biochemical activity of compound was determined by incubation with Ret kinase and the substrate in the presence of ATP/ [gamma-33P] ATP. After in... | Bioorg Med Chem Lett 17: 5886-93 (2007) Article DOI: 10.1016/j.bmcl.2007.07.104 BindingDB Entry DOI: 10.7270/Q2H1309S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proto-oncogene tyrosine-protein kinase receptor Ret (Homo sapiens (Human)) | BDBM25196 (4-[(5-hydroxy-2-methylphenyl)amino]-N-(propan-2-yl...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | 95 | n/a | n/a | n/a | n/a | 7.4 | 22 |
GlaxoSmithKline | Assay Description The biochemical activity of compound was determined by incubation with Ret kinase and the substrate in the presence of ATP/ [gamma-33P] ATP. After in... | Bioorg Med Chem Lett 17: 5886-93 (2007) Article DOI: 10.1016/j.bmcl.2007.07.104 BindingDB Entry DOI: 10.7270/Q2H1309S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proto-oncogene tyrosine-protein kinase receptor Ret (Homo sapiens (Human)) | BDBM25188 (3-{[3-chloro-7-(1,3-thiazol-5-yl)quinolin-4-yl]ami...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 100 | n/a | n/a | n/a | n/a | 7.4 | 22 |
GlaxoSmithKline | Assay Description The biochemical activity of compound was determined by incubation with Ret kinase and the substrate in the presence of ATP/ [gamma-33P] ATP. After in... | Bioorg Med Chem Lett 17: 5886-93 (2007) Article DOI: 10.1016/j.bmcl.2007.07.104 BindingDB Entry DOI: 10.7270/Q2H1309S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proto-oncogene tyrosine-protein kinase receptor Ret (Homo sapiens (Human)) | BDBM25189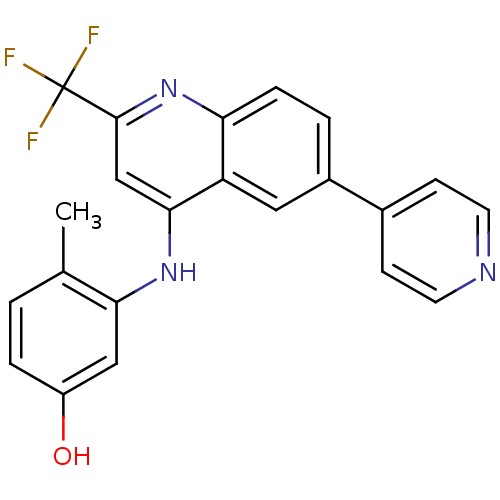 (4-methyl-3-{[6-(pyridin-4-yl)-2-(trifluoromethyl)q...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a | 7.4 | 22 |
GlaxoSmithKline | Assay Description The biochemical activity of compound was determined by incubation with Ret kinase and the substrate in the presence of ATP/ [gamma-33P] ATP. After in... | Bioorg Med Chem Lett 17: 5886-93 (2007) Article DOI: 10.1016/j.bmcl.2007.07.104 BindingDB Entry DOI: 10.7270/Q2H1309S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proto-oncogene tyrosine-protein kinase receptor Ret (Homo sapiens (Human)) | BDBM25201 (4-Anilino-6,7-disubstituted quinoline, 41 | 4-chlo...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a | 7.4 | 22 |
GlaxoSmithKline | Assay Description The biochemical activity of compound was determined by incubation with Ret kinase and the substrate in the presence of ATP/ [gamma-33P] ATP. After in... | Bioorg Med Chem Lett 17: 5886-93 (2007) Article DOI: 10.1016/j.bmcl.2007.07.104 BindingDB Entry DOI: 10.7270/Q2H1309S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||