 Found 38 hits of Enzyme Inhibition Constant Data
Found 38 hits of Enzyme Inhibition Constant Data Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Hydroxycarboxylic acid receptor 2
(Homo sapiens (Human)) | BDBM23515

(CHEMBL573 | Niacin | Nicotinic Acid | [5, 6-3H]-ni...)Show InChI InChI=1S/C6H5NO2/c8-6(9)5-2-1-3-7-4-5/h1-4H,(H,8,9) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
Article
PubMed
| n/a | n/a | 140 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [3H]niacin from human GPR109A |
Bioorg Med Chem Lett 17: 6723-8 (2008)
Article DOI: 10.1016/j.bmcl.2007.10.055
BindingDB Entry DOI: 10.7270/Q2R2114Q |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Hydroxycarboxylic acid receptor 2
(Homo sapiens (Human)) | BDBM50227775

(2-(1-(6-hydroxyquinoxalin-2-yl)piperazine-4-carbox...)Show SMILES OC(=O)c1ccccc1NC(=O)N1CCN(CC1)c1cnc2cc(O)ccc2n1 Show InChI InChI=1S/C20H19N5O4/c26-13-5-6-16-17(11-13)21-12-18(22-16)24-7-9-25(10-8-24)20(29)23-15-4-2-1-3-14(15)19(27)28/h1-6,11-12,26H,7-10H2,(H,23,29)(H,27,28) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 140 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [3H]niacin from human GPR109A |
Bioorg Med Chem Lett 17: 6723-8 (2008)
Article DOI: 10.1016/j.bmcl.2007.10.055
BindingDB Entry DOI: 10.7270/Q2R2114Q |
More data for this
Ligand-Target Pair | |
Hydroxycarboxylic acid receptor 2
(Homo sapiens (Human)) | BDBM50227783

(2-(1-(5-methoxyquinoxalin-2-yl)piperazine-4-carbox...)Show SMILES COc1cccc2nc(cnc12)N1CCN(CC1)C(=O)Nc1ccccc1C(O)=O Show InChI InChI=1S/C21H21N5O4/c1-30-17-8-4-7-16-19(17)22-13-18(23-16)25-9-11-26(12-10-25)21(29)24-15-6-3-2-5-14(15)20(27)28/h2-8,13H,9-12H2,1H3,(H,24,29)(H,27,28) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 290 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [3H]niacin from human GPR109A |
Bioorg Med Chem Lett 17: 6723-8 (2008)
Article DOI: 10.1016/j.bmcl.2007.10.055
BindingDB Entry DOI: 10.7270/Q2R2114Q |
More data for this
Ligand-Target Pair | |
Hydroxycarboxylic acid receptor 2
(Homo sapiens (Human)) | BDBM50227780

(2-(1-(quinoxalin-2-yl)piperazine-4-carboxamido)ben...)Show SMILES OC(=O)c1ccccc1NC(=O)N1CCN(CC1)c1cnc2ccccc2n1 Show InChI InChI=1S/C20H19N5O3/c26-19(27)14-5-1-2-6-15(14)23-20(28)25-11-9-24(10-12-25)18-13-21-16-7-3-4-8-17(16)22-18/h1-8,13H,9-12H2,(H,23,28)(H,26,27) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 550 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [3H]niacin from human GPR109A |
Bioorg Med Chem Lett 17: 6723-8 (2008)
Article DOI: 10.1016/j.bmcl.2007.10.055
BindingDB Entry DOI: 10.7270/Q2R2114Q |
More data for this
Ligand-Target Pair | |
Hydroxycarboxylic acid receptor 2
(Homo sapiens (Human)) | BDBM50227784
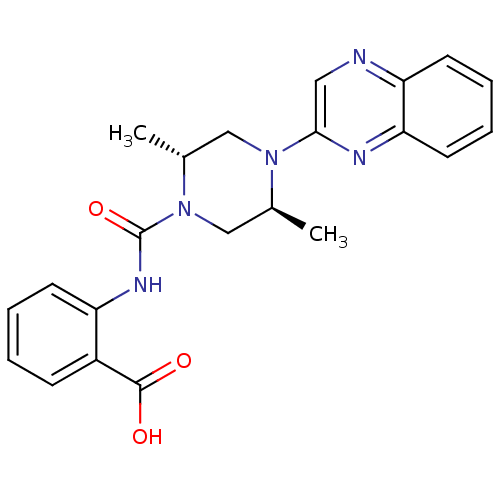
(2-((2S,5R)-2,5-dimethyl-1-(quinoxalin-2-yl)piperaz...)Show SMILES C[C@@H]1CN([C@@H](C)CN1C(=O)Nc1ccccc1C(O)=O)c1cnc2ccccc2n1 Show InChI InChI=1S/C22H23N5O3/c1-14-13-27(22(30)25-17-8-4-3-7-16(17)21(28)29)15(2)12-26(14)20-11-23-18-9-5-6-10-19(18)24-20/h3-11,14-15H,12-13H2,1-2H3,(H,25,30)(H,28,29)/t14-,15+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 650 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [3H]niacin from human GPR109A |
Bioorg Med Chem Lett 17: 6723-8 (2008)
Article DOI: 10.1016/j.bmcl.2007.10.055
BindingDB Entry DOI: 10.7270/Q2R2114Q |
More data for this
Ligand-Target Pair | |
Hydroxycarboxylic acid receptor 2
(Homo sapiens (Human)) | BDBM50227776
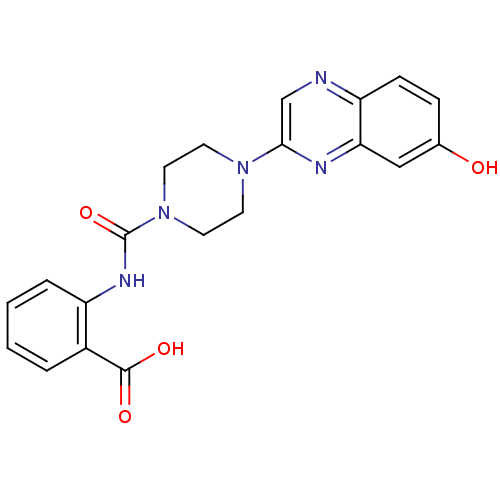
(2-(1-(7-hydroxyquinoxalin-2-yl)piperazine-4-carbox...)Show SMILES OC(=O)c1ccccc1NC(=O)N1CCN(CC1)c1cnc2ccc(O)cc2n1 Show InChI InChI=1S/C20H19N5O4/c26-13-5-6-16-17(11-13)22-18(12-21-16)24-7-9-25(10-8-24)20(29)23-15-4-2-1-3-14(15)19(27)28/h1-6,11-12,26H,7-10H2,(H,23,29)(H,27,28) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 790 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [3H]niacin from human GPR109A |
Bioorg Med Chem Lett 17: 6723-8 (2008)
Article DOI: 10.1016/j.bmcl.2007.10.055
BindingDB Entry DOI: 10.7270/Q2R2114Q |
More data for this
Ligand-Target Pair | |
Hydroxycarboxylic acid receptor 2
(Homo sapiens (Human)) | BDBM50227779

(2-(1-(quinoxalin-2-yl)-1,4-diazepane-4-carboxamido...)Show SMILES OC(=O)c1ccccc1NC(=O)N1CCCN(CC1)c1cnc2ccccc2n1 Show InChI InChI=1S/C21H21N5O3/c27-20(28)15-6-1-2-7-16(15)24-21(29)26-11-5-10-25(12-13-26)19-14-22-17-8-3-4-9-18(17)23-19/h1-4,6-9,14H,5,10-13H2,(H,24,29)(H,27,28) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 800 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [3H]niacin from human GPR109A |
Bioorg Med Chem Lett 17: 6723-8 (2008)
Article DOI: 10.1016/j.bmcl.2007.10.055
BindingDB Entry DOI: 10.7270/Q2R2114Q |
More data for this
Ligand-Target Pair | |
Hydroxycarboxylic acid receptor 2
(Homo sapiens (Human)) | BDBM50227785

(2-(1-(8-hydroxyquinoxalin-2-yl)piperazine-4-carbox...)Show SMILES OC(=O)c1ccccc1NC(=O)N1CCN(CC1)c1cnc2cccc(O)c2n1 Show InChI InChI=1S/C20H19N5O4/c26-16-7-3-6-15-18(16)23-17(12-21-15)24-8-10-25(11-9-24)20(29)22-14-5-2-1-4-13(14)19(27)28/h1-7,12,26H,8-11H2,(H,22,29)(H,27,28) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 870 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [3H]niacin from human GPR109A |
Bioorg Med Chem Lett 17: 6723-8 (2008)
Article DOI: 10.1016/j.bmcl.2007.10.055
BindingDB Entry DOI: 10.7270/Q2R2114Q |
More data for this
Ligand-Target Pair | |
Hydroxycarboxylic acid receptor 2
(Homo sapiens (Human)) | BDBM50227768
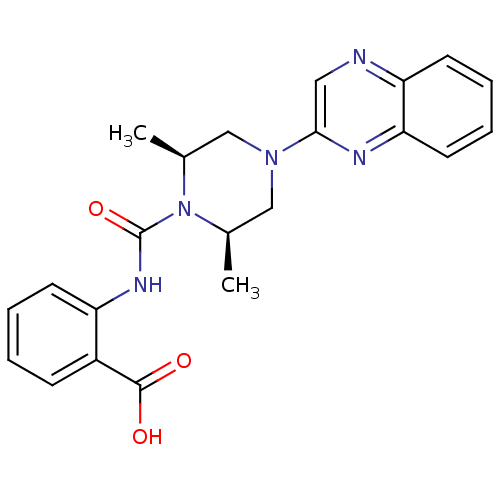
(2-((3S,5R)-3,5-dimethyl-1-(quinoxalin-2-yl)piperaz...)Show SMILES C[C@H]1CN(C[C@@H](C)N1C(=O)Nc1ccccc1C(O)=O)c1cnc2ccccc2n1 Show InChI InChI=1S/C22H23N5O3/c1-14-12-26(20-11-23-18-9-5-6-10-19(18)24-20)13-15(2)27(14)22(30)25-17-8-4-3-7-16(17)21(28)29/h3-11,14-15H,12-13H2,1-2H3,(H,25,30)(H,28,29)/t14-,15+ | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [3H]niacin from human GPR109A |
Bioorg Med Chem Lett 17: 6723-8 (2008)
Article DOI: 10.1016/j.bmcl.2007.10.055
BindingDB Entry DOI: 10.7270/Q2R2114Q |
More data for this
Ligand-Target Pair | |
Hydroxycarboxylic acid receptor 2
(Homo sapiens (Human)) | BDBM50227770
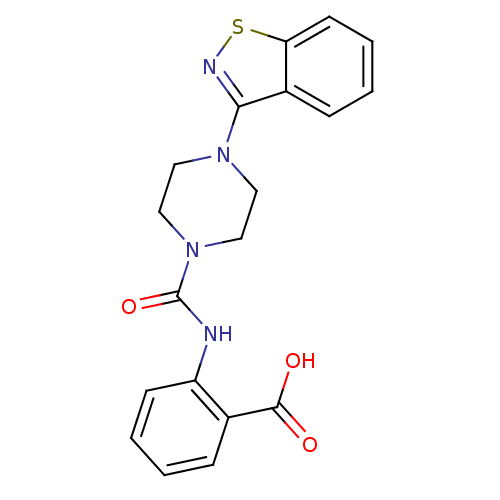
(2-(1-(benzo[d]isothiazol-3-yl)piperazine-4-carboxa...)Show SMILES OC(=O)c1ccccc1NC(=O)N1CCN(CC1)c1nsc2ccccc12 Show InChI InChI=1S/C19H18N4O3S/c24-18(25)13-5-1-3-7-15(13)20-19(26)23-11-9-22(10-12-23)17-14-6-2-4-8-16(14)27-21-17/h1-8H,9-12H2,(H,20,26)(H,24,25) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | 1.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [3H]niacin from human GPR109A |
Bioorg Med Chem Lett 17: 6723-8 (2008)
Article DOI: 10.1016/j.bmcl.2007.10.055
BindingDB Entry DOI: 10.7270/Q2R2114Q |
More data for this
Ligand-Target Pair | |
Hydroxycarboxylic acid receptor 2
(Homo sapiens (Human)) | BDBM50227781

(2-(1-(quinoxalin-2-yl)piperidine-4-carboxamido)ben...)Show SMILES OC(=O)c1ccccc1NC(=O)C1CCN(CC1)c1cnc2ccccc2n1 Show InChI InChI=1S/C21H20N4O3/c26-20(24-16-6-2-1-5-15(16)21(27)28)14-9-11-25(12-10-14)19-13-22-17-7-3-4-8-18(17)23-19/h1-8,13-14H,9-12H2,(H,24,26)(H,27,28) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [3H]niacin from human GPR109A |
Bioorg Med Chem Lett 17: 6723-8 (2008)
Article DOI: 10.1016/j.bmcl.2007.10.055
BindingDB Entry DOI: 10.7270/Q2R2114Q |
More data for this
Ligand-Target Pair | |
Hydroxycarboxylic acid receptor 2
(Homo sapiens (Human)) | BDBM50227777

(2-(1-(4-phenylpyrimidin-2-yl)piperazine-4-carboxam...)Show SMILES OC(=O)c1ccccc1NC(=O)N1CCN(CC1)c1nccc(n1)-c1ccccc1 Show InChI InChI=1S/C22H21N5O3/c28-20(29)17-8-4-5-9-19(17)25-22(30)27-14-12-26(13-15-27)21-23-11-10-18(24-21)16-6-2-1-3-7-16/h1-11H,12-15H2,(H,25,30)(H,28,29) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | 2.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [3H]niacin from human GPR109A |
Bioorg Med Chem Lett 17: 6723-8 (2008)
Article DOI: 10.1016/j.bmcl.2007.10.055
BindingDB Entry DOI: 10.7270/Q2R2114Q |
More data for this
Ligand-Target Pair | |
Hydroxycarboxylic acid receptor 2
(Homo sapiens (Human)) | BDBM50227771

(2-(1-(isoquinolin-1-yl)piperazine-4-carboxamido)be...)Show SMILES OC(=O)c1ccccc1NC(=O)N1CCN(CC1)c1nccc2ccccc12 Show InChI InChI=1S/C21H20N4O3/c26-20(27)17-7-3-4-8-18(17)23-21(28)25-13-11-24(12-14-25)19-16-6-2-1-5-15(16)9-10-22-19/h1-10H,11-14H2,(H,23,28)(H,26,27) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [3H]niacin from human GPR109A |
Bioorg Med Chem Lett 17: 6723-8 (2008)
Article DOI: 10.1016/j.bmcl.2007.10.055
BindingDB Entry DOI: 10.7270/Q2R2114Q |
More data for this
Ligand-Target Pair | |
Hydroxycarboxylic acid receptor 2
(Homo sapiens (Human)) | BDBM50227767

(2-(1-(quinolin-2-yl)piperazine-4-carboxamido)benzo...)Show SMILES OC(=O)c1ccccc1NC(=O)N1CCN(CC1)c1ccc2ccccc2n1 Show InChI InChI=1S/C21H20N4O3/c26-20(27)16-6-2-4-8-18(16)23-21(28)25-13-11-24(12-14-25)19-10-9-15-5-1-3-7-17(15)22-19/h1-10H,11-14H2,(H,23,28)(H,26,27) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [3H]niacin from human GPR109A |
Bioorg Med Chem Lett 17: 6723-8 (2008)
Article DOI: 10.1016/j.bmcl.2007.10.055
BindingDB Entry DOI: 10.7270/Q2R2114Q |
More data for this
Ligand-Target Pair | |
Hydroxycarboxylic acid receptor 2
(Homo sapiens (Human)) | BDBM50227778
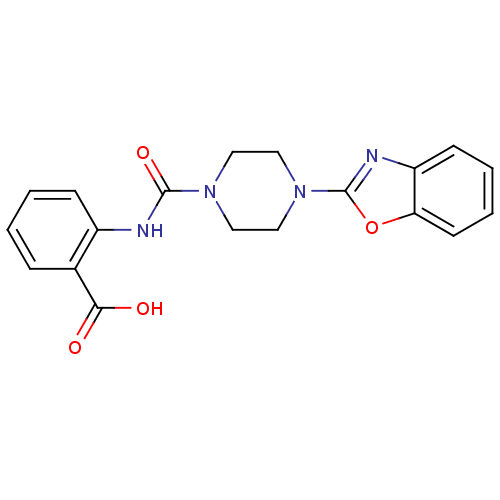
(2-(1-(benzo[d]oxazol-2-yl)piperazine-4-carboxamido...)Show SMILES OC(=O)c1ccccc1NC(=O)N1CCN(CC1)c1nc2ccccc2o1 Show InChI InChI=1S/C19H18N4O4/c24-17(25)13-5-1-2-6-14(13)20-18(26)22-9-11-23(12-10-22)19-21-15-7-3-4-8-16(15)27-19/h1-8H,9-12H2,(H,20,26)(H,24,25) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | 5.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [3H]niacin from human GPR109A |
Bioorg Med Chem Lett 17: 6723-8 (2008)
Article DOI: 10.1016/j.bmcl.2007.10.055
BindingDB Entry DOI: 10.7270/Q2R2114Q |
More data for this
Ligand-Target Pair | |
Hydroxycarboxylic acid receptor 2
(Homo sapiens (Human)) | BDBM50227769
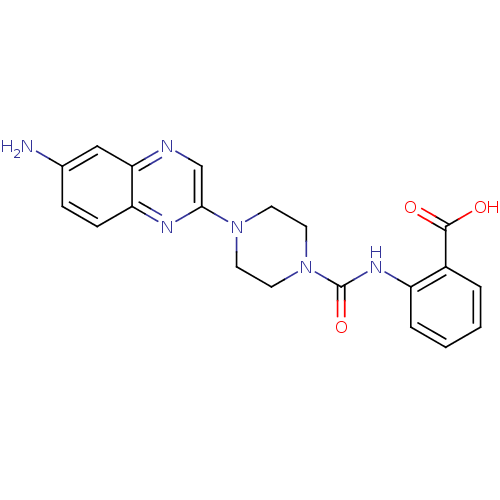
(2-(1-(6-aminoquinoxalin-2-yl)piperazine-4-carboxam...)Show SMILES Nc1ccc2nc(cnc2c1)N1CCN(CC1)C(=O)Nc1ccccc1C(O)=O Show InChI InChI=1S/C20H20N6O3/c21-13-5-6-16-17(11-13)22-12-18(23-16)25-7-9-26(10-8-25)20(29)24-15-4-2-1-3-14(15)19(27)28/h1-6,11-12H,7-10,21H2,(H,24,29)(H,27,28) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 6.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [3H]niacin from human GPR109A |
Bioorg Med Chem Lett 17: 6723-8 (2008)
Article DOI: 10.1016/j.bmcl.2007.10.055
BindingDB Entry DOI: 10.7270/Q2R2114Q |
More data for this
Ligand-Target Pair | |
Hydroxycarboxylic acid receptor 2
(Homo sapiens (Human)) | BDBM50227786
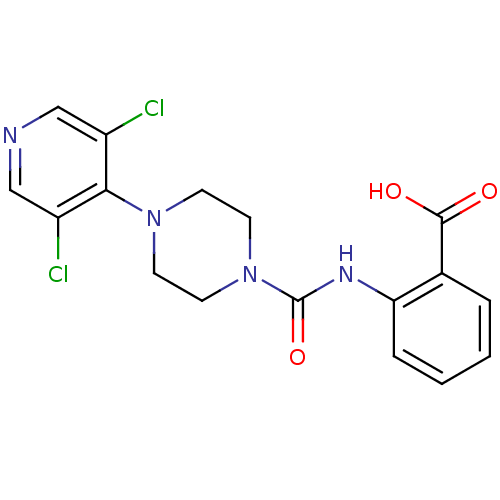
(2-(1-(3,5-dichloropyridin-4-yl)piperazine-4-carbox...)Show SMILES OC(=O)c1ccccc1NC(=O)N1CCN(CC1)c1c(Cl)cncc1Cl Show InChI InChI=1S/C17H16Cl2N4O3/c18-12-9-20-10-13(19)15(12)22-5-7-23(8-6-22)17(26)21-14-4-2-1-3-11(14)16(24)25/h1-4,9-10H,5-8H2,(H,21,26)(H,24,25) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | 2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [3H]niacin from human GPR109A |
Bioorg Med Chem Lett 17: 6723-8 (2008)
Article DOI: 10.1016/j.bmcl.2007.10.055
BindingDB Entry DOI: 10.7270/Q2R2114Q |
More data for this
Ligand-Target Pair | |
Hydroxycarboxylic acid receptor 2
(Homo sapiens (Human)) | BDBM50227774

(2-(1-(naphthalen-2-yl)piperazine-4-carboxamido)ben...)Show SMILES OC(=O)c1ccccc1NC(=O)N1CCN(CC1)c1ccc2ccccc2c1 Show InChI InChI=1S/C22H21N3O3/c26-21(27)19-7-3-4-8-20(19)23-22(28)25-13-11-24(12-14-25)18-10-9-16-5-1-2-6-17(16)15-18/h1-10,15H,11-14H2,(H,23,28)(H,26,27) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | 7.60E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [3H]niacin from human GPR109A |
Bioorg Med Chem Lett 17: 6723-8 (2008)
Article DOI: 10.1016/j.bmcl.2007.10.055
BindingDB Entry DOI: 10.7270/Q2R2114Q |
More data for this
Ligand-Target Pair | |
Hydroxycarboxylic acid receptor 2
(Homo sapiens (Human)) | BDBM50227773
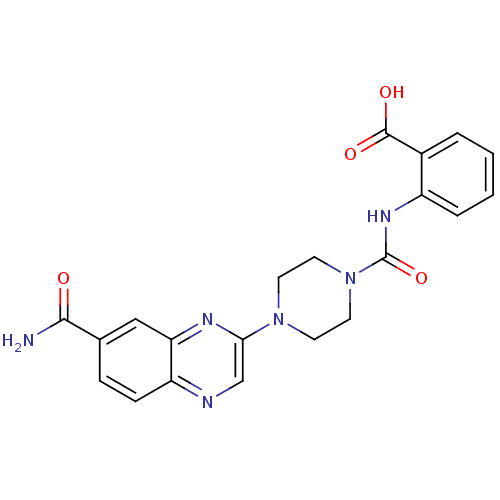
(2-(1-(7-carbamoylquinoxalin-2-yl)piperazine-4-carb...)Show SMILES NC(=O)c1ccc2ncc(nc2c1)N1CCN(CC1)C(=O)Nc1ccccc1C(O)=O Show InChI InChI=1S/C21H20N6O4/c22-19(28)13-5-6-16-17(11-13)24-18(12-23-16)26-7-9-27(10-8-26)21(31)25-15-4-2-1-3-14(15)20(29)30/h1-6,11-12H,7-10H2,(H2,22,28)(H,25,31)(H,29,30) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 7.90E+3 | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Agonist activity at human GPR106A by [35S]GTPgammaS binding assay |
Bioorg Med Chem Lett 17: 6723-8 (2008)
Article DOI: 10.1016/j.bmcl.2007.10.055
BindingDB Entry DOI: 10.7270/Q2R2114Q |
More data for this
Ligand-Target Pair | |
Hydroxycarboxylic acid receptor 2
(Homo sapiens (Human)) | BDBM50227778

(2-(1-(benzo[d]oxazol-2-yl)piperazine-4-carboxamido...)Show SMILES OC(=O)c1ccccc1NC(=O)N1CCN(CC1)c1nc2ccccc2o1 Show InChI InChI=1S/C19H18N4O4/c24-17(25)13-5-1-2-6-14(13)20-18(26)22-9-11-23(12-10-22)19-21-15-7-3-4-8-16(15)27-19/h1-8H,9-12H2,(H,20,26)(H,24,25) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | n/a | n/a | 2.20E+4 | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Agonist activity at human GPR106A by [35S]GTPgammaS binding assay |
Bioorg Med Chem Lett 17: 6723-8 (2008)
Article DOI: 10.1016/j.bmcl.2007.10.055
BindingDB Entry DOI: 10.7270/Q2R2114Q |
More data for this
Ligand-Target Pair | |
Hydroxycarboxylic acid receptor 2
(Homo sapiens (Human)) | BDBM50227775

(2-(1-(6-hydroxyquinoxalin-2-yl)piperazine-4-carbox...)Show SMILES OC(=O)c1ccccc1NC(=O)N1CCN(CC1)c1cnc2cc(O)ccc2n1 Show InChI InChI=1S/C20H19N5O4/c26-13-5-6-16-17(11-13)21-12-18(22-16)24-7-9-25(10-8-24)20(29)23-15-4-2-1-3-14(15)19(27)28/h1-6,11-12,26H,7-10H2,(H,23,29)(H,27,28) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 470 | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Agonist activity at human GPR106A by [35S]GTPgammaS binding assay |
Bioorg Med Chem Lett 17: 6723-8 (2008)
Article DOI: 10.1016/j.bmcl.2007.10.055
BindingDB Entry DOI: 10.7270/Q2R2114Q |
More data for this
Ligand-Target Pair | |
Hydroxycarboxylic acid receptor 2
(Homo sapiens (Human)) | BDBM50227774

(2-(1-(naphthalen-2-yl)piperazine-4-carboxamido)ben...)Show SMILES OC(=O)c1ccccc1NC(=O)N1CCN(CC1)c1ccc2ccccc2c1 Show InChI InChI=1S/C22H21N3O3/c26-21(27)19-7-3-4-8-20(19)23-22(28)25-13-11-24(12-14-25)18-10-9-16-5-1-2-6-17(16)15-18/h1-10,15H,11-14H2,(H,23,28)(H,26,27) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | n/a | n/a | 1.30E+4 | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Agonist activity at human GPR106A by [35S]GTPgammaS binding assay |
Bioorg Med Chem Lett 17: 6723-8 (2008)
Article DOI: 10.1016/j.bmcl.2007.10.055
BindingDB Entry DOI: 10.7270/Q2R2114Q |
More data for this
Ligand-Target Pair | |
Hydroxycarboxylic acid receptor 2
(Homo sapiens (Human)) | BDBM50227772

(2-(1-(quinazolin-2-yl)piperazine-4-carboxamido)ben...)Show SMILES OC(=O)c1ccccc1NC(=O)N1CCN(CC1)c1ncc2ccccc2n1 Show InChI InChI=1S/C20H19N5O3/c26-18(27)15-6-2-4-8-17(15)23-20(28)25-11-9-24(10-12-25)19-21-13-14-5-1-3-7-16(14)22-19/h1-8,13H,9-12H2,(H,23,28)(H,26,27) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | n/a | n/a | 7.80E+3 | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Agonist activity at human GPR106A by [35S]GTPgammaS binding assay |
Bioorg Med Chem Lett 17: 6723-8 (2008)
Article DOI: 10.1016/j.bmcl.2007.10.055
BindingDB Entry DOI: 10.7270/Q2R2114Q |
More data for this
Ligand-Target Pair | |
Hydroxycarboxylic acid receptor 2
(Homo sapiens (Human)) | BDBM50227782

(2-(1-(8-methoxyquinoxalin-2-yl)piperazine-4-carbox...)Show SMILES COc1cccc2ncc(nc12)N1CCN(CC1)C(=O)Nc1ccccc1C(O)=O Show InChI InChI=1S/C21H21N5O4/c1-30-17-8-4-7-16-19(17)24-18(13-22-16)25-9-11-26(12-10-25)21(29)23-15-6-3-2-5-14(15)20(27)28/h2-8,13H,9-12H2,1H3,(H,23,29)(H,27,28) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 1.10E+4 | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Agonist activity at human GPR106A by [35S]GTPgammaS binding assay |
Bioorg Med Chem Lett 17: 6723-8 (2008)
Article DOI: 10.1016/j.bmcl.2007.10.055
BindingDB Entry DOI: 10.7270/Q2R2114Q |
More data for this
Ligand-Target Pair | |
Hydroxycarboxylic acid receptor 2
(Homo sapiens (Human)) | BDBM50227767

(2-(1-(quinolin-2-yl)piperazine-4-carboxamido)benzo...)Show SMILES OC(=O)c1ccccc1NC(=O)N1CCN(CC1)c1ccc2ccccc2n1 Show InChI InChI=1S/C21H20N4O3/c26-20(27)16-6-2-4-8-18(16)23-21(28)25-13-11-24(12-14-25)19-10-9-15-5-1-3-7-17(15)22-19/h1-10H,11-14H2,(H,23,28)(H,26,27) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 5.90E+3 | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Agonist activity at human GPR106A by [35S]GTPgammaS binding assay |
Bioorg Med Chem Lett 17: 6723-8 (2008)
Article DOI: 10.1016/j.bmcl.2007.10.055
BindingDB Entry DOI: 10.7270/Q2R2114Q |
More data for this
Ligand-Target Pair | |
Hydroxycarboxylic acid receptor 2
(Homo sapiens (Human)) | BDBM50227776

(2-(1-(7-hydroxyquinoxalin-2-yl)piperazine-4-carbox...)Show SMILES OC(=O)c1ccccc1NC(=O)N1CCN(CC1)c1cnc2ccc(O)cc2n1 Show InChI InChI=1S/C20H19N5O4/c26-13-5-6-16-17(11-13)22-18(12-21-16)24-7-9-25(10-8-24)20(29)23-15-4-2-1-3-14(15)19(27)28/h1-6,11-12,26H,7-10H2,(H,23,29)(H,27,28) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 5.80E+3 | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Agonist activity at human GPR106A by [35S]GTPgammaS binding assay |
Bioorg Med Chem Lett 17: 6723-8 (2008)
Article DOI: 10.1016/j.bmcl.2007.10.055
BindingDB Entry DOI: 10.7270/Q2R2114Q |
More data for this
Ligand-Target Pair | |
Hydroxycarboxylic acid receptor 2
(Homo sapiens (Human)) | BDBM50227779

(2-(1-(quinoxalin-2-yl)-1,4-diazepane-4-carboxamido...)Show SMILES OC(=O)c1ccccc1NC(=O)N1CCCN(CC1)c1cnc2ccccc2n1 Show InChI InChI=1S/C21H21N5O3/c27-20(28)15-6-1-2-7-16(15)24-21(29)26-11-5-10-25(12-13-26)19-14-22-17-8-3-4-9-18(17)23-19/h1-4,6-9,14H,5,10-13H2,(H,24,29)(H,27,28) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 4.60E+3 | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Agonist activity at human GPR106A by [35S]GTPgammaS binding assay |
Bioorg Med Chem Lett 17: 6723-8 (2008)
Article DOI: 10.1016/j.bmcl.2007.10.055
BindingDB Entry DOI: 10.7270/Q2R2114Q |
More data for this
Ligand-Target Pair | |
Hydroxycarboxylic acid receptor 2
(Homo sapiens (Human)) | BDBM50227785

(2-(1-(8-hydroxyquinoxalin-2-yl)piperazine-4-carbox...)Show SMILES OC(=O)c1ccccc1NC(=O)N1CCN(CC1)c1cnc2cccc(O)c2n1 Show InChI InChI=1S/C20H19N5O4/c26-16-7-3-6-15-18(16)23-17(12-21-15)24-8-10-25(11-9-24)20(29)22-14-5-2-1-4-13(14)19(27)28/h1-7,12,26H,8-11H2,(H,22,29)(H,27,28) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 1.10E+4 | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Agonist activity at human GPR106A by [35S]GTPgammaS binding assay |
Bioorg Med Chem Lett 17: 6723-8 (2008)
Article DOI: 10.1016/j.bmcl.2007.10.055
BindingDB Entry DOI: 10.7270/Q2R2114Q |
More data for this
Ligand-Target Pair | |
Hydroxycarboxylic acid receptor 2
(Homo sapiens (Human)) | BDBM50227769

(2-(1-(6-aminoquinoxalin-2-yl)piperazine-4-carboxam...)Show SMILES Nc1ccc2nc(cnc2c1)N1CCN(CC1)C(=O)Nc1ccccc1C(O)=O Show InChI InChI=1S/C20H20N6O3/c21-13-5-6-16-17(11-13)22-12-18(23-16)25-7-9-26(10-8-25)20(29)24-15-4-2-1-3-14(15)19(27)28/h1-6,11-12H,7-10,21H2,(H,24,29)(H,27,28) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 1.70E+4 | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Agonist activity at human GPR106A by [35S]GTPgammaS binding assay |
Bioorg Med Chem Lett 17: 6723-8 (2008)
Article DOI: 10.1016/j.bmcl.2007.10.055
BindingDB Entry DOI: 10.7270/Q2R2114Q |
More data for this
Ligand-Target Pair | |
Hydroxycarboxylic acid receptor 2
(Homo sapiens (Human)) | BDBM50227770

(2-(1-(benzo[d]isothiazol-3-yl)piperazine-4-carboxa...)Show SMILES OC(=O)c1ccccc1NC(=O)N1CCN(CC1)c1nsc2ccccc12 Show InChI InChI=1S/C19H18N4O3S/c24-18(25)13-5-1-3-7-15(13)20-19(26)23-11-9-22(10-12-23)17-14-6-2-4-8-16(14)27-21-17/h1-8H,9-12H2,(H,20,26)(H,24,25) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | n/a | n/a | 5.00E+3 | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Agonist activity at human GPR106A by [35S]GTPgammaS binding assay |
Bioorg Med Chem Lett 17: 6723-8 (2008)
Article DOI: 10.1016/j.bmcl.2007.10.055
BindingDB Entry DOI: 10.7270/Q2R2114Q |
More data for this
Ligand-Target Pair | |
Hydroxycarboxylic acid receptor 2
(Homo sapiens (Human)) | BDBM50227768

(2-((3S,5R)-3,5-dimethyl-1-(quinoxalin-2-yl)piperaz...)Show SMILES C[C@H]1CN(C[C@@H](C)N1C(=O)Nc1ccccc1C(O)=O)c1cnc2ccccc2n1 Show InChI InChI=1S/C22H23N5O3/c1-14-12-26(20-11-23-18-9-5-6-10-19(18)24-20)13-15(2)27(14)22(30)25-17-8-4-3-7-16(17)21(28)29/h3-11,14-15H,12-13H2,1-2H3,(H,25,30)(H,28,29)/t14-,15+ | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 9.10E+3 | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Agonist activity at human GPR106A by [35S]GTPgammaS binding assay |
Bioorg Med Chem Lett 17: 6723-8 (2008)
Article DOI: 10.1016/j.bmcl.2007.10.055
BindingDB Entry DOI: 10.7270/Q2R2114Q |
More data for this
Ligand-Target Pair | |
Hydroxycarboxylic acid receptor 2
(Homo sapiens (Human)) | BDBM50227780

(2-(1-(quinoxalin-2-yl)piperazine-4-carboxamido)ben...)Show SMILES OC(=O)c1ccccc1NC(=O)N1CCN(CC1)c1cnc2ccccc2n1 Show InChI InChI=1S/C20H19N5O3/c26-19(27)14-5-1-2-6-15(14)23-20(28)25-11-9-24(10-12-25)18-13-21-16-7-3-4-8-17(16)22-18/h1-8,13H,9-12H2,(H,23,28)(H,26,27) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 1.60E+3 | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Agonist activity at human GPR106A by [35S]GTPgammaS binding assay |
Bioorg Med Chem Lett 17: 6723-8 (2008)
Article DOI: 10.1016/j.bmcl.2007.10.055
BindingDB Entry DOI: 10.7270/Q2R2114Q |
More data for this
Ligand-Target Pair | |
Hydroxycarboxylic acid receptor 2
(Homo sapiens (Human)) | BDBM50227783

(2-(1-(5-methoxyquinoxalin-2-yl)piperazine-4-carbox...)Show SMILES COc1cccc2nc(cnc12)N1CCN(CC1)C(=O)Nc1ccccc1C(O)=O Show InChI InChI=1S/C21H21N5O4/c1-30-17-8-4-7-16-19(17)22-13-18(23-16)25-9-11-26(12-10-25)21(29)24-15-6-3-2-5-14(15)20(27)28/h2-8,13H,9-12H2,1H3,(H,24,29)(H,27,28) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 3.30E+3 | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Agonist activity at human GPR106A by [35S]GTPgammaS binding assay |
Bioorg Med Chem Lett 17: 6723-8 (2008)
Article DOI: 10.1016/j.bmcl.2007.10.055
BindingDB Entry DOI: 10.7270/Q2R2114Q |
More data for this
Ligand-Target Pair | |
Hydroxycarboxylic acid receptor 2
(Homo sapiens (Human)) | BDBM50227777

(2-(1-(4-phenylpyrimidin-2-yl)piperazine-4-carboxam...)Show SMILES OC(=O)c1ccccc1NC(=O)N1CCN(CC1)c1nccc(n1)-c1ccccc1 Show InChI InChI=1S/C22H21N5O3/c28-20(29)17-8-4-5-9-19(17)25-22(30)27-14-12-26(13-15-27)21-23-11-10-18(24-21)16-6-2-1-3-7-16/h1-11H,12-15H2,(H,25,30)(H,28,29) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | n/a | n/a | 1.30E+4 | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Agonist activity at human GPR106A by [35S]GTPgammaS binding assay |
Bioorg Med Chem Lett 17: 6723-8 (2008)
Article DOI: 10.1016/j.bmcl.2007.10.055
BindingDB Entry DOI: 10.7270/Q2R2114Q |
More data for this
Ligand-Target Pair | |
Hydroxycarboxylic acid receptor 2
(Homo sapiens (Human)) | BDBM50227784

(2-((2S,5R)-2,5-dimethyl-1-(quinoxalin-2-yl)piperaz...)Show SMILES C[C@@H]1CN([C@@H](C)CN1C(=O)Nc1ccccc1C(O)=O)c1cnc2ccccc2n1 Show InChI InChI=1S/C22H23N5O3/c1-14-13-27(22(30)25-17-8-4-3-7-16(17)21(28)29)15(2)12-26(14)20-11-23-18-9-5-6-10-19(18)24-20/h3-11,14-15H,12-13H2,1-2H3,(H,25,30)(H,28,29)/t14-,15+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 4.40E+3 | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Agonist activity at human GPR106A by [35S]GTPgammaS binding assay |
Bioorg Med Chem Lett 17: 6723-8 (2008)
Article DOI: 10.1016/j.bmcl.2007.10.055
BindingDB Entry DOI: 10.7270/Q2R2114Q |
More data for this
Ligand-Target Pair | |
Hydroxycarboxylic acid receptor 2
(Homo sapiens (Human)) | BDBM23515

(CHEMBL573 | Niacin | Nicotinic Acid | [5, 6-3H]-ni...)Show InChI InChI=1S/C6H5NO2/c8-6(9)5-2-1-3-7-4-5/h1-4H,(H,8,9) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
Article
PubMed
| n/a | n/a | n/a | n/a | 1.00E+3 | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Agonist activity at human GPR106A by [35S]GTPgammaS binding assay |
Bioorg Med Chem Lett 17: 6723-8 (2008)
Article DOI: 10.1016/j.bmcl.2007.10.055
BindingDB Entry DOI: 10.7270/Q2R2114Q |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Hydroxycarboxylic acid receptor 2
(Homo sapiens (Human)) | BDBM50227771

(2-(1-(isoquinolin-1-yl)piperazine-4-carboxamido)be...)Show SMILES OC(=O)c1ccccc1NC(=O)N1CCN(CC1)c1nccc2ccccc12 Show InChI InChI=1S/C21H20N4O3/c26-20(27)17-7-3-4-8-18(17)23-21(28)25-13-11-24(12-14-25)19-16-6-2-1-5-15(16)9-10-22-19/h1-10H,11-14H2,(H,23,28)(H,26,27) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 9.00E+3 | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Agonist activity at human GPR106A by [35S]GTPgammaS binding assay |
Bioorg Med Chem Lett 17: 6723-8 (2008)
Article DOI: 10.1016/j.bmcl.2007.10.055
BindingDB Entry DOI: 10.7270/Q2R2114Q |
More data for this
Ligand-Target Pair | |
Hydroxycarboxylic acid receptor 2
(Homo sapiens (Human)) | BDBM50227781

(2-(1-(quinoxalin-2-yl)piperidine-4-carboxamido)ben...)Show SMILES OC(=O)c1ccccc1NC(=O)C1CCN(CC1)c1cnc2ccccc2n1 Show InChI InChI=1S/C21H20N4O3/c26-20(24-16-6-2-1-5-15(16)21(27)28)14-9-11-25(12-10-14)19-13-22-17-7-3-4-8-18(17)23-19/h1-8,13-14H,9-12H2,(H,24,26)(H,27,28) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | n/a | 6.30E+3 | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Agonist activity at human GPR106A by [35S]GTPgammaS binding assay |
Bioorg Med Chem Lett 17: 6723-8 (2008)
Article DOI: 10.1016/j.bmcl.2007.10.055
BindingDB Entry DOI: 10.7270/Q2R2114Q |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data