

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Carbonic anhydrase 2 (Homo sapiens (Human)) | BDBM50376374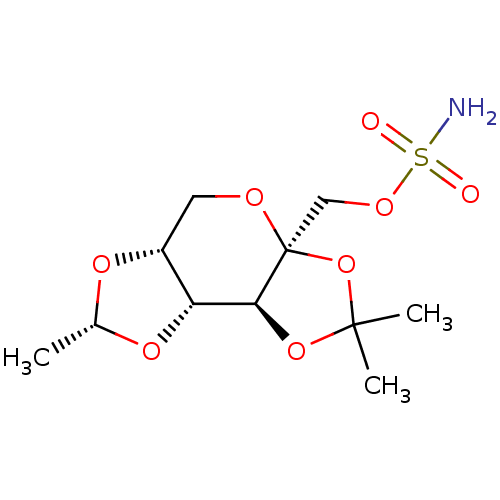 (CHEMBL26303) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development Curated by ChEMBL | Assay Description Inhibition of human carbonic anhydrase 2 by 4-NPA hydrolysis assay | J Med Chem 51: 2518-21 (2008) Article DOI: 10.1021/jm7015649 BindingDB Entry DOI: 10.7270/Q22R3SJN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 2 (Homo sapiens (Human)) | BDBM50376372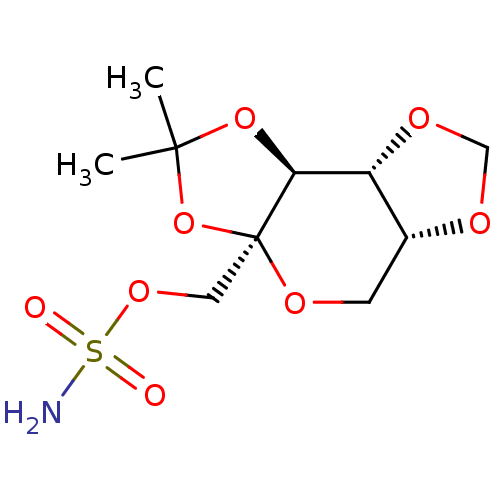 (CHEMBL26590) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 350 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development Curated by ChEMBL | Assay Description Inhibition of human carbonic anhydrase 2 by 4-NPA hydrolysis assay | J Med Chem 51: 2518-21 (2008) Article DOI: 10.1021/jm7015649 BindingDB Entry DOI: 10.7270/Q22R3SJN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 2 (Homo sapiens (Human)) | BDBM10887 (Sulfamate 7 | Topiramate (TPM) | US11535599, Examp...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank PDB Article PubMed | 450 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development Curated by ChEMBL | Assay Description Inhibition of human carbonic anhydrase 2 by 4-NPA hydrolysis assay | J Med Chem 51: 2518-21 (2008) Article DOI: 10.1021/jm7015649 BindingDB Entry DOI: 10.7270/Q22R3SJN | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Carbonic anhydrase 2 (Homo sapiens (Human)) | BDBM50376370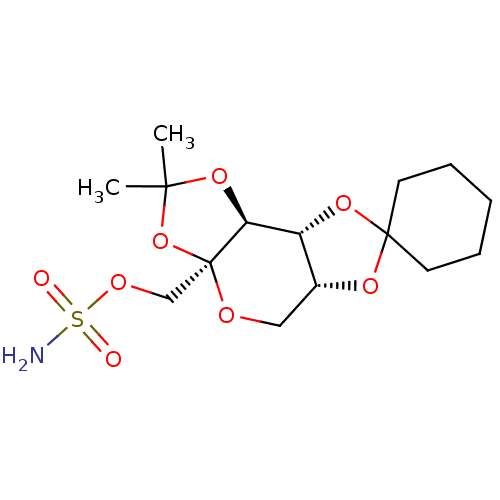 (CHEMBL410329) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development Curated by ChEMBL | Assay Description Inhibition of human carbonic anhydrase 2 by 4-NPA hydrolysis assay | J Med Chem 51: 2518-21 (2008) Article DOI: 10.1021/jm7015649 BindingDB Entry DOI: 10.7270/Q22R3SJN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 2 (Homo sapiens (Human)) | BDBM50376368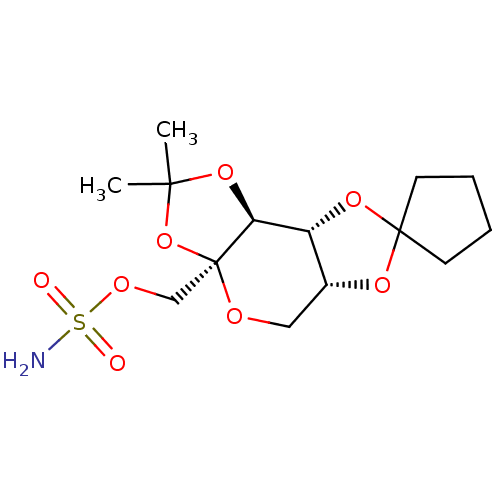 (CHEMBL26780) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development Curated by ChEMBL | Assay Description Inhibition of human carbonic anhydrase 2 by 4-NPA hydrolysis assay | J Med Chem 51: 2518-21 (2008) Article DOI: 10.1021/jm7015649 BindingDB Entry DOI: 10.7270/Q22R3SJN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 2 (Homo sapiens (Human)) | BDBM10888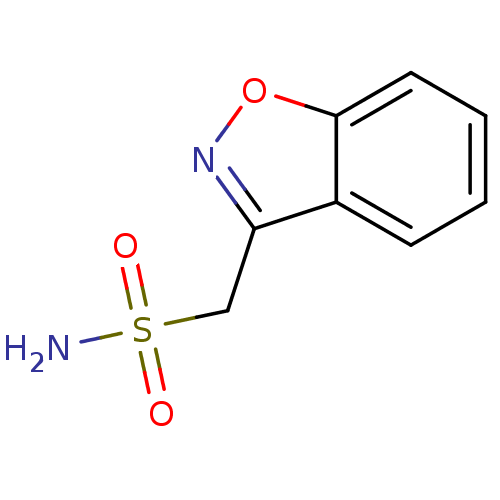 (1,2-benzoxazol-3-ylmethanesulfonamide | CHEMBL750 ...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank Article PubMed | 6.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development Curated by ChEMBL | Assay Description Inhibition of human carbonic anhydrase 2 by 4-NPA hydrolysis assay | J Med Chem 51: 2518-21 (2008) Article DOI: 10.1021/jm7015649 BindingDB Entry DOI: 10.7270/Q22R3SJN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 2 (Homo sapiens (Human)) | BDBM50376371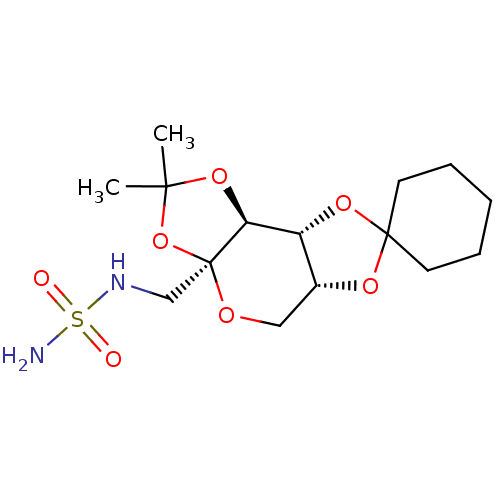 (CHEMBL264966) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | >3.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development Curated by ChEMBL | Assay Description Inhibition of human carbonic anhydrase 2 by 4-NPA hydrolysis assay | J Med Chem 51: 2518-21 (2008) Article DOI: 10.1021/jm7015649 BindingDB Entry DOI: 10.7270/Q22R3SJN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 2 (Homo sapiens (Human)) | BDBM50376373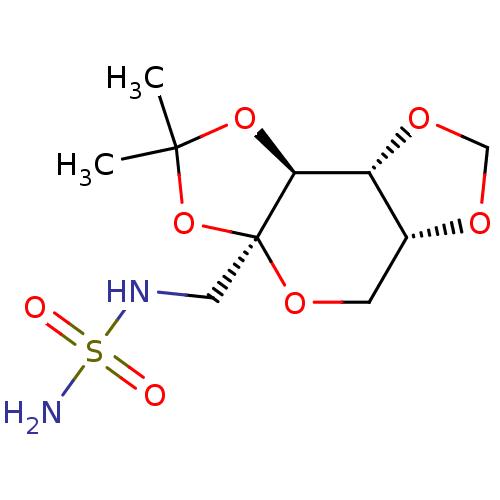 (CHEMBL260866) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development Curated by ChEMBL | Assay Description Inhibition of human carbonic anhydrase 2 by 4-NPA hydrolysis assay | J Med Chem 51: 2518-21 (2008) Article DOI: 10.1021/jm7015649 BindingDB Entry DOI: 10.7270/Q22R3SJN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 2 (Homo sapiens (Human)) | BDBM50376369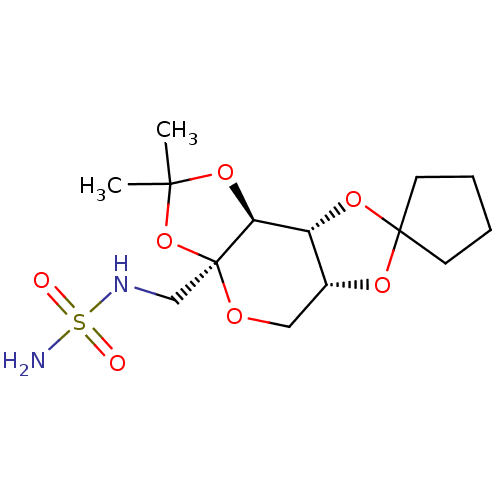 (CHEMBL410328) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | >3.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development Curated by ChEMBL | Assay Description Inhibition of human carbonic anhydrase 2 by 4-NPA hydrolysis assay | J Med Chem 51: 2518-21 (2008) Article DOI: 10.1021/jm7015649 BindingDB Entry DOI: 10.7270/Q22R3SJN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 2 (Homo sapiens (Human)) | BDBM50376375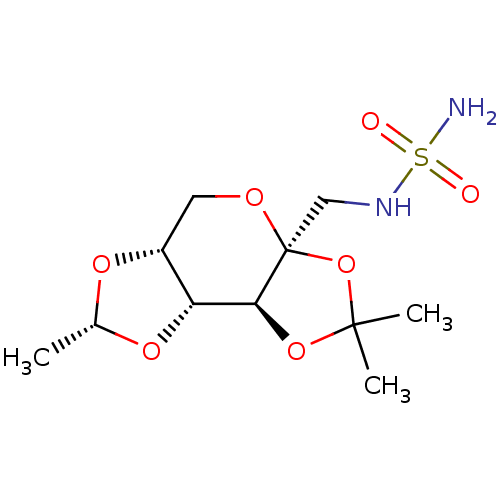 (CHEMBL412256) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development Curated by ChEMBL | Assay Description Inhibition of human carbonic anhydrase 2 by 4-NPA hydrolysis assay | J Med Chem 51: 2518-21 (2008) Article DOI: 10.1021/jm7015649 BindingDB Entry DOI: 10.7270/Q22R3SJN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 2 (Homo sapiens (Human)) | BDBM13055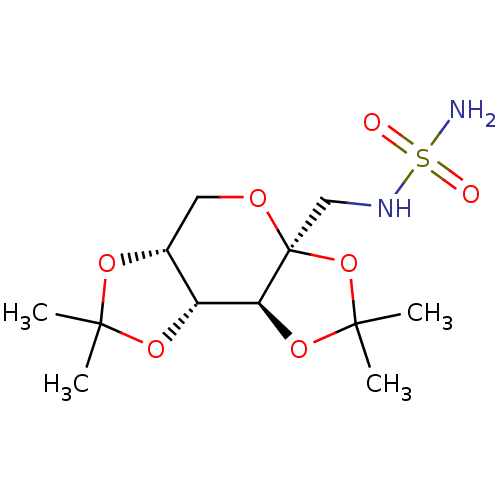 (CHEMBL283695 | Compound 8 | Topiramate Sulfamide A...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB PDB Article PubMed | 3.40E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development Curated by ChEMBL | Assay Description Inhibition of human carbonic anhydrase 2 by 4-NPA hydrolysis assay | J Med Chem 51: 2518-21 (2008) Article DOI: 10.1021/jm7015649 BindingDB Entry DOI: 10.7270/Q22R3SJN | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Carbonic anhydrase 2 (Homo sapiens (Human)) | BDBM50376370 (CHEMBL410329) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | 800 | n/a | n/a | n/a | 7.0 | n/a |
Johnson& Johnson Pharmaceutical Research& Development Curated by ChEMBL | Assay Description Binding affinity to human carbonic anhydrase 2 in presence of 10 mM PIPES and 100 uM Na2SO4 at pH 7.0 by ThermoFluor method | J Med Chem 51: 2518-21 (2008) Article DOI: 10.1021/jm7015649 BindingDB Entry DOI: 10.7270/Q22R3SJN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 2 (Homo sapiens (Human)) | BDBM50376369 (CHEMBL410328) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | >5.70E+4 | n/a | n/a | n/a | 7.0 | n/a |
Johnson& Johnson Pharmaceutical Research& Development Curated by ChEMBL | Assay Description Binding affinity to human carbonic anhydrase 2 in presence of 10 mM PIPES and 200 uM NaCl at pH 7.0 by ThermoFluor method | J Med Chem 51: 2518-21 (2008) Article DOI: 10.1021/jm7015649 BindingDB Entry DOI: 10.7270/Q22R3SJN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 2 (Homo sapiens (Human)) | BDBM10887 (Sulfamate 7 | Topiramate (TPM) | US11535599, Examp...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank PDB Article PubMed | n/a | n/a | n/a | 290 | n/a | n/a | n/a | 7.5 | n/a |
Johnson& Johnson Pharmaceutical Research& Development Curated by ChEMBL | Assay Description Binding affinity to human carbonic anhydrase 2 in presence of 10 mM HEPES and 100 uM Na2SO4 at pH 7.5 by ThermoFluor method | J Med Chem 51: 2518-21 (2008) Article DOI: 10.1021/jm7015649 BindingDB Entry DOI: 10.7270/Q22R3SJN | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Carbonic anhydrase 2 (Homo sapiens (Human)) | BDBM50376373 (CHEMBL260866) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | >5.70E+4 | n/a | n/a | n/a | 7.5 | n/a |
Johnson& Johnson Pharmaceutical Research& Development Curated by ChEMBL | Assay Description Binding affinity to human carbonic anhydrase 2 in presence of 10 mM HEPES and 100 uM Na2SO4 at pH 7.5 by ThermoFluor method | J Med Chem 51: 2518-21 (2008) Article DOI: 10.1021/jm7015649 BindingDB Entry DOI: 10.7270/Q22R3SJN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 2 (Homo sapiens (Human)) | BDBM50376372 (CHEMBL26590) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | 300 | n/a | n/a | n/a | 7.5 | n/a |
Johnson& Johnson Pharmaceutical Research& Development Curated by ChEMBL | Assay Description Binding affinity to human carbonic anhydrase 2 in presence of 10 mM HEPES without Na+ at pH 7.5 by ThermoFluor method | J Med Chem 51: 2518-21 (2008) Article DOI: 10.1021/jm7015649 BindingDB Entry DOI: 10.7270/Q22R3SJN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 2 (Homo sapiens (Human)) | BDBM50376371 (CHEMBL264966) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | 5.00E+4 | n/a | n/a | n/a | 7.5 | n/a |
Johnson& Johnson Pharmaceutical Research& Development Curated by ChEMBL | Assay Description Binding affinity to human carbonic anhydrase 2 in presence of 10 mM HEPES without Na+ at pH 7.5 by ThermoFluor method | J Med Chem 51: 2518-21 (2008) Article DOI: 10.1021/jm7015649 BindingDB Entry DOI: 10.7270/Q22R3SJN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 2 (Homo sapiens (Human)) | BDBM50376369 (CHEMBL410328) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | >5.70E+4 | n/a | n/a | n/a | 7.5 | n/a |
Johnson& Johnson Pharmaceutical Research& Development Curated by ChEMBL | Assay Description Binding affinity to human carbonic anhydrase 2 in presence of 10 mM HEPES and 100 uM Na2SO4 at pH 7.5 by ThermoFluor method | J Med Chem 51: 2518-21 (2008) Article DOI: 10.1021/jm7015649 BindingDB Entry DOI: 10.7270/Q22R3SJN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 2 (Homo sapiens (Human)) | BDBM50376374 (CHEMBL26303) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | 300 | n/a | n/a | n/a | 7.5 | n/a |
Johnson& Johnson Pharmaceutical Research& Development Curated by ChEMBL | Assay Description Binding affinity to human carbonic anhydrase 2 in presence of 10 mM HEPES and 100 uM Na2SO4 at pH 7.5 by ThermoFluor method | J Med Chem 51: 2518-21 (2008) Article DOI: 10.1021/jm7015649 BindingDB Entry DOI: 10.7270/Q22R3SJN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 2 (Homo sapiens (Human)) | BDBM50376375 (CHEMBL412256) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | >5.70E+4 | n/a | n/a | n/a | 7.5 | n/a |
Johnson& Johnson Pharmaceutical Research& Development Curated by ChEMBL | Assay Description Binding affinity to human carbonic anhydrase 2 in presence of 10 mM HEPES without Na+ at pH 7.5 by ThermoFluor method | J Med Chem 51: 2518-21 (2008) Article DOI: 10.1021/jm7015649 BindingDB Entry DOI: 10.7270/Q22R3SJN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 2 (Homo sapiens (Human)) | BDBM50376369 (CHEMBL410328) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | 5.00E+4 | n/a | n/a | n/a | 7.0 | n/a |
Johnson& Johnson Pharmaceutical Research& Development Curated by ChEMBL | Assay Description Binding affinity to human carbonic anhydrase 2 in presence of 10 mM PIPES without Na+ at pH 7.0 by ThermoFluor method | J Med Chem 51: 2518-21 (2008) Article DOI: 10.1021/jm7015649 BindingDB Entry DOI: 10.7270/Q22R3SJN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 2 (Homo sapiens (Human)) | BDBM50376372 (CHEMBL26590) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | 300 | n/a | n/a | n/a | 7.0 | n/a |
Johnson& Johnson Pharmaceutical Research& Development Curated by ChEMBL | Assay Description Binding affinity to human carbonic anhydrase 2 in presence of 10 mM PIPES without Na+ at pH 7.0 by ThermoFluor method | J Med Chem 51: 2518-21 (2008) Article DOI: 10.1021/jm7015649 BindingDB Entry DOI: 10.7270/Q22R3SJN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 2 (Homo sapiens (Human)) | BDBM50376368 (CHEMBL26780) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | 500 | n/a | n/a | n/a | 7.5 | n/a |
Johnson& Johnson Pharmaceutical Research& Development Curated by ChEMBL | Assay Description Binding affinity to human carbonic anhydrase 2 in presence of 10 mM HEPES without Na+ at pH 7.5 by ThermoFluor method | J Med Chem 51: 2518-21 (2008) Article DOI: 10.1021/jm7015649 BindingDB Entry DOI: 10.7270/Q22R3SJN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 2 (Homo sapiens (Human)) | BDBM50376368 (CHEMBL26780) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | 500 | n/a | n/a | n/a | 7.5 | n/a |
Johnson& Johnson Pharmaceutical Research& Development Curated by ChEMBL | Assay Description Binding affinity to human carbonic anhydrase 2 in presence of 10 mM HEPES and 100 uM Na2SO4 at pH 7.5 by ThermoFluor method | J Med Chem 51: 2518-21 (2008) Article DOI: 10.1021/jm7015649 BindingDB Entry DOI: 10.7270/Q22R3SJN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 2 (Homo sapiens (Human)) | BDBM50376375 (CHEMBL412256) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | >5.70E+4 | n/a | n/a | n/a | 7.0 | n/a |
Johnson& Johnson Pharmaceutical Research& Development Curated by ChEMBL | Assay Description Binding affinity to human carbonic anhydrase 2 in presence of 10 mM PIPES and 100 uM Na2SO4 at pH 7.0 by ThermoFluor method | J Med Chem 51: 2518-21 (2008) Article DOI: 10.1021/jm7015649 BindingDB Entry DOI: 10.7270/Q22R3SJN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 2 (Homo sapiens (Human)) | BDBM50376371 (CHEMBL264966) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | 5.00E+4 | n/a | n/a | n/a | 7.0 | n/a |
Johnson& Johnson Pharmaceutical Research& Development Curated by ChEMBL | Assay Description Binding affinity to human carbonic anhydrase 2 in presence of 10 mM PIPES and 100 uM Na2SO4 at pH 7.0 by ThermoFluor method | J Med Chem 51: 2518-21 (2008) Article DOI: 10.1021/jm7015649 BindingDB Entry DOI: 10.7270/Q22R3SJN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 2 (Homo sapiens (Human)) | BDBM50376372 (CHEMBL26590) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | 700 | n/a | n/a | n/a | 7.0 | n/a |
Johnson& Johnson Pharmaceutical Research& Development Curated by ChEMBL | Assay Description Binding affinity to human carbonic anhydrase 2 in presence of 10 mM PIPES and 200 uM NaCl at pH 7.0 by ThermoFluor method | J Med Chem 51: 2518-21 (2008) Article DOI: 10.1021/jm7015649 BindingDB Entry DOI: 10.7270/Q22R3SJN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 2 (Homo sapiens (Human)) | BDBM50376373 (CHEMBL260866) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | >5.70E+4 | n/a | n/a | n/a | 7.0 | n/a |
Johnson& Johnson Pharmaceutical Research& Development Curated by ChEMBL | Assay Description Binding affinity to human carbonic anhydrase 2 in presence of 10 mM PIPES without Na+ at pH 7.0 by ThermoFluor method | J Med Chem 51: 2518-21 (2008) Article DOI: 10.1021/jm7015649 BindingDB Entry DOI: 10.7270/Q22R3SJN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 2 (Homo sapiens (Human)) | BDBM50376373 (CHEMBL260866) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | >5.70E+4 | n/a | n/a | n/a | 7.5 | n/a |
Johnson& Johnson Pharmaceutical Research& Development Curated by ChEMBL | Assay Description Binding affinity to human carbonic anhydrase 2 in presence of 10 mM HEPES without Na+ at pH 7.5 by ThermoFluor method | J Med Chem 51: 2518-21 (2008) Article DOI: 10.1021/jm7015649 BindingDB Entry DOI: 10.7270/Q22R3SJN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 2 (Homo sapiens (Human)) | BDBM50376372 (CHEMBL26590) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | 400 | n/a | n/a | n/a | 7.0 | n/a |
Johnson& Johnson Pharmaceutical Research& Development Curated by ChEMBL | Assay Description Binding affinity to human carbonic anhydrase 2 in presence of 10 mM PIPES and 100 uM Na2SO4 at pH 7.0 by ThermoFluor method | J Med Chem 51: 2518-21 (2008) Article DOI: 10.1021/jm7015649 BindingDB Entry DOI: 10.7270/Q22R3SJN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 2 (Homo sapiens (Human)) | BDBM50376371 (CHEMBL264966) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | 5.00E+4 | n/a | n/a | n/a | 7.0 | n/a |
Johnson& Johnson Pharmaceutical Research& Development Curated by ChEMBL | Assay Description Binding affinity to human carbonic anhydrase 2 in presence of 10 mM PIPES and 200 uM NaCl at pH 7.0 by ThermoFluor method | J Med Chem 51: 2518-21 (2008) Article DOI: 10.1021/jm7015649 BindingDB Entry DOI: 10.7270/Q22R3SJN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 2 (Homo sapiens (Human)) | BDBM50376371 (CHEMBL264966) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | 5.00E+4 | n/a | n/a | n/a | 7.0 | n/a |
Johnson& Johnson Pharmaceutical Research& Development Curated by ChEMBL | Assay Description Binding affinity to human carbonic anhydrase 2 in presence of 10 mM PIPES without Na+ at pH 7.0 by ThermoFluor method | J Med Chem 51: 2518-21 (2008) Article DOI: 10.1021/jm7015649 BindingDB Entry DOI: 10.7270/Q22R3SJN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 2 (Homo sapiens (Human)) | BDBM50376374 (CHEMBL26303) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | 200 | n/a | n/a | n/a | 7.0 | n/a |
Johnson& Johnson Pharmaceutical Research& Development Curated by ChEMBL | Assay Description Binding affinity to human carbonic anhydrase 2 in presence of 10 mM PIPES without Na+ at pH 7.0 by ThermoFluor method | J Med Chem 51: 2518-21 (2008) Article DOI: 10.1021/jm7015649 BindingDB Entry DOI: 10.7270/Q22R3SJN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 2 (Homo sapiens (Human)) | BDBM50376375 (CHEMBL412256) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | 3.30E+4 | n/a | n/a | n/a | 7.0 | n/a |
Johnson& Johnson Pharmaceutical Research& Development Curated by ChEMBL | Assay Description Binding affinity to human carbonic anhydrase 2 in presence of 10 mM PIPES and 200 uM NaCl at pH 7.0 by ThermoFluor method | J Med Chem 51: 2518-21 (2008) Article DOI: 10.1021/jm7015649 BindingDB Entry DOI: 10.7270/Q22R3SJN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 2 (Homo sapiens (Human)) | BDBM50376370 (CHEMBL410329) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | 900 | n/a | n/a | n/a | 7.5 | n/a |
Johnson& Johnson Pharmaceutical Research& Development Curated by ChEMBL | Assay Description Binding affinity to human carbonic anhydrase 2 in presence of 10 mM HEPES and 100 uM Na2SO4 at pH 7.5 by ThermoFluor method | J Med Chem 51: 2518-21 (2008) Article DOI: 10.1021/jm7015649 BindingDB Entry DOI: 10.7270/Q22R3SJN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 2 (Homo sapiens (Human)) | BDBM50376375 (CHEMBL412256) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | >5.70E+4 | n/a | n/a | n/a | 7.5 | n/a |
Johnson& Johnson Pharmaceutical Research& Development Curated by ChEMBL | Assay Description Binding affinity to human carbonic anhydrase 2 in presence of 10 mM HEPES and 100 uM Na2SO4 at pH 7.5 by ThermoFluor method | J Med Chem 51: 2518-21 (2008) Article DOI: 10.1021/jm7015649 BindingDB Entry DOI: 10.7270/Q22R3SJN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 2 (Homo sapiens (Human)) | BDBM50376369 (CHEMBL410328) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | >5.70E+4 | n/a | n/a | n/a | 7.5 | n/a |
Johnson& Johnson Pharmaceutical Research& Development Curated by ChEMBL | Assay Description Binding affinity to human carbonic anhydrase 2 in presence of 10 mM HEPES without Na+ at pH 7.5 by ThermoFluor method | J Med Chem 51: 2518-21 (2008) Article DOI: 10.1021/jm7015649 BindingDB Entry DOI: 10.7270/Q22R3SJN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 2 (Homo sapiens (Human)) | BDBM50376368 (CHEMBL26780) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | 700 | n/a | n/a | n/a | 7.0 | n/a |
Johnson& Johnson Pharmaceutical Research& Development Curated by ChEMBL | Assay Description Binding affinity to human carbonic anhydrase 2 in presence of 10 mM PIPES and 100 uM Na2SO4 at pH 7.0 by ThermoFluor method | J Med Chem 51: 2518-21 (2008) Article DOI: 10.1021/jm7015649 BindingDB Entry DOI: 10.7270/Q22R3SJN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 2 (Homo sapiens (Human)) | BDBM50376370 (CHEMBL410329) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | 800 | n/a | n/a | n/a | 7.0 | n/a |
Johnson& Johnson Pharmaceutical Research& Development Curated by ChEMBL | Assay Description Binding affinity to human carbonic anhydrase 2 in presence of 10 mM PIPES and 200 uM NaCl at pH 7.0 by ThermoFluor method | J Med Chem 51: 2518-21 (2008) Article DOI: 10.1021/jm7015649 BindingDB Entry DOI: 10.7270/Q22R3SJN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 2 (Homo sapiens (Human)) | BDBM50376368 (CHEMBL26780) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | 400 | n/a | n/a | n/a | 7.0 | n/a |
Johnson& Johnson Pharmaceutical Research& Development Curated by ChEMBL | Assay Description Binding affinity to human carbonic anhydrase 2 in presence of 10 mM PIPES without Na+ at pH 7.0 by ThermoFluor method | J Med Chem 51: 2518-21 (2008) Article DOI: 10.1021/jm7015649 BindingDB Entry DOI: 10.7270/Q22R3SJN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 2 (Homo sapiens (Human)) | BDBM50376373 (CHEMBL260866) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | >5.70E+4 | n/a | n/a | n/a | 7.0 | n/a |
Johnson& Johnson Pharmaceutical Research& Development Curated by ChEMBL | Assay Description Binding affinity to human carbonic anhydrase 2 in presence of 10 mM PIPES and 100 uM Na2SO4 at pH 7.0 by ThermoFluor method | J Med Chem 51: 2518-21 (2008) Article DOI: 10.1021/jm7015649 BindingDB Entry DOI: 10.7270/Q22R3SJN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 2 (Homo sapiens (Human)) | BDBM50376370 (CHEMBL410329) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | 800 | n/a | n/a | n/a | 7.0 | n/a |
Johnson& Johnson Pharmaceutical Research& Development Curated by ChEMBL | Assay Description Binding affinity to human carbonic anhydrase 2 in presence of 10 mM PIPES without Na+ at pH 7.0 by ThermoFluor method | J Med Chem 51: 2518-21 (2008) Article DOI: 10.1021/jm7015649 BindingDB Entry DOI: 10.7270/Q22R3SJN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 2 (Homo sapiens (Human)) | BDBM50376374 (CHEMBL26303) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | 300 | n/a | n/a | n/a | 7.0 | n/a |
Johnson& Johnson Pharmaceutical Research& Development Curated by ChEMBL | Assay Description Binding affinity to human carbonic anhydrase 2 in presence of 10 mM PIPES and 200 uM NaCl at pH 7.0 by ThermoFluor method | J Med Chem 51: 2518-21 (2008) Article DOI: 10.1021/jm7015649 BindingDB Entry DOI: 10.7270/Q22R3SJN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 2 (Homo sapiens (Human)) | BDBM50376374 (CHEMBL26303) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | 200 | n/a | n/a | n/a | 7.5 | n/a |
Johnson& Johnson Pharmaceutical Research& Development Curated by ChEMBL | Assay Description Binding affinity to human carbonic anhydrase 2 in presence of 10 mM HEPES without Na+ at pH 7.5 by ThermoFluor method | J Med Chem 51: 2518-21 (2008) Article DOI: 10.1021/jm7015649 BindingDB Entry DOI: 10.7270/Q22R3SJN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 2 (Homo sapiens (Human)) | BDBM50376370 (CHEMBL410329) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | 500 | n/a | n/a | n/a | 7.5 | n/a |
Johnson& Johnson Pharmaceutical Research& Development Curated by ChEMBL | Assay Description Binding affinity to human carbonic anhydrase 2 in presence of 10 mM HEPES without Na+ at pH 7.5 by ThermoFluor method | J Med Chem 51: 2518-21 (2008) Article DOI: 10.1021/jm7015649 BindingDB Entry DOI: 10.7270/Q22R3SJN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 2 (Homo sapiens (Human)) | BDBM50376371 (CHEMBL264966) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | 5.00E+4 | n/a | n/a | n/a | 7.5 | n/a |
Johnson& Johnson Pharmaceutical Research& Development Curated by ChEMBL | Assay Description Binding affinity to human carbonic anhydrase 2 in presence of 10 mM HEPES and 100 uM Na2SO4 at pH 7.5 by ThermoFluor method | J Med Chem 51: 2518-21 (2008) Article DOI: 10.1021/jm7015649 BindingDB Entry DOI: 10.7270/Q22R3SJN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 2 (Homo sapiens (Human)) | BDBM50376373 (CHEMBL260866) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | >5.70E+4 | n/a | n/a | n/a | 7.0 | n/a |
Johnson& Johnson Pharmaceutical Research& Development Curated by ChEMBL | Assay Description Binding affinity to human carbonic anhydrase 2 in presence of 10 mM PIPES and 200 uM NaCl at pH 7.0 by ThermoFluor method | J Med Chem 51: 2518-21 (2008) Article DOI: 10.1021/jm7015649 BindingDB Entry DOI: 10.7270/Q22R3SJN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 2 (Homo sapiens (Human)) | BDBM50376368 (CHEMBL26780) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | 700 | n/a | n/a | n/a | 7.0 | n/a |
Johnson& Johnson Pharmaceutical Research& Development Curated by ChEMBL | Assay Description Binding affinity to human carbonic anhydrase 2 in presence of 10 mM PIPES and 200 uM NaCl at pH 7.0 by ThermoFluor method | J Med Chem 51: 2518-21 (2008) Article DOI: 10.1021/jm7015649 BindingDB Entry DOI: 10.7270/Q22R3SJN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 2 (Homo sapiens (Human)) | BDBM50376374 (CHEMBL26303) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | 300 | n/a | n/a | n/a | 7.0 | n/a |
Johnson& Johnson Pharmaceutical Research& Development Curated by ChEMBL | Assay Description Binding affinity to human carbonic anhydrase 2 in presence of 10 mM PIPES and 100 uM Na2SO4 at pH 7.0 by ThermoFluor method | J Med Chem 51: 2518-21 (2008) Article DOI: 10.1021/jm7015649 BindingDB Entry DOI: 10.7270/Q22R3SJN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 2 (Homo sapiens (Human)) | BDBM50376369 (CHEMBL410328) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | >5.70E+4 | n/a | n/a | n/a | 7.0 | n/a |
Johnson& Johnson Pharmaceutical Research& Development Curated by ChEMBL | Assay Description Binding affinity to human carbonic anhydrase 2 in presence of 10 mM PIPES and 100 uM Na2SO4 at pH 7.0 by ThermoFluor method | J Med Chem 51: 2518-21 (2008) Article DOI: 10.1021/jm7015649 BindingDB Entry DOI: 10.7270/Q22R3SJN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 2 (Homo sapiens (Human)) | BDBM13055 (CHEMBL283695 | Compound 8 | Topiramate Sulfamide A...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB PDB Article PubMed | n/a | n/a | n/a | 2.50E+4 | n/a | n/a | n/a | 7.5 | n/a |
Johnson& Johnson Pharmaceutical Research& Development Curated by ChEMBL | Assay Description Binding affinity to human carbonic anhydrase 2 in presence of 10 mM HEPES and 100 uM Na2SO4 at pH 7.5 by ThermoFluor method | J Med Chem 51: 2518-21 (2008) Article DOI: 10.1021/jm7015649 BindingDB Entry DOI: 10.7270/Q22R3SJN | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Carbonic anhydrase 2 (Homo sapiens (Human)) | BDBM50376372 (CHEMBL26590) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | 300 | n/a | n/a | n/a | 7.5 | n/a |
Johnson& Johnson Pharmaceutical Research& Development Curated by ChEMBL | Assay Description Binding affinity to human carbonic anhydrase 2 in presence of 10 mM HEPES and 100 uM Na2SO4 at pH 7.5 by ThermoFluor method | J Med Chem 51: 2518-21 (2008) Article DOI: 10.1021/jm7015649 BindingDB Entry DOI: 10.7270/Q22R3SJN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 2 (Homo sapiens (Human)) | BDBM50376375 (CHEMBL412256) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | 3.30E+4 | n/a | n/a | n/a | 7.0 | n/a |
Johnson& Johnson Pharmaceutical Research& Development Curated by ChEMBL | Assay Description Binding affinity to human carbonic anhydrase 2 in presence of 10 mM PIPES without Na+ at pH 7.0 by ThermoFluor method | J Med Chem 51: 2518-21 (2008) Article DOI: 10.1021/jm7015649 BindingDB Entry DOI: 10.7270/Q22R3SJN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||