

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM21975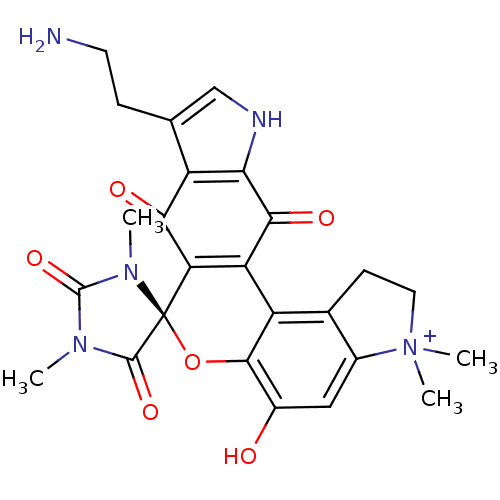 ((4S)-16'-(2-aminoethyl)-9'-hydroxy-1,3,6',6'-tetra...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 41 | -43.9 | n/a | n/a | n/a | n/a | n/a | 6.5 | 37 |
University of British Columbia | Assay Description The ability of the compounds prepared in this study to inhibit purified recombinant human IDO was evaluated with a steady state spectrophotometric as... | J Med Chem 51: 2634-7 (2008) Article DOI: 10.1021/jm800143h BindingDB Entry DOI: 10.7270/Q2MK6B65 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM21979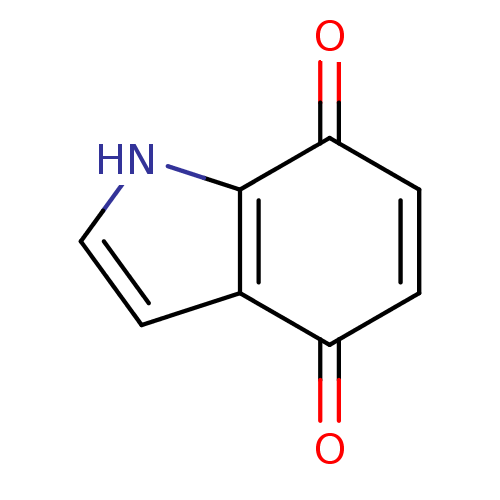 (4,7-dihydro-1H-indole-4,7-dione | Indolequinone, 1...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents | Article PubMed | 190 | -39.9 | n/a | n/a | n/a | n/a | n/a | 6.5 | 37 |
University of British Columbia | Assay Description The ability of the compounds prepared in this study to inhibit purified recombinant human IDO was evaluated with a steady state spectrophotometric as... | J Med Chem 51: 2634-7 (2008) Article DOI: 10.1021/jm800143h BindingDB Entry DOI: 10.7270/Q2MK6B65 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM21982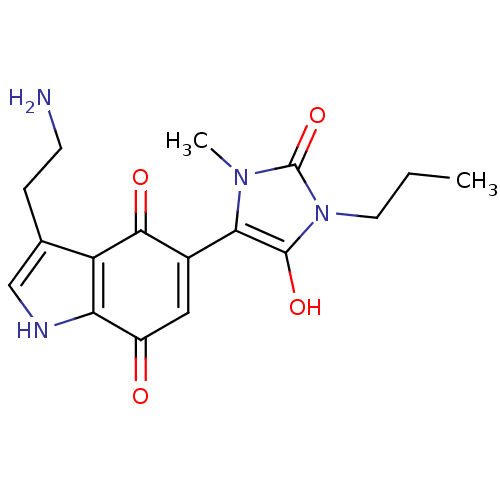 (3-(2-aminoethyl)-5-(3-methyl-2,5-dioxo-1-propylimi...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 200 | -39.8 | n/a | n/a | n/a | n/a | n/a | 6.5 | 37 |
University of British Columbia | Assay Description The ability of the compounds prepared in this study to inhibit purified recombinant human IDO was evaluated with a steady state spectrophotometric as... | J Med Chem 51: 2634-7 (2008) Article DOI: 10.1021/jm800143h BindingDB Entry DOI: 10.7270/Q2MK6B65 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM21981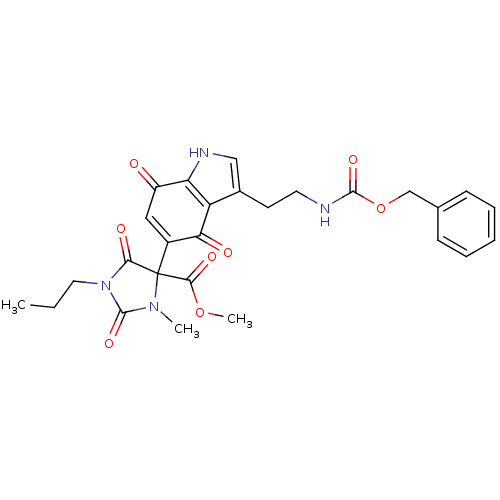 (Tryptamine quinone, 21 | methyl 4-[3-(2-{[(benzylo...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 260 | -39.1 | n/a | n/a | n/a | n/a | n/a | 6.5 | 37 |
University of British Columbia | Assay Description The ability of the compounds prepared in this study to inhibit purified recombinant human IDO was evaluated with a steady state spectrophotometric as... | J Med Chem 51: 2634-7 (2008) Article DOI: 10.1021/jm800143h BindingDB Entry DOI: 10.7270/Q2MK6B65 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM21984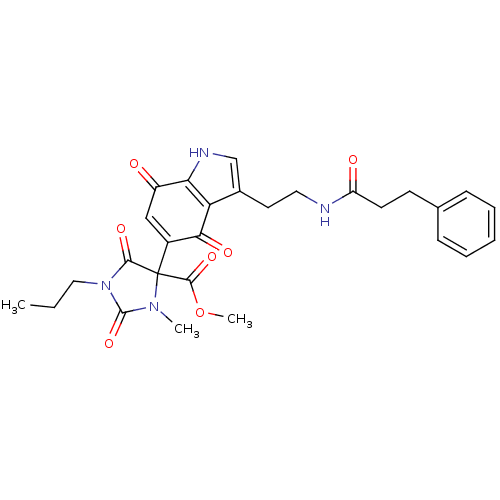 (Tryptamine quinone, 25 | methyl 4-{4,7-dioxo-3-[2-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 260 | -39.1 | n/a | n/a | n/a | n/a | n/a | 6.5 | 37 |
University of British Columbia | Assay Description The ability of the compounds prepared in this study to inhibit purified recombinant human IDO was evaluated with a steady state spectrophotometric as... | J Med Chem 51: 2634-7 (2008) Article DOI: 10.1021/jm800143h BindingDB Entry DOI: 10.7270/Q2MK6B65 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM21980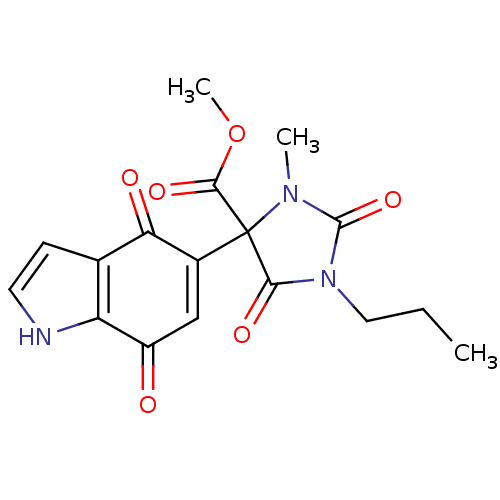 (Indolequinone, 20 | methyl 4-(4,7-dioxo-4,7-dihydr...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 420 | -37.9 | n/a | n/a | n/a | n/a | n/a | 6.5 | 37 |
University of British Columbia | Assay Description The ability of the compounds prepared in this study to inhibit purified recombinant human IDO was evaluated with a steady state spectrophotometric as... | J Med Chem 51: 2634-7 (2008) Article DOI: 10.1021/jm800143h BindingDB Entry DOI: 10.7270/Q2MK6B65 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM21976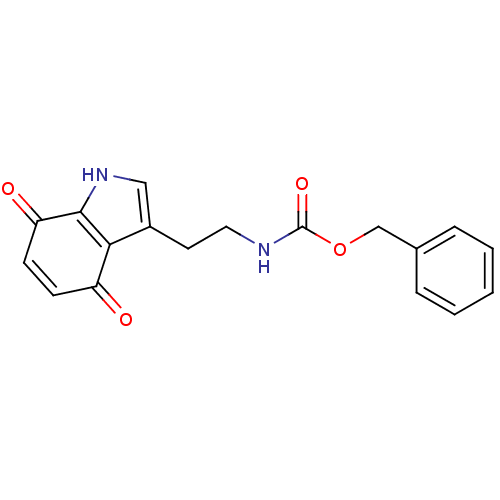 (Tryptamine quinone, 9 | benzyl N-[2-(4,7-dioxo-4,7...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.49E+3 | -34.6 | n/a | n/a | n/a | n/a | n/a | 6.5 | 37 |
University of British Columbia | Assay Description The ability of the compounds prepared in this study to inhibit purified recombinant human IDO was evaluated with a steady state spectrophotometric as... | J Med Chem 51: 2634-7 (2008) Article DOI: 10.1021/jm800143h BindingDB Entry DOI: 10.7270/Q2MK6B65 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM21977 (Tryptamine quinone, 13 | benzyl N-{2-[5-(1,3-dioxo...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.09E+4 | -29.5 | n/a | n/a | n/a | n/a | n/a | 6.5 | 37 |
University of British Columbia | Assay Description The ability of the compounds prepared in this study to inhibit purified recombinant human IDO was evaluated with a steady state spectrophotometric as... | J Med Chem 51: 2634-7 (2008) Article DOI: 10.1021/jm800143h BindingDB Entry DOI: 10.7270/Q2MK6B65 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM21973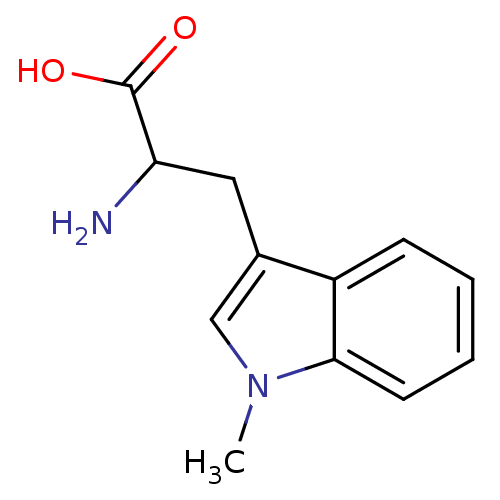 (1-Methyltryptophan, 1 | 2-amino-3-(1-methyl-1H-ind...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 6.20E+4 | -25.0 | n/a | n/a | n/a | n/a | n/a | 6.5 | 37 |
University of British Columbia | Assay Description The ability of the compounds prepared in this study to inhibit purified recombinant human IDO was evaluated with a steady state spectrophotometric as... | J Med Chem 51: 2634-7 (2008) Article DOI: 10.1021/jm800143h BindingDB Entry DOI: 10.7270/Q2MK6B65 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM21978 (1H-indol-4-ol | 4-hydroxyindole, 18) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | n/a | n/a | n/a | 6.5 | 37 |
University of British Columbia | Assay Description The ability of the compounds prepared in this study to inhibit purified recombinant human IDO was evaluated with a steady state spectrophotometric as... | J Med Chem 51: 2634-7 (2008) Article DOI: 10.1021/jm800143h BindingDB Entry DOI: 10.7270/Q2MK6B65 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM21983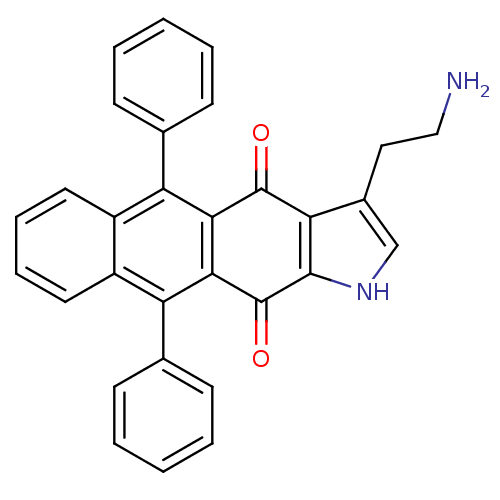 (3-(2-aminoethyl)-5,10-diphenyl-1H,4H,11H-anthracen...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | n/a | n/a | n/a | 6.5 | 37 |
University of British Columbia | Assay Description The ability of the compounds prepared in this study to inhibit purified recombinant human IDO was evaluated with a steady state spectrophotometric as... | J Med Chem 51: 2634-7 (2008) Article DOI: 10.1021/jm800143h BindingDB Entry DOI: 10.7270/Q2MK6B65 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||