 Found 28 hits of Enzyme Inhibition Constant Data
Found 28 hits of Enzyme Inhibition Constant Data Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Proto-oncogene tyrosine-protein kinase Src
(Homo sapiens (Human)) | BDBM4552

(4-[(2,4-Dichloro-5-methoxyphenyl)amino]-6-methoxy-...)Show SMILES COc1cc(Nc2c(cnc3cc(OCCCN4CCN(C)CC4)c(OC)cc23)C#N)c(Cl)cc1Cl Show InChI InChI=1S/C26H29Cl2N5O3/c1-32-6-8-33(9-7-32)5-4-10-36-25-13-21-18(11-24(25)35-3)26(17(15-29)16-30-21)31-22-14-23(34-2)20(28)12-19(22)27/h11-14,16H,4-10H2,1-3H3,(H,30,31) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
Article
PubMed
| n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University
Curated by ChEMBL
| Assay Description
Inhibition of cSrc (unknown origin) by ELISA |
Bioorg Med Chem Lett 18: 6206-9 (2008)
Article DOI: 10.1016/j.bmcl.2008.10.006
BindingDB Entry DOI: 10.7270/Q2K0743J |
More data for this
Ligand-Target Pair | |
Proto-oncogene tyrosine-protein kinase Src
(Homo sapiens (Human)) | BDBM4552

(4-[(2,4-Dichloro-5-methoxyphenyl)amino]-6-methoxy-...)Show SMILES COc1cc(Nc2c(cnc3cc(OCCCN4CCN(C)CC4)c(OC)cc23)C#N)c(Cl)cc1Cl Show InChI InChI=1S/C26H29Cl2N5O3/c1-32-6-8-33(9-7-32)5-4-10-36-25-13-21-18(11-24(25)35-3)26(17(15-29)16-30-21)31-22-14-23(34-2)20(28)12-19(22)27/h11-14,16H,4-10H2,1-3H3,(H,30,31) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
Article
PubMed
| n/a | n/a | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University
Curated by ChEMBL
| Assay Description
Inhibition of cSrc (unknown origin) |
Bioorg Med Chem Lett 18: 6206-9 (2008)
Article DOI: 10.1016/j.bmcl.2008.10.006
BindingDB Entry DOI: 10.7270/Q2K0743J |
More data for this
Ligand-Target Pair | |
Proto-oncogene tyrosine-protein kinase Src
(Homo sapiens (Human)) | BDBM50271896
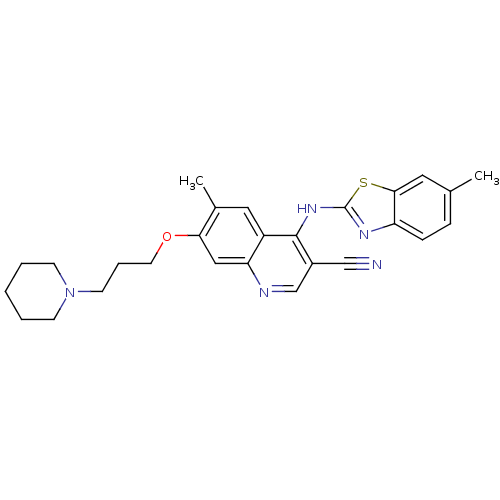
(6-Methyl-7-[3-(piperidin-1-yl)-propoxy]-4-(6-methy...)Show SMILES Cc1ccc2nc(Nc3c(cnc4cc(OCCCN5CCCCC5)c(C)cc34)C#N)sc2c1 Show InChI InChI=1S/C27H29N5OS/c1-18-7-8-22-25(13-18)34-27(30-22)31-26-20(16-28)17-29-23-15-24(19(2)14-21(23)26)33-12-6-11-32-9-4-3-5-10-32/h7-8,13-15,17H,3-6,9-12H2,1-2H3,(H,29,30,31) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 15.4 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University
Curated by ChEMBL
| Assay Description
Inhibition of cSrc (unknown origin) |
Bioorg Med Chem Lett 18: 6206-9 (2008)
Article DOI: 10.1016/j.bmcl.2008.10.006
BindingDB Entry DOI: 10.7270/Q2K0743J |
More data for this
Ligand-Target Pair | |
Proto-oncogene tyrosine-protein kinase Src
(Homo sapiens (Human)) | BDBM50275877

(4-(2-aminobenzo[d]thiazol-6-ylamino)-6-methoxy-7-(...)Show SMILES COc1cc2c(Nc3ccc4nc(N)sc4c3)c(cnc2cc1OCCCN1CCNCC1)C#N Show InChI InChI=1S/C25H27N7O2S/c1-33-21-12-18-20(13-22(21)34-10-2-7-32-8-5-28-6-9-32)29-15-16(14-26)24(18)30-17-3-4-19-23(11-17)35-25(27)31-19/h3-4,11-13,15,28H,2,5-10H2,1H3,(H2,27,31)(H,29,30) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 20.7 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University
Curated by ChEMBL
| Assay Description
Inhibition of cSrc (unknown origin) |
Bioorg Med Chem Lett 18: 6206-9 (2008)
Article DOI: 10.1016/j.bmcl.2008.10.006
BindingDB Entry DOI: 10.7270/Q2K0743J |
More data for this
Ligand-Target Pair | |
Proto-oncogene tyrosine-protein kinase Src
(Homo sapiens (Human)) | BDBM50275756
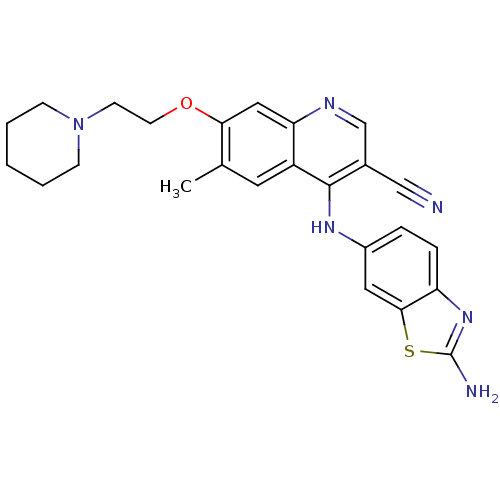
(4-(2-aminobenzo[d]thiazol-6-ylamino)-6-methyl-7-(2...)Show SMILES Cc1cc2c(Nc3ccc4nc(N)sc4c3)c(cnc2cc1OCCN1CCCCC1)C#N Show InChI InChI=1S/C25H26N6OS/c1-16-11-19-21(13-22(16)32-10-9-31-7-3-2-4-8-31)28-15-17(14-26)24(19)29-18-5-6-20-23(12-18)33-25(27)30-20/h5-6,11-13,15H,2-4,7-10H2,1H3,(H2,27,30)(H,28,29) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 34.8 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University
Curated by ChEMBL
| Assay Description
Inhibition of cSrc (unknown origin) |
Bioorg Med Chem Lett 18: 6206-9 (2008)
Article DOI: 10.1016/j.bmcl.2008.10.006
BindingDB Entry DOI: 10.7270/Q2K0743J |
More data for this
Ligand-Target Pair | |
Proto-oncogene tyrosine-protein kinase Src
(Homo sapiens (Human)) | BDBM50275812
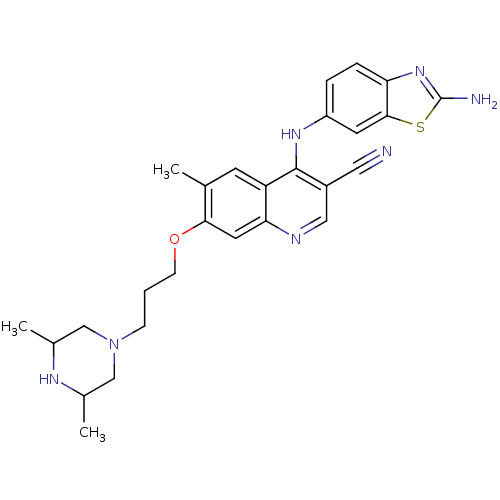
(4-(2-aminobenzo[d]thiazol-6-ylamino)-7-(3-(3,5-dim...)Show SMILES CC1CN(CCCOc2cc3ncc(C#N)c(Nc4ccc5nc(N)sc5c4)c3cc2C)CC(C)N1 Show InChI InChI=1S/C27H31N7OS/c1-16-9-21-23(11-24(16)35-8-4-7-34-14-17(2)31-18(3)15-34)30-13-19(12-28)26(21)32-20-5-6-22-25(10-20)36-27(29)33-22/h5-6,9-11,13,17-18,31H,4,7-8,14-15H2,1-3H3,(H2,29,33)(H,30,32) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 36.6 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University
Curated by ChEMBL
| Assay Description
Inhibition of cSrc (unknown origin) |
Bioorg Med Chem Lett 18: 6206-9 (2008)
Article DOI: 10.1016/j.bmcl.2008.10.006
BindingDB Entry DOI: 10.7270/Q2K0743J |
More data for this
Ligand-Target Pair | |
Proto-oncogene tyrosine-protein kinase Src
(Homo sapiens (Human)) | BDBM50275813
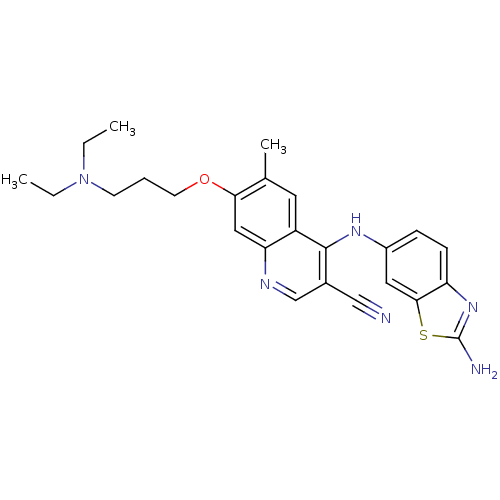
(4-(2-aminobenzo[d]thiazol-6-ylamino)-7-(3-(diethyl...)Show SMILES CCN(CC)CCCOc1cc2ncc(C#N)c(Nc3ccc4nc(N)sc4c3)c2cc1C Show InChI InChI=1S/C25H28N6OS/c1-4-31(5-2)9-6-10-32-22-13-21-19(11-16(22)3)24(17(14-26)15-28-21)29-18-7-8-20-23(12-18)33-25(27)30-20/h7-8,11-13,15H,4-6,9-10H2,1-3H3,(H2,27,30)(H,28,29) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 42 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University
Curated by ChEMBL
| Assay Description
Inhibition of cSrc (unknown origin) |
Bioorg Med Chem Lett 18: 6206-9 (2008)
Article DOI: 10.1016/j.bmcl.2008.10.006
BindingDB Entry DOI: 10.7270/Q2K0743J |
More data for this
Ligand-Target Pair | |
Proto-oncogene tyrosine-protein kinase Src
(Homo sapiens (Human)) | BDBM50275757
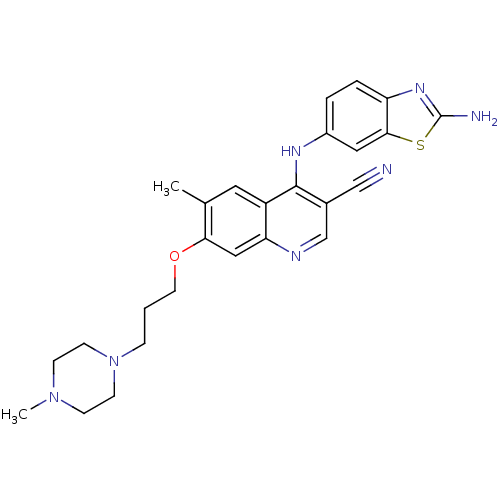
(4-(2-aminobenzo[d]thiazol-6-ylamino)-6-methyl-7-(3...)Show SMILES CN1CCN(CCCOc2cc3ncc(C#N)c(Nc4ccc5nc(N)sc5c4)c3cc2C)CC1 Show InChI InChI=1S/C26H29N7OS/c1-17-12-20-22(14-23(17)34-11-3-6-33-9-7-32(2)8-10-33)29-16-18(15-27)25(20)30-19-4-5-21-24(13-19)35-26(28)31-21/h4-5,12-14,16H,3,6-11H2,1-2H3,(H2,28,31)(H,29,30) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 50.6 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University
Curated by ChEMBL
| Assay Description
Inhibition of cSrc (unknown origin) |
Bioorg Med Chem Lett 18: 6206-9 (2008)
Article DOI: 10.1016/j.bmcl.2008.10.006
BindingDB Entry DOI: 10.7270/Q2K0743J |
More data for this
Ligand-Target Pair | |
Proto-oncogene tyrosine-protein kinase Src
(Homo sapiens (Human)) | BDBM50275811
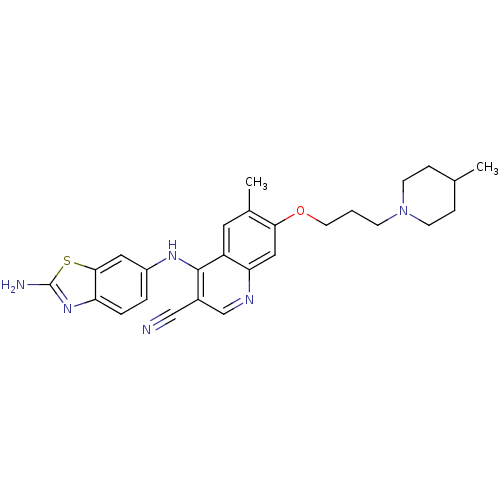
(4-(2-aminobenzo[d]thiazol-6-ylamino)-6-methyl-7-(3...)Show SMILES CC1CCN(CCCOc2cc3ncc(C#N)c(Nc4ccc5nc(N)sc5c4)c3cc2C)CC1 Show InChI InChI=1S/C27H30N6OS/c1-17-6-9-33(10-7-17)8-3-11-34-24-14-23-21(12-18(24)2)26(19(15-28)16-30-23)31-20-4-5-22-25(13-20)35-27(29)32-22/h4-5,12-14,16-17H,3,6-11H2,1-2H3,(H2,29,32)(H,30,31) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 53.4 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University
Curated by ChEMBL
| Assay Description
Inhibition of cSrc (unknown origin) |
Bioorg Med Chem Lett 18: 6206-9 (2008)
Article DOI: 10.1016/j.bmcl.2008.10.006
BindingDB Entry DOI: 10.7270/Q2K0743J |
More data for this
Ligand-Target Pair | |
Proto-oncogene tyrosine-protein kinase Src
(Homo sapiens (Human)) | BDBM50275758
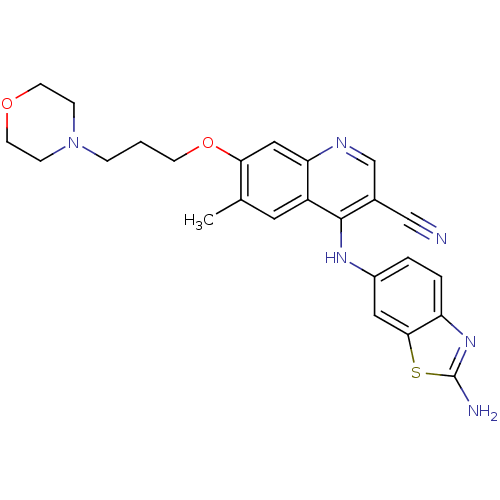
(4-(2-aminobenzo[d]thiazol-6-ylamino)-6-methyl-7-(3...)Show SMILES Cc1cc2c(Nc3ccc4nc(N)sc4c3)c(cnc2cc1OCCCN1CCOCC1)C#N Show InChI InChI=1S/C25H26N6O2S/c1-16-11-19-21(13-22(16)33-8-2-5-31-6-9-32-10-7-31)28-15-17(14-26)24(19)29-18-3-4-20-23(12-18)34-25(27)30-20/h3-4,11-13,15H,2,5-10H2,1H3,(H2,27,30)(H,28,29) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 81.1 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University
Curated by ChEMBL
| Assay Description
Inhibition of cSrc (unknown origin) |
Bioorg Med Chem Lett 18: 6206-9 (2008)
Article DOI: 10.1016/j.bmcl.2008.10.006
BindingDB Entry DOI: 10.7270/Q2K0743J |
More data for this
Ligand-Target Pair | |
Proto-oncogene tyrosine-protein kinase Src
(Homo sapiens (Human)) | BDBM50275759

(4-(2-aminobenzo[d]thiazol-6-ylamino)-6-methyl-7-(3...)Show SMILES Cc1cc2c(Nc3ccc4nc(N)sc4c3)c(cnc2cc1OCCCN1CCNCC1)C#N Show InChI InChI=1S/C25H27N7OS/c1-16-11-19-21(13-22(16)33-10-2-7-32-8-5-28-6-9-32)29-15-17(14-26)24(19)30-18-3-4-20-23(12-18)34-25(27)31-20/h3-4,11-13,15,28H,2,5-10H2,1H3,(H2,27,31)(H,29,30) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 129 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University
Curated by ChEMBL
| Assay Description
Inhibition of cSrc (unknown origin) |
Bioorg Med Chem Lett 18: 6206-9 (2008)
Article DOI: 10.1016/j.bmcl.2008.10.006
BindingDB Entry DOI: 10.7270/Q2K0743J |
More data for this
Ligand-Target Pair | |
Proto-oncogene tyrosine-protein kinase Src
(Homo sapiens (Human)) | BDBM50275875
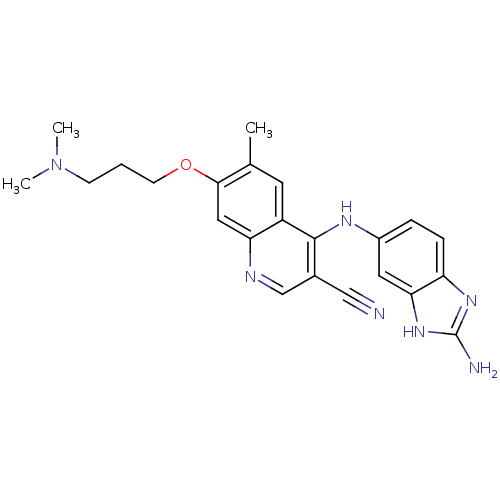
(4-(2-amino-1H-benzo[d]imidazol-6-ylamino)-7-(3-(di...)Show SMILES CN(C)CCCOc1cc2ncc(C#N)c(Nc3ccc4nc(N)[nH]c4c3)c2cc1C Show InChI InChI=1S/C23H25N7O/c1-14-9-17-19(11-21(14)31-8-4-7-30(2)3)26-13-15(12-24)22(17)27-16-5-6-18-20(10-16)29-23(25)28-18/h5-6,9-11,13H,4,7-8H2,1-3H3,(H,26,27)(H3,25,28,29) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 143 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University
Curated by ChEMBL
| Assay Description
Inhibition of cSrc (unknown origin) |
Bioorg Med Chem Lett 18: 6206-9 (2008)
Article DOI: 10.1016/j.bmcl.2008.10.006
BindingDB Entry DOI: 10.7270/Q2K0743J |
More data for this
Ligand-Target Pair | |
Proto-oncogene tyrosine-protein kinase Src
(Homo sapiens (Human)) | BDBM50275876

(4-(2-amino-1H-benzo[d]imidazol-6-ylamino)-6-methyl...)Show SMILES Cc1cc2c(Nc3ccc4nc(N)[nH]c4c3)c(cnc2cc1OCCCN1CCCC1)C#N Show InChI InChI=1S/C25H27N7O/c1-16-11-19-21(13-23(16)33-10-4-9-32-7-2-3-8-32)28-15-17(14-26)24(19)29-18-5-6-20-22(12-18)31-25(27)30-20/h5-6,11-13,15H,2-4,7-10H2,1H3,(H,28,29)(H3,27,30,31) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 159 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University
Curated by ChEMBL
| Assay Description
Inhibition of cSrc (unknown origin) |
Bioorg Med Chem Lett 18: 6206-9 (2008)
Article DOI: 10.1016/j.bmcl.2008.10.006
BindingDB Entry DOI: 10.7270/Q2K0743J |
More data for this
Ligand-Target Pair | |
Proto-oncogene tyrosine-protein kinase Src
(Homo sapiens (Human)) | BDBM50275814
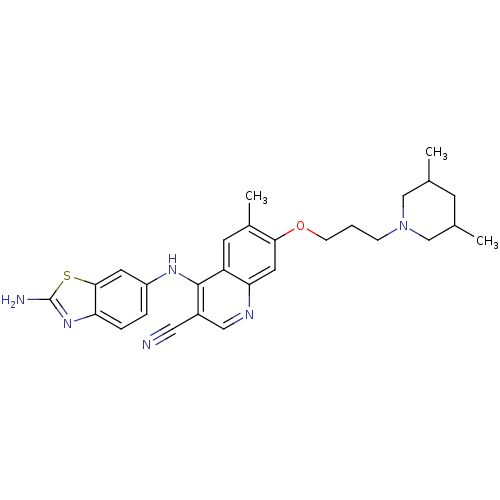
(4-(2-aminobenzo[d]thiazol-6-ylamino)-7-(3-(3,5-dim...)Show SMILES CC1CC(C)CN(CCCOc2cc3ncc(C#N)c(Nc4ccc5nc(N)sc5c4)c3cc2C)C1 Show InChI InChI=1S/C28H32N6OS/c1-17-9-18(2)16-34(15-17)7-4-8-35-25-12-24-22(10-19(25)3)27(20(13-29)14-31-24)32-21-5-6-23-26(11-21)36-28(30)33-23/h5-6,10-12,14,17-18H,4,7-9,15-16H2,1-3H3,(H2,30,33)(H,31,32) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 267 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University
Curated by ChEMBL
| Assay Description
Inhibition of cSrc (unknown origin) |
Bioorg Med Chem Lett 18: 6206-9 (2008)
Article DOI: 10.1016/j.bmcl.2008.10.006
BindingDB Entry DOI: 10.7270/Q2K0743J |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, inducible
(Mus musculus (mouse)) | BDBM50275814

(4-(2-aminobenzo[d]thiazol-6-ylamino)-7-(3-(3,5-dim...)Show SMILES CC1CC(C)CN(CCCOc2cc3ncc(C#N)c(Nc4ccc5nc(N)sc5c4)c3cc2C)C1 Show InChI InChI=1S/C28H32N6OS/c1-17-9-18(2)16-34(15-17)7-4-8-35-25-12-24-22(10-19(25)3)27(20(13-29)14-31-24)32-21-5-6-23-26(11-21)36-28(30)33-23/h5-6,10-12,14,17-18H,4,7-9,15-16H2,1-3H3,(H2,30,33)(H,31,32) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.05E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University
Curated by ChEMBL
| Assay Description
Inhibition of iNOS in mouse ANA1 cells |
Bioorg Med Chem Lett 18: 6206-9 (2008)
Article DOI: 10.1016/j.bmcl.2008.10.006
BindingDB Entry DOI: 10.7270/Q2K0743J |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, inducible
(Mus musculus (mouse)) | BDBM50275811

(4-(2-aminobenzo[d]thiazol-6-ylamino)-6-methyl-7-(3...)Show SMILES CC1CCN(CCCOc2cc3ncc(C#N)c(Nc4ccc5nc(N)sc5c4)c3cc2C)CC1 Show InChI InChI=1S/C27H30N6OS/c1-17-6-9-33(10-7-17)8-3-11-34-24-14-23-21(12-18(24)2)26(19(15-28)16-30-23)31-20-4-5-22-25(13-20)35-27(29)32-22/h4-5,12-14,16-17H,3,6-11H2,1-2H3,(H2,29,32)(H,30,31) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.27E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University
Curated by ChEMBL
| Assay Description
Inhibition of iNOS in mouse ANA1 cells |
Bioorg Med Chem Lett 18: 6206-9 (2008)
Article DOI: 10.1016/j.bmcl.2008.10.006
BindingDB Entry DOI: 10.7270/Q2K0743J |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, inducible
(Mus musculus (mouse)) | BDBM50275875

(4-(2-amino-1H-benzo[d]imidazol-6-ylamino)-7-(3-(di...)Show SMILES CN(C)CCCOc1cc2ncc(C#N)c(Nc3ccc4nc(N)[nH]c4c3)c2cc1C Show InChI InChI=1S/C23H25N7O/c1-14-9-17-19(11-21(14)31-8-4-7-30(2)3)26-13-15(12-24)22(17)27-16-5-6-18-20(10-16)29-23(25)28-18/h5-6,9-11,13H,4,7-8H2,1-3H3,(H,26,27)(H3,25,28,29) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.32E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University
Curated by ChEMBL
| Assay Description
Inhibition of iNOS in mouse ANA1 cells |
Bioorg Med Chem Lett 18: 6206-9 (2008)
Article DOI: 10.1016/j.bmcl.2008.10.006
BindingDB Entry DOI: 10.7270/Q2K0743J |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, inducible
(Mus musculus (mouse)) | BDBM50275759

(4-(2-aminobenzo[d]thiazol-6-ylamino)-6-methyl-7-(3...)Show SMILES Cc1cc2c(Nc3ccc4nc(N)sc4c3)c(cnc2cc1OCCCN1CCNCC1)C#N Show InChI InChI=1S/C25H27N7OS/c1-16-11-19-21(13-22(16)33-10-2-7-32-8-5-28-6-9-32)29-15-17(14-26)24(19)30-18-3-4-20-23(12-18)34-25(27)31-20/h3-4,11-13,15,28H,2,5-10H2,1H3,(H2,27,31)(H,29,30) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.73E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University
Curated by ChEMBL
| Assay Description
Inhibition of iNOS in mouse ANA1 cells |
Bioorg Med Chem Lett 18: 6206-9 (2008)
Article DOI: 10.1016/j.bmcl.2008.10.006
BindingDB Entry DOI: 10.7270/Q2K0743J |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, inducible
(Mus musculus (mouse)) | BDBM50240843
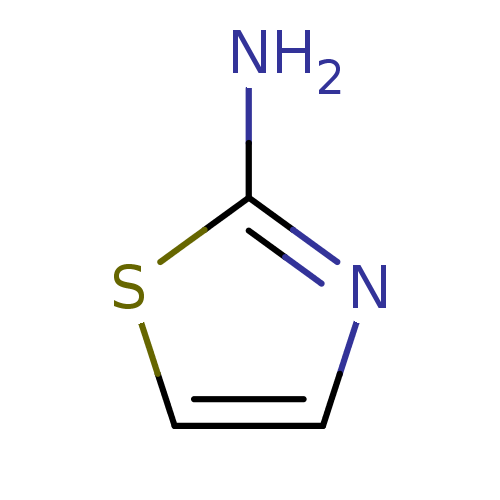
(2-Aminothiazole | Aminothiazole | Aminothiazoline ...)Show InChI InChI=1S/C3H4N2S/c4-3-5-1-2-6-3/h1-2H,(H2,4,5) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
| Article
PubMed
| n/a | n/a | 1.80E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University
Curated by ChEMBL
| Assay Description
Inhibition of iNOS in mouse ANA1 cells |
Bioorg Med Chem Lett 18: 6206-9 (2008)
Article DOI: 10.1016/j.bmcl.2008.10.006
BindingDB Entry DOI: 10.7270/Q2K0743J |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, inducible
(Mus musculus (mouse)) | BDBM50275876

(4-(2-amino-1H-benzo[d]imidazol-6-ylamino)-6-methyl...)Show SMILES Cc1cc2c(Nc3ccc4nc(N)[nH]c4c3)c(cnc2cc1OCCCN1CCCC1)C#N Show InChI InChI=1S/C25H27N7O/c1-16-11-19-21(13-23(16)33-10-4-9-32-7-2-3-8-32)28-15-17(14-26)24(19)29-18-5-6-20-22(12-18)31-25(27)30-20/h5-6,11-13,15H,2-4,7-10H2,1H3,(H,28,29)(H3,27,30,31) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.48E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University
Curated by ChEMBL
| Assay Description
Inhibition of iNOS in mouse ANA1 cells |
Bioorg Med Chem Lett 18: 6206-9 (2008)
Article DOI: 10.1016/j.bmcl.2008.10.006
BindingDB Entry DOI: 10.7270/Q2K0743J |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, inducible
(Mus musculus (mouse)) | BDBM50275756

(4-(2-aminobenzo[d]thiazol-6-ylamino)-6-methyl-7-(2...)Show SMILES Cc1cc2c(Nc3ccc4nc(N)sc4c3)c(cnc2cc1OCCN1CCCCC1)C#N Show InChI InChI=1S/C25H26N6OS/c1-16-11-19-21(13-22(16)32-10-9-31-7-3-2-4-8-31)28-15-17(14-26)24(19)29-18-5-6-20-23(12-18)33-25(27)30-20/h5-6,11-13,15H,2-4,7-10H2,1H3,(H2,27,30)(H,28,29) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.67E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University
Curated by ChEMBL
| Assay Description
Inhibition of iNOS in mouse ANA1 cells |
Bioorg Med Chem Lett 18: 6206-9 (2008)
Article DOI: 10.1016/j.bmcl.2008.10.006
BindingDB Entry DOI: 10.7270/Q2K0743J |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, inducible
(Mus musculus (mouse)) | BDBM50275758

(4-(2-aminobenzo[d]thiazol-6-ylamino)-6-methyl-7-(3...)Show SMILES Cc1cc2c(Nc3ccc4nc(N)sc4c3)c(cnc2cc1OCCCN1CCOCC1)C#N Show InChI InChI=1S/C25H26N6O2S/c1-16-11-19-21(13-22(16)33-8-2-5-31-6-9-32-10-7-31)28-15-17(14-26)24(19)29-18-3-4-20-23(12-18)34-25(27)30-20/h3-4,11-13,15H,2,5-10H2,1H3,(H2,27,30)(H,28,29) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.18E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University
Curated by ChEMBL
| Assay Description
Inhibition of iNOS in mouse ANA1 cells |
Bioorg Med Chem Lett 18: 6206-9 (2008)
Article DOI: 10.1016/j.bmcl.2008.10.006
BindingDB Entry DOI: 10.7270/Q2K0743J |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, inducible
(Mus musculus (mouse)) | BDBM50275757

(4-(2-aminobenzo[d]thiazol-6-ylamino)-6-methyl-7-(3...)Show SMILES CN1CCN(CCCOc2cc3ncc(C#N)c(Nc4ccc5nc(N)sc5c4)c3cc2C)CC1 Show InChI InChI=1S/C26H29N7OS/c1-17-12-20-22(14-23(17)34-11-3-6-33-9-7-32(2)8-10-33)29-16-18(15-27)25(20)30-19-4-5-21-24(13-19)35-26(28)31-21/h4-5,12-14,16H,3,6-11H2,1-2H3,(H2,28,31)(H,29,30) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.71E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University
Curated by ChEMBL
| Assay Description
Inhibition of iNOS in mouse ANA1 cells |
Bioorg Med Chem Lett 18: 6206-9 (2008)
Article DOI: 10.1016/j.bmcl.2008.10.006
BindingDB Entry DOI: 10.7270/Q2K0743J |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, inducible
(Mus musculus (mouse)) | BDBM50275813

(4-(2-aminobenzo[d]thiazol-6-ylamino)-7-(3-(diethyl...)Show SMILES CCN(CC)CCCOc1cc2ncc(C#N)c(Nc3ccc4nc(N)sc4c3)c2cc1C Show InChI InChI=1S/C25H28N6OS/c1-4-31(5-2)9-6-10-32-22-13-21-19(11-16(22)3)24(17(14-26)15-28-21)29-18-7-8-20-23(12-18)33-25(27)30-20/h7-8,11-13,15H,4-6,9-10H2,1-3H3,(H2,27,30)(H,28,29) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.68E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University
Curated by ChEMBL
| Assay Description
Inhibition of iNOS in mouse ANA1 cells |
Bioorg Med Chem Lett 18: 6206-9 (2008)
Article DOI: 10.1016/j.bmcl.2008.10.006
BindingDB Entry DOI: 10.7270/Q2K0743J |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, inducible
(Mus musculus (mouse)) | BDBM50271982
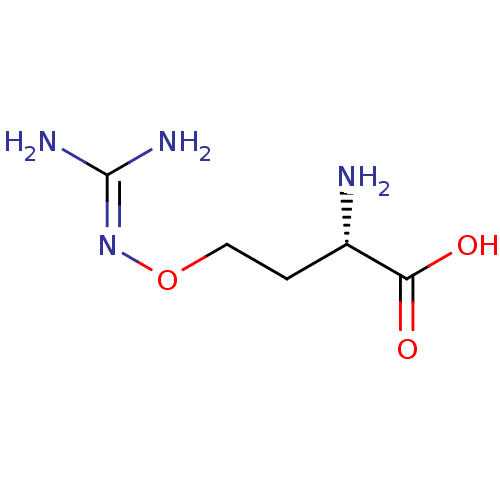
(CHEMBL443732 | L-canavanine)Show SMILES [#7]-[#6@@H](-[#6]-[#6]-[#8]\[#7]=[#6](\[#7])-[#7])-[#6](-[#8])=O |r| Show InChI InChI=1S/C5H12N4O3/c6-3(4(10)11)1-2-12-9-5(7)8/h3H,1-2,6H2,(H,10,11)(H4,7,8,9)/t3-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MMDB
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 6.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University
Curated by ChEMBL
| Assay Description
Inhibition of iNOS in mouse ANA1 cells |
Bioorg Med Chem Lett 18: 6206-9 (2008)
Article DOI: 10.1016/j.bmcl.2008.10.006
BindingDB Entry DOI: 10.7270/Q2K0743J |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, inducible
(Mus musculus (mouse)) | BDBM50275812

(4-(2-aminobenzo[d]thiazol-6-ylamino)-7-(3-(3,5-dim...)Show SMILES CC1CN(CCCOc2cc3ncc(C#N)c(Nc4ccc5nc(N)sc5c4)c3cc2C)CC(C)N1 Show InChI InChI=1S/C27H31N7OS/c1-16-9-21-23(11-24(16)35-8-4-7-34-14-17(2)31-18(3)15-34)30-13-19(12-28)26(21)32-20-5-6-22-25(10-20)36-27(29)33-22/h5-6,9-11,13,17-18,31H,4,7-8,14-15H2,1-3H3,(H2,29,33)(H,30,32) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 8.91E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University
Curated by ChEMBL
| Assay Description
Inhibition of iNOS in mouse ANA1 cells |
Bioorg Med Chem Lett 18: 6206-9 (2008)
Article DOI: 10.1016/j.bmcl.2008.10.006
BindingDB Entry DOI: 10.7270/Q2K0743J |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, inducible
(Mus musculus (mouse)) | BDBM50275877

(4-(2-aminobenzo[d]thiazol-6-ylamino)-6-methoxy-7-(...)Show SMILES COc1cc2c(Nc3ccc4nc(N)sc4c3)c(cnc2cc1OCCCN1CCNCC1)C#N Show InChI InChI=1S/C25H27N7O2S/c1-33-21-12-18-20(13-22(21)34-10-2-7-32-8-5-28-6-9-32)29-15-16(14-26)24(18)30-17-3-4-19-23(11-17)35-25(27)31-19/h3-4,11-13,15,28H,2,5-10H2,1H3,(H2,27,31)(H,29,30) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.05E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University
Curated by ChEMBL
| Assay Description
Inhibition of iNOS in mouse ANA1 cells |
Bioorg Med Chem Lett 18: 6206-9 (2008)
Article DOI: 10.1016/j.bmcl.2008.10.006
BindingDB Entry DOI: 10.7270/Q2K0743J |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, inducible
(Mus musculus (mouse)) | BDBM50271896

(6-Methyl-7-[3-(piperidin-1-yl)-propoxy]-4-(6-methy...)Show SMILES Cc1ccc2nc(Nc3c(cnc4cc(OCCCN5CCCCC5)c(C)cc34)C#N)sc2c1 Show InChI InChI=1S/C27H29N5OS/c1-18-7-8-22-25(13-18)34-27(30-22)31-26-20(16-28)17-29-23-15-24(19(2)14-21(23)26)33-12-6-11-32-9-4-3-5-10-32/h7-8,13-15,17H,3-6,9-12H2,1-2H3,(H,29,30,31) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.13E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University
Curated by ChEMBL
| Assay Description
Inhibition of iNOS in mouse ANA1 cells |
Bioorg Med Chem Lett 18: 6206-9 (2008)
Article DOI: 10.1016/j.bmcl.2008.10.006
BindingDB Entry DOI: 10.7270/Q2K0743J |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data