 Found 50 hits of Enzyme Inhibition Constant Data
Found 50 hits of Enzyme Inhibition Constant Data Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Bone morphogenetic protein 1
(Homo sapiens (Human)) | BDBM50241177
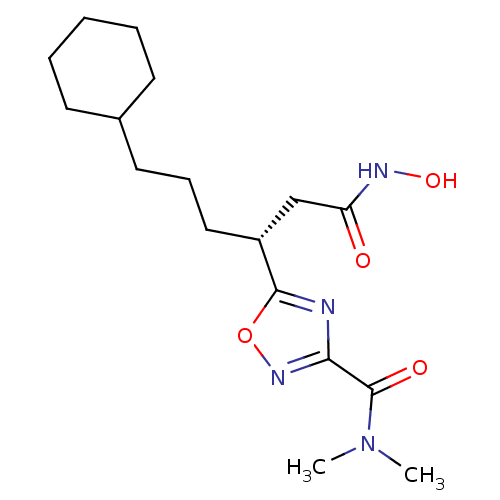
((R)-5-(6-cyclohexyl-1-(hydroxyamino)-1-oxohexan-3-...)Show SMILES CN(C)C(=O)c1noc(n1)[C@H](CCCC1CCCCC1)CC(=O)NO |r| Show InChI InChI=1S/C17H28N4O4/c1-21(2)17(23)15-18-16(25-20-15)13(11-14(22)19-24)10-6-9-12-7-4-3-5-8-12/h12-13,24H,3-11H2,1-2H3,(H,19,22)/t13-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 9.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human procollagen C-proteinase assessed as [3H]procollagen turnover by scintillation counting |
Bioorg Med Chem Lett 18: 6562-7 (2008)
Article DOI: 10.1016/j.bmcl.2008.10.036
BindingDB Entry DOI: 10.7270/Q2J1031M |
More data for this
Ligand-Target Pair | |
Bone morphogenetic protein 1
(Homo sapiens (Human)) | BDBM50241177

((R)-5-(6-cyclohexyl-1-(hydroxyamino)-1-oxohexan-3-...)Show SMILES CN(C)C(=O)c1noc(n1)[C@H](CCCC1CCCCC1)CC(=O)NO |r| Show InChI InChI=1S/C17H28N4O4/c1-21(2)17(23)15-18-16(25-20-15)13(11-14(22)19-24)10-6-9-12-7-4-3-5-8-12/h12-13,24H,3-11H2,1-2H3,(H,19,22)/t13-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human procollagen C-proteinase expressed in CHO cells by fluorescence assay |
Bioorg Med Chem Lett 18: 6562-7 (2008)
Article DOI: 10.1016/j.bmcl.2008.10.036
BindingDB Entry DOI: 10.7270/Q2J1031M |
More data for this
Ligand-Target Pair | |
Bone morphogenetic protein 1
(Homo sapiens (Human)) | BDBM50256367

((R)-2-(6-cyclohexyl-1-(hydroxyamino)-1-oxohexan-3-...)Show SMILES Cc1oc(nc1C(N)=O)[C@H](CCCC1CCCCC1)CC(=O)NO |r| Show InChI InChI=1S/C17H27N3O4/c1-11-15(16(18)22)19-17(24-11)13(10-14(21)20-23)9-5-8-12-6-3-2-4-7-12/h12-13,23H,2-10H2,1H3,(H2,18,22)(H,20,21)/t13-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 17 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human procollagen C-proteinase assessed as [3H]procollagen turnover by scintillation counting |
Bioorg Med Chem Lett 18: 6562-7 (2008)
Article DOI: 10.1016/j.bmcl.2008.10.036
BindingDB Entry DOI: 10.7270/Q2J1031M |
More data for this
Ligand-Target Pair | |
Bone morphogenetic protein 1
(Homo sapiens (Human)) | BDBM50256368
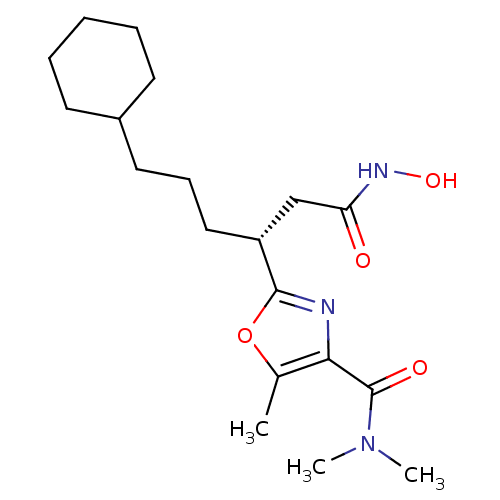
((R)-2-(6-cyclohexyl-1-(hydroxyamino)-1-oxohexan-3-...)Show SMILES CN(C)C(=O)c1nc(oc1C)[C@H](CCCC1CCCCC1)CC(=O)NO |r| Show InChI InChI=1S/C19H31N3O4/c1-13-17(19(24)22(2)3)20-18(26-13)15(12-16(23)21-25)11-7-10-14-8-5-4-6-9-14/h14-15,25H,4-12H2,1-3H3,(H,21,23)/t15-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 17 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human procollagen C-proteinase assessed as [3H]procollagen turnover by scintillation counting |
Bioorg Med Chem Lett 18: 6562-7 (2008)
Article DOI: 10.1016/j.bmcl.2008.10.036
BindingDB Entry DOI: 10.7270/Q2J1031M |
More data for this
Ligand-Target Pair | |
Bone morphogenetic protein 1
(Homo sapiens (Human)) | BDBM50256203
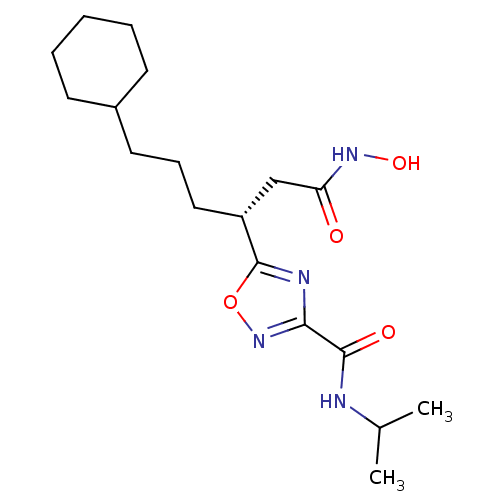
((R)-5-(6-cyclohexyl-1-(hydroxyamino)-1-oxohexan-3-...)Show SMILES CC(C)NC(=O)c1noc(n1)[C@H](CCCC1CCCCC1)CC(=O)NO |r| Show InChI InChI=1S/C18H30N4O4/c1-12(2)19-17(24)16-20-18(26-22-16)14(11-15(23)21-25)10-6-9-13-7-4-3-5-8-13/h12-14,25H,3-11H2,1-2H3,(H,19,24)(H,21,23)/t14-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 21 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human procollagen C-proteinase assessed as [3H]procollagen turnover by scintillation counting |
Bioorg Med Chem Lett 18: 6562-7 (2008)
Article DOI: 10.1016/j.bmcl.2008.10.036
BindingDB Entry DOI: 10.7270/Q2J1031M |
More data for this
Ligand-Target Pair | |
Bone morphogenetic protein 1
(Homo sapiens (Human)) | BDBM50256303

((R)-2-(5-(6-cyclohexyl-1-(hydroxyamino)-1-oxohexan...)Show SMILES ONC(=O)C[C@@H](CCCC1CCCCC1)c1nc(no1)C(=O)NCC(O)=O |r| Show InChI InChI=1S/C17H26N4O6/c22-13(20-26)9-12(8-4-7-11-5-2-1-3-6-11)17-19-15(21-27-17)16(25)18-10-14(23)24/h11-12,26H,1-10H2,(H,18,25)(H,20,22)(H,23,24)/t12-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 21 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human procollagen C-proteinase assessed as [3H]procollagen turnover by scintillation counting |
Bioorg Med Chem Lett 18: 6562-7 (2008)
Article DOI: 10.1016/j.bmcl.2008.10.036
BindingDB Entry DOI: 10.7270/Q2J1031M |
More data for this
Ligand-Target Pair | |
Bone morphogenetic protein 1
(Homo sapiens (Human)) | BDBM50256306
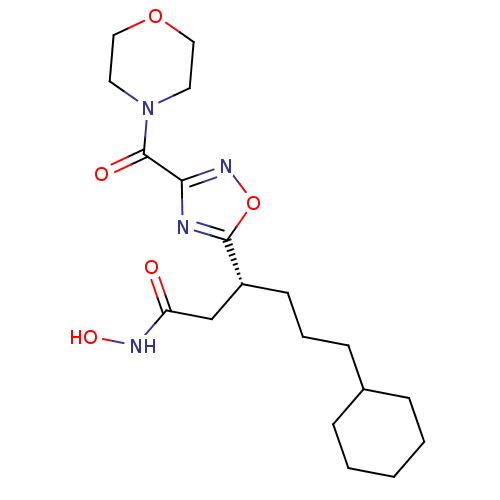
((R)-6-cyclohexyl-N-hydroxy-3-(3-(morpholine-4-carb...)Show SMILES ONC(=O)C[C@@H](CCCC1CCCCC1)c1nc(no1)C(=O)N1CCOCC1 |r| Show InChI InChI=1S/C19H30N4O5/c24-16(21-26)13-15(8-4-7-14-5-2-1-3-6-14)18-20-17(22-28-18)19(25)23-9-11-27-12-10-23/h14-15,26H,1-13H2,(H,21,24)/t15-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 25 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human procollagen C-proteinase assessed as [3H]procollagen turnover by scintillation counting |
Bioorg Med Chem Lett 18: 6562-7 (2008)
Article DOI: 10.1016/j.bmcl.2008.10.036
BindingDB Entry DOI: 10.7270/Q2J1031M |
More data for this
Ligand-Target Pair | |
Bone morphogenetic protein 1
(Homo sapiens (Human)) | BDBM50256305
((R)-6-cyclohexyl-N-hydroxy-3-(3-(piperidine-1-carb...)Show SMILES ONC(=O)C[C@@H](CCCC1CCCCC1)c1nc(no1)C(=O)N1CCCCC1 |r| Show InChI InChI=1S/C20H32N4O4/c25-17(22-27)14-16(11-7-10-15-8-3-1-4-9-15)19-21-18(23-28-19)20(26)24-12-5-2-6-13-24/h15-16,27H,1-14H2,(H,22,25)/t16-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 26 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human procollagen C-proteinase assessed as [3H]procollagen turnover by scintillation counting |
Bioorg Med Chem Lett 18: 6562-7 (2008)
Article DOI: 10.1016/j.bmcl.2008.10.036
BindingDB Entry DOI: 10.7270/Q2J1031M |
More data for this
Ligand-Target Pair | |
Bone morphogenetic protein 1
(Homo sapiens (Human)) | BDBM50216795

((3R)-6-cyclohexyl-N-hydroxy-3-(1,2,4-oxadiazol-5-y...)Show SMILES NC(=O)c1noc(n1)[C@H](CCCC1CCCCC1)CC(=O)NO |r| Show InChI InChI=1S/C15H24N4O4/c16-13(21)14-17-15(23-19-14)11(9-12(20)18-22)8-4-7-10-5-2-1-3-6-10/h10-11,22H,1-9H2,(H2,16,21)(H,18,20)/t11-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 26 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human procollagen C-proteinase assessed as [3H]procollagen turnover by scintillation counting |
Bioorg Med Chem Lett 18: 6562-7 (2008)
Article DOI: 10.1016/j.bmcl.2008.10.036
BindingDB Entry DOI: 10.7270/Q2J1031M |
More data for this
Ligand-Target Pair | |
Bone morphogenetic protein 1
(Homo sapiens (Human)) | BDBM50256150

((R)-5-(6-cyclohexyl-1-(hydroxyamino)-1-oxohexan-3-...)Show SMILES CNC(=O)c1noc(n1)[C@H](CCCC1CCCCC1)CC(=O)NO |r| Show InChI InChI=1S/C16H26N4O4/c1-17-15(22)14-18-16(24-20-14)12(10-13(21)19-23)9-5-8-11-6-3-2-4-7-11/h11-12,23H,2-10H2,1H3,(H,17,22)(H,19,21)/t12-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 28 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human procollagen C-proteinase assessed as [3H]procollagen turnover by scintillation counting |
Bioorg Med Chem Lett 18: 6562-7 (2008)
Article DOI: 10.1016/j.bmcl.2008.10.036
BindingDB Entry DOI: 10.7270/Q2J1031M |
More data for this
Ligand-Target Pair | |
Bone morphogenetic protein 1
(Homo sapiens (Human)) | BDBM50256151
((R)-5-(6-cyclohexyl-1-(hydroxyamino)-1-oxohexan-3-...)Show SMILES CCCNC(=O)c1noc(n1)[C@H](CCCC1CCCCC1)CC(=O)NO |r| Show InChI InChI=1S/C18H30N4O4/c1-2-11-19-17(24)16-20-18(26-22-16)14(12-15(23)21-25)10-6-9-13-7-4-3-5-8-13/h13-14,25H,2-12H2,1H3,(H,19,24)(H,21,23)/t14-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 32 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human procollagen C-proteinase assessed as [3H]procollagen turnover by scintillation counting |
Bioorg Med Chem Lett 18: 6562-7 (2008)
Article DOI: 10.1016/j.bmcl.2008.10.036
BindingDB Entry DOI: 10.7270/Q2J1031M |
More data for this
Ligand-Target Pair | |
Bone morphogenetic protein 1
(Homo sapiens (Human)) | BDBM50256204

((R)-5-(6-cyclohexyl-1-(hydroxyamino)-1-oxohexan-3-...)Show SMILES ONC(=O)C[C@@H](CCCC1CCCCC1)c1nc(no1)C(=O)NCC1CC1 |r| Show InChI InChI=1S/C19H30N4O4/c24-16(22-26)11-15(8-4-7-13-5-2-1-3-6-13)19-21-17(23-27-19)18(25)20-12-14-9-10-14/h13-15,26H,1-12H2,(H,20,25)(H,22,24)/t15-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 33 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human procollagen C-proteinase assessed as [3H]procollagen turnover by scintillation counting |
Bioorg Med Chem Lett 18: 6562-7 (2008)
Article DOI: 10.1016/j.bmcl.2008.10.036
BindingDB Entry DOI: 10.7270/Q2J1031M |
More data for this
Ligand-Target Pair | |
Bone morphogenetic protein 1
(Homo sapiens (Human)) | BDBM50216798
((R)-ethyl 5-(6-cyclohexyl-1-(hydroxyamino)-1-oxohe...)Show SMILES CCOC(=O)c1noc(n1)[C@H](CCCC1CCCCC1)CC(=O)NO |r| Show InChI InChI=1S/C17H27N3O5/c1-2-24-17(22)15-18-16(25-20-15)13(11-14(21)19-23)10-6-9-12-7-4-3-5-8-12/h12-13,23H,2-11H2,1H3,(H,19,21)/t13-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 36 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human procollagen C-proteinase assessed as [3H]procollagen turnover by scintillation counting |
Bioorg Med Chem Lett 18: 6562-7 (2008)
Article DOI: 10.1016/j.bmcl.2008.10.036
BindingDB Entry DOI: 10.7270/Q2J1031M |
More data for this
Ligand-Target Pair | |
Bone morphogenetic protein 1
(Homo sapiens (Human)) | BDBM50256302

((R)-5-(6-cyclohexyl-1-(hydroxyamino)-1-oxohexan-3-...)Show SMILES ONC(=O)C[C@@H](CCCC1CCCCC1)c1nc(no1)C(=O)NCc1ccccn1 |r| Show InChI InChI=1S/C21H29N5O4/c27-18(25-29)13-16(10-6-9-15-7-2-1-3-8-15)21-24-19(26-30-21)20(28)23-14-17-11-4-5-12-22-17/h4-5,11-12,15-16,29H,1-3,6-10,13-14H2,(H,23,28)(H,25,27)/t16-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 37 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human procollagen C-proteinase assessed as [3H]procollagen turnover by scintillation counting |
Bioorg Med Chem Lett 18: 6562-7 (2008)
Article DOI: 10.1016/j.bmcl.2008.10.036
BindingDB Entry DOI: 10.7270/Q2J1031M |
More data for this
Ligand-Target Pair | |
Bone morphogenetic protein 1
(Homo sapiens (Human)) | BDBM50256096
((R)-5-(6-cyclohexyl-1-(hydroxyamino)-1-oxohexan-3-...)Show SMILES CN(Cc1ccccn1)C(=O)c1noc(n1)[C@H](CCCC1CCCCC1)CC(=O)NO |r| Show InChI InChI=1S/C22H31N5O4/c1-27(15-18-12-5-6-13-23-18)22(29)20-24-21(31-26-20)17(14-19(28)25-30)11-7-10-16-8-3-2-4-9-16/h5-6,12-13,16-17,30H,2-4,7-11,14-15H2,1H3,(H,25,28)/t17-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human procollagen C-proteinase assessed as [3H]procollagen turnover by scintillation counting |
Bioorg Med Chem Lett 18: 6562-7 (2008)
Article DOI: 10.1016/j.bmcl.2008.10.036
BindingDB Entry DOI: 10.7270/Q2J1031M |
More data for this
Ligand-Target Pair | |
Bone morphogenetic protein 1
(Homo sapiens (Human)) | BDBM50256304
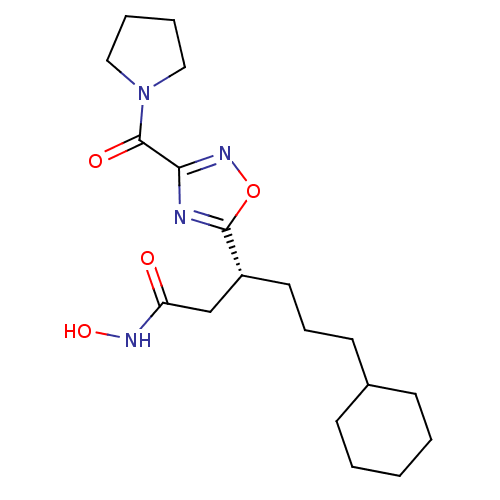
((R)-6-cyclohexyl-N-hydroxy-3-(3-(pyrrolidine-1-car...)Show SMILES ONC(=O)C[C@@H](CCCC1CCCCC1)c1nc(no1)C(=O)N1CCCC1 |r| Show InChI InChI=1S/C19H30N4O4/c24-16(21-26)13-15(10-6-9-14-7-2-1-3-8-14)18-20-17(22-27-18)19(25)23-11-4-5-12-23/h14-15,26H,1-13H2,(H,21,24)/t15-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 43 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human procollagen C-proteinase assessed as [3H]procollagen turnover by scintillation counting |
Bioorg Med Chem Lett 18: 6562-7 (2008)
Article DOI: 10.1016/j.bmcl.2008.10.036
BindingDB Entry DOI: 10.7270/Q2J1031M |
More data for this
Ligand-Target Pair | |
Bone morphogenetic protein 1
(Homo sapiens (Human)) | BDBM50216795

((3R)-6-cyclohexyl-N-hydroxy-3-(1,2,4-oxadiazol-5-y...)Show SMILES NC(=O)c1noc(n1)[C@H](CCCC1CCCCC1)CC(=O)NO |r| Show InChI InChI=1S/C15H24N4O4/c16-13(21)14-17-15(23-19-14)11(9-12(20)18-22)8-4-7-10-5-2-1-3-6-10/h10-11,22H,1-9H2,(H2,16,21)(H,18,20)/t11-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 44 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human procollagen C-proteinase assessed as [3H]procollagen turnover by scintillation counting |
Bioorg Med Chem Lett 18: 6562-7 (2008)
Article DOI: 10.1016/j.bmcl.2008.10.036
BindingDB Entry DOI: 10.7270/Q2J1031M |
More data for this
Ligand-Target Pair | |
Bone morphogenetic protein 1
(Homo sapiens (Human)) | BDBM50256207
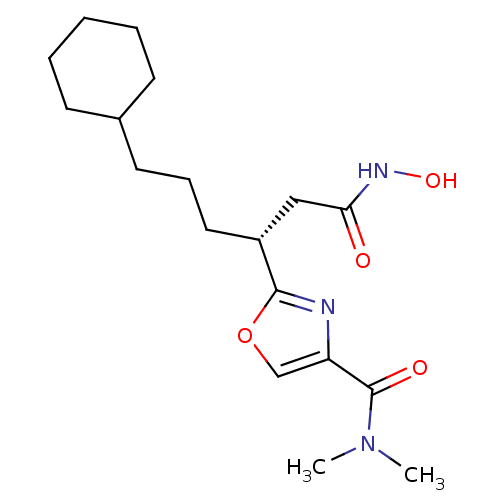
((R)-2-(6-cyclohexyl-1-(hydroxyamino)-1-oxohexan-3-...)Show SMILES CN(C)C(=O)c1coc(n1)[C@H](CCCC1CCCCC1)CC(=O)NO |r| Show InChI InChI=1S/C18H29N3O4/c1-21(2)18(23)15-12-25-17(19-15)14(11-16(22)20-24)10-6-9-13-7-4-3-5-8-13/h12-14,24H,3-11H2,1-2H3,(H,20,22)/t14-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 45 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human procollagen C-proteinase assessed as [3H]procollagen turnover by scintillation counting |
Bioorg Med Chem Lett 18: 6562-7 (2008)
Article DOI: 10.1016/j.bmcl.2008.10.036
BindingDB Entry DOI: 10.7270/Q2J1031M |
More data for this
Ligand-Target Pair | |
Bone morphogenetic protein 1
(Homo sapiens (Human)) | BDBM50256365

((R)-5-(6-cyclohexyl-1-(hydroxyamino)-1-oxohexan-3-...)Show SMILES CN(CN1CCN(C)CC1)C(=O)c1noc(n1)[C@H](CCCC1CCCCC1)CC(=O)NO |r| Show InChI InChI=1S/C22H38N6O4/c1-26-11-13-28(14-12-26)16-27(2)22(30)20-23-21(32-25-20)18(15-19(29)24-31)10-6-9-17-7-4-3-5-8-17/h17-18,31H,3-16H2,1-2H3,(H,24,29)/t18-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 54 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human procollagen C-proteinase assessed as [3H]procollagen turnover by scintillation counting |
Bioorg Med Chem Lett 18: 6562-7 (2008)
Article DOI: 10.1016/j.bmcl.2008.10.036
BindingDB Entry DOI: 10.7270/Q2J1031M |
More data for this
Ligand-Target Pair | |
Bone morphogenetic protein 1
(Homo sapiens (Human)) | BDBM50256205

((R)-N-benzyl-5-(6-cyclohexyl-1-(hydroxyamino)-1-ox...)Show SMILES ONC(=O)C[C@@H](CCCC1CCCCC1)c1nc(no1)C(=O)NCc1ccccc1 |r| Show InChI InChI=1S/C22H30N4O4/c27-19(25-29)14-18(13-7-12-16-8-3-1-4-9-16)22-24-20(26-30-22)21(28)23-15-17-10-5-2-6-11-17/h2,5-6,10-11,16,18,29H,1,3-4,7-9,12-15H2,(H,23,28)(H,25,27)/t18-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 57 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human procollagen C-proteinase assessed as [3H]procollagen turnover by scintillation counting |
Bioorg Med Chem Lett 18: 6562-7 (2008)
Article DOI: 10.1016/j.bmcl.2008.10.036
BindingDB Entry DOI: 10.7270/Q2J1031M |
More data for this
Ligand-Target Pair | |
Bone morphogenetic protein 1
(Homo sapiens (Human)) | BDBM50256366
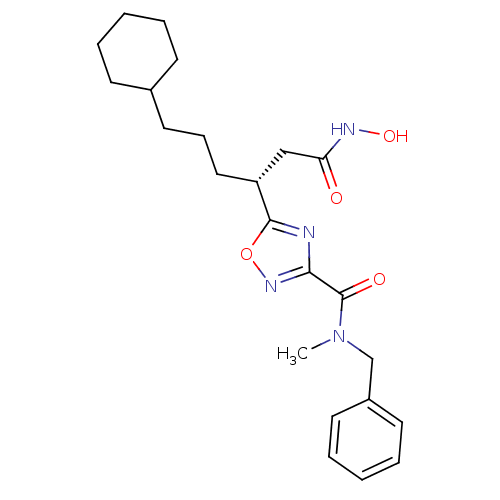
((R)-N-benzyl-5-(6-cyclohexyl-1-(hydroxyamino)-1-ox...)Show SMILES CN(Cc1ccccc1)C(=O)c1noc(n1)[C@H](CCCC1CCCCC1)CC(=O)NO |r| Show InChI InChI=1S/C23H32N4O4/c1-27(16-18-11-6-3-7-12-18)23(29)21-24-22(31-26-21)19(15-20(28)25-30)14-8-13-17-9-4-2-5-10-17/h3,6-7,11-12,17,19,30H,2,4-5,8-10,13-16H2,1H3,(H,25,28)/t19-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 65 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human procollagen C-proteinase assessed as [3H]procollagen turnover by scintillation counting |
Bioorg Med Chem Lett 18: 6562-7 (2008)
Article DOI: 10.1016/j.bmcl.2008.10.036
BindingDB Entry DOI: 10.7270/Q2J1031M |
More data for this
Ligand-Target Pair | |
Bone morphogenetic protein 1
(Homo sapiens (Human)) | BDBM50256206

((R)-2-(6-cyclohexyl-1-(hydroxyamino)-1-oxohexan-3-...)Show SMILES NC(=O)c1coc(n1)[C@H](CCCC1CCCCC1)CC(=O)NO |r| Show InChI InChI=1S/C16H25N3O4/c17-15(21)13-10-23-16(18-13)12(9-14(20)19-22)8-4-7-11-5-2-1-3-6-11/h10-12,22H,1-9H2,(H2,17,21)(H,19,20)/t12-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 73 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human procollagen C-proteinase assessed as [3H]procollagen turnover by scintillation counting |
Bioorg Med Chem Lett 18: 6562-7 (2008)
Article DOI: 10.1016/j.bmcl.2008.10.036
BindingDB Entry DOI: 10.7270/Q2J1031M |
More data for this
Ligand-Target Pair | |
72 kDa type IV collagenase
(Homo sapiens (Human)) | BDBM50097252
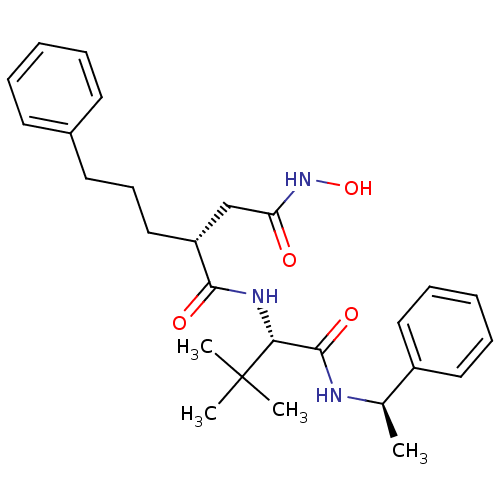
((R)-N'-((S)-3,3-dimethyl-1-oxo-1-((R)-1-phenylethy...)Show SMILES C[C@@H](NC(=O)[C@@H](NC(=O)[C@H](CCCc1ccccc1)CC(=O)NO)C(C)(C)C)c1ccccc1 |r| Show InChI InChI=1S/C27H37N3O4/c1-19(21-15-9-6-10-16-21)28-26(33)24(27(2,3)4)29-25(32)22(18-23(31)30-34)17-11-14-20-12-7-5-8-13-20/h5-10,12-13,15-16,19,22,24,34H,11,14,17-18H2,1-4H3,(H,28,33)(H,29,32)(H,30,31)/t19-,22-,24-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 135 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human MMP2 by fluorescence assay |
Bioorg Med Chem Lett 18: 6562-7 (2008)
Article DOI: 10.1016/j.bmcl.2008.10.036
BindingDB Entry DOI: 10.7270/Q2J1031M |
More data for this
Ligand-Target Pair | |
Bone morphogenetic protein 1
(Homo sapiens (Human)) | BDBM50097259
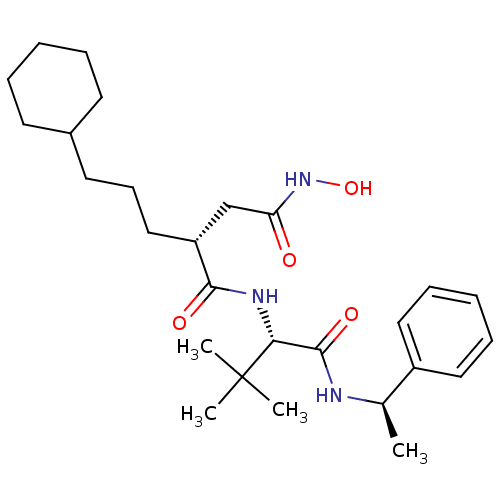
((R)-2-(3-Cyclohexyl-propyl)-N*1*-[(S)-2,2-dimethyl...)Show SMILES C[C@@H](NC(=O)[C@@H](NC(=O)[C@H](CCCC1CCCCC1)CC(=O)NO)C(C)(C)C)c1ccccc1 |r| Show InChI InChI=1S/C27H43N3O4/c1-19(21-15-9-6-10-16-21)28-26(33)24(27(2,3)4)29-25(32)22(18-23(31)30-34)17-11-14-20-12-7-5-8-13-20/h6,9-10,15-16,19-20,22,24,34H,5,7-8,11-14,17-18H2,1-4H3,(H,28,33)(H,29,32)(H,30,31)/t19-,22-,24-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 820 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human procollagen C-proteinase assessed as [3H]procollagen turnover by scintillation counting |
Bioorg Med Chem Lett 18: 6562-7 (2008)
Article DOI: 10.1016/j.bmcl.2008.10.036
BindingDB Entry DOI: 10.7270/Q2J1031M |
More data for this
Ligand-Target Pair | |
cAMP-specific 3',5'-cyclic phosphodiesterase 4D
(Homo sapiens (Human)) | BDBM50216795

((3R)-6-cyclohexyl-N-hydroxy-3-(1,2,4-oxadiazol-5-y...)Show SMILES NC(=O)c1noc(n1)[C@H](CCCC1CCCCC1)CC(=O)NO |r| Show InChI InChI=1S/C15H24N4O4/c16-13(21)14-17-15(23-19-14)11(9-12(20)18-22)8-4-7-10-5-2-1-3-6-10/h10-11,22H,1-9H2,(H2,16,21)(H,18,20)/t11-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 900 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of PDE4D |
Bioorg Med Chem Lett 18: 6562-7 (2008)
Article DOI: 10.1016/j.bmcl.2008.10.036
BindingDB Entry DOI: 10.7270/Q2J1031M |
More data for this
Ligand-Target Pair | |
72 kDa type IV collagenase
(Homo sapiens (Human)) | BDBM50097259

((R)-2-(3-Cyclohexyl-propyl)-N*1*-[(S)-2,2-dimethyl...)Show SMILES C[C@@H](NC(=O)[C@@H](NC(=O)[C@H](CCCC1CCCCC1)CC(=O)NO)C(C)(C)C)c1ccccc1 |r| Show InChI InChI=1S/C27H43N3O4/c1-19(21-15-9-6-10-16-21)28-26(33)24(27(2,3)4)29-25(32)22(18-23(31)30-34)17-11-14-20-12-7-5-8-13-20/h6,9-10,15-16,19-20,22,24,34H,5,7-8,11-14,17-18H2,1-4H3,(H,28,33)(H,29,32)(H,30,31)/t19-,22-,24-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human MMP2 by fluorescence assay |
Bioorg Med Chem Lett 18: 6562-7 (2008)
Article DOI: 10.1016/j.bmcl.2008.10.036
BindingDB Entry DOI: 10.7270/Q2J1031M |
More data for this
Ligand-Target Pair | |
cAMP-specific 3',5'-cyclic phosphodiesterase 4B
(Homo sapiens (Human)) | BDBM50216795

((3R)-6-cyclohexyl-N-hydroxy-3-(1,2,4-oxadiazol-5-y...)Show SMILES NC(=O)c1noc(n1)[C@H](CCCC1CCCCC1)CC(=O)NO |r| Show InChI InChI=1S/C15H24N4O4/c16-13(21)14-17-15(23-19-14)11(9-12(20)18-22)8-4-7-10-5-2-1-3-6-10/h10-11,22H,1-9H2,(H2,16,21)(H,18,20)/t11-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of PDE4B |
Bioorg Med Chem Lett 18: 6562-7 (2008)
Article DOI: 10.1016/j.bmcl.2008.10.036
BindingDB Entry DOI: 10.7270/Q2J1031M |
More data for this
Ligand-Target Pair | |
cAMP-specific 3',5'-cyclic phosphodiesterase 4A
(Homo sapiens (Human)) | BDBM50216795

((3R)-6-cyclohexyl-N-hydroxy-3-(1,2,4-oxadiazol-5-y...)Show SMILES NC(=O)c1noc(n1)[C@H](CCCC1CCCCC1)CC(=O)NO |r| Show InChI InChI=1S/C15H24N4O4/c16-13(21)14-17-15(23-19-14)11(9-12(20)18-22)8-4-7-10-5-2-1-3-6-10/h10-11,22H,1-9H2,(H2,16,21)(H,18,20)/t11-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of PDE4A |
Bioorg Med Chem Lett 18: 6562-7 (2008)
Article DOI: 10.1016/j.bmcl.2008.10.036
BindingDB Entry DOI: 10.7270/Q2J1031M |
More data for this
Ligand-Target Pair | |
Bone morphogenetic protein 1
(Homo sapiens (Human)) | BDBM50256149
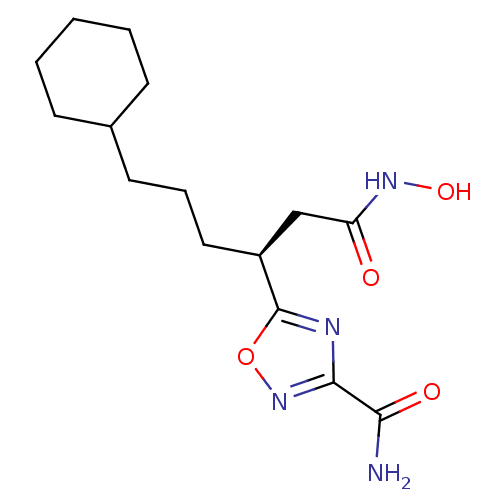
((S)-5-(6-cyclohexyl-1-(hydroxyamino)-1-oxohexan-3-...)Show SMILES NC(=O)c1noc(n1)[C@@H](CCCC1CCCCC1)CC(=O)NO |r| Show InChI InChI=1S/C15H24N4O4/c16-13(21)14-17-15(23-19-14)11(9-12(20)18-22)8-4-7-10-5-2-1-3-6-10/h10-11,22H,1-9H2,(H2,16,21)(H,18,20)/t11-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human procollagen C-proteinase assessed as [3H]procollagen turnover by scintillation counting |
Bioorg Med Chem Lett 18: 6562-7 (2008)
Article DOI: 10.1016/j.bmcl.2008.10.036
BindingDB Entry DOI: 10.7270/Q2J1031M |
More data for this
Ligand-Target Pair | |
cAMP-specific 3',5'-cyclic phosphodiesterase 4C
(Homo sapiens (Human)) | BDBM50216795

((3R)-6-cyclohexyl-N-hydroxy-3-(1,2,4-oxadiazol-5-y...)Show SMILES NC(=O)c1noc(n1)[C@H](CCCC1CCCCC1)CC(=O)NO |r| Show InChI InChI=1S/C15H24N4O4/c16-13(21)14-17-15(23-19-14)11(9-12(20)18-22)8-4-7-10-5-2-1-3-6-10/h10-11,22H,1-9H2,(H2,16,21)(H,18,20)/t11-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of PDE4C |
Bioorg Med Chem Lett 18: 6562-7 (2008)
Article DOI: 10.1016/j.bmcl.2008.10.036
BindingDB Entry DOI: 10.7270/Q2J1031M |
More data for this
Ligand-Target Pair | |
72 kDa type IV collagenase
(Homo sapiens (Human)) | BDBM50216835
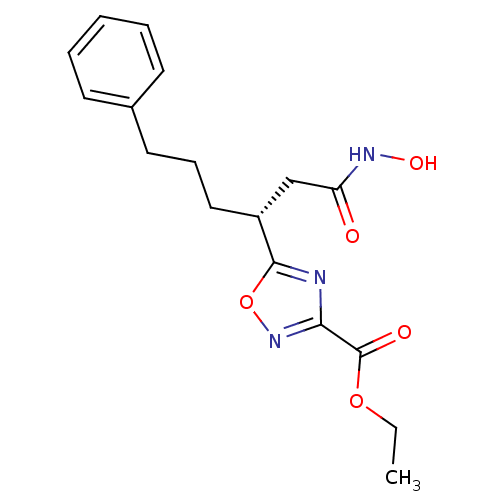
((R)-ethyl 5-(1-(hydroxyamino)-1-oxo-6-phenylhexan-...)Show SMILES CCOC(=O)c1noc(n1)[C@H](CCCc1ccccc1)CC(=O)NO |r| Show InChI InChI=1S/C17H21N3O5/c1-2-24-17(22)15-18-16(25-20-15)13(11-14(21)19-23)10-6-9-12-7-4-3-5-8-12/h3-5,7-8,13,23H,2,6,9-11H2,1H3,(H,19,21)/t13-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human MMP2 by fluorescence assay |
Bioorg Med Chem Lett 18: 6562-7 (2008)
Article DOI: 10.1016/j.bmcl.2008.10.036
BindingDB Entry DOI: 10.7270/Q2J1031M |
More data for this
Ligand-Target Pair | |
Bone morphogenetic protein 1
(Homo sapiens (Human)) | BDBM50216835

((R)-ethyl 5-(1-(hydroxyamino)-1-oxo-6-phenylhexan-...)Show SMILES CCOC(=O)c1noc(n1)[C@H](CCCc1ccccc1)CC(=O)NO |r| Show InChI InChI=1S/C17H21N3O5/c1-2-24-17(22)15-18-16(25-20-15)13(11-14(21)19-23)10-6-9-12-7-4-3-5-8-12/h3-5,7-8,13,23H,2,6,9-11H2,1H3,(H,19,21)/t13-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human procollagen C-proteinase assessed as [3H]procollagen turnover by scintillation counting |
Bioorg Med Chem Lett 18: 6562-7 (2008)
Article DOI: 10.1016/j.bmcl.2008.10.036
BindingDB Entry DOI: 10.7270/Q2J1031M |
More data for this
Ligand-Target Pair | |
Bone morphogenetic protein 1
(Homo sapiens (Human)) | BDBM50097252

((R)-N'-((S)-3,3-dimethyl-1-oxo-1-((R)-1-phenylethy...)Show SMILES C[C@@H](NC(=O)[C@@H](NC(=O)[C@H](CCCc1ccccc1)CC(=O)NO)C(C)(C)C)c1ccccc1 |r| Show InChI InChI=1S/C27H37N3O4/c1-19(21-15-9-6-10-16-21)28-26(33)24(27(2,3)4)29-25(32)22(18-23(31)30-34)17-11-14-20-12-7-5-8-13-20/h5-10,12-13,15-16,19,22,24,34H,11,14,17-18H2,1-4H3,(H,28,33)(H,29,32)(H,30,31)/t19-,22-,24-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 8.77E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human procollagen C-proteinase assessed as [3H]procollagen turnover by scintillation counting |
Bioorg Med Chem Lett 18: 6562-7 (2008)
Article DOI: 10.1016/j.bmcl.2008.10.036
BindingDB Entry DOI: 10.7270/Q2J1031M |
More data for this
Ligand-Target Pair | |
Stromelysin-1
(Homo sapiens (Human)) | BDBM50216795

((3R)-6-cyclohexyl-N-hydroxy-3-(1,2,4-oxadiazol-5-y...)Show SMILES NC(=O)c1noc(n1)[C@H](CCCC1CCCCC1)CC(=O)NO |r| Show InChI InChI=1S/C15H24N4O4/c16-13(21)14-17-15(23-19-14)11(9-12(20)18-22)8-4-7-10-5-2-1-3-6-10/h10-11,22H,1-9H2,(H2,16,21)(H,18,20)/t11-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human MMP3 by fluorescence assay |
Bioorg Med Chem Lett 18: 6562-7 (2008)
Article DOI: 10.1016/j.bmcl.2008.10.036
BindingDB Entry DOI: 10.7270/Q2J1031M |
More data for this
Ligand-Target Pair | |
Matrix metalloproteinase-14
(Homo sapiens (Human)) | BDBM50216795

((3R)-6-cyclohexyl-N-hydroxy-3-(1,2,4-oxadiazol-5-y...)Show SMILES NC(=O)c1noc(n1)[C@H](CCCC1CCCCC1)CC(=O)NO |r| Show InChI InChI=1S/C15H24N4O4/c16-13(21)14-17-15(23-19-14)11(9-12(20)18-22)8-4-7-10-5-2-1-3-6-10/h10-11,22H,1-9H2,(H2,16,21)(H,18,20)/t11-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human MMP14 catalytic domain by fluorescence assay |
Bioorg Med Chem Lett 18: 6562-7 (2008)
Article DOI: 10.1016/j.bmcl.2008.10.036
BindingDB Entry DOI: 10.7270/Q2J1031M |
More data for this
Ligand-Target Pair | |
Matrix metalloproteinase-9
(Homo sapiens (Human)) | BDBM50216795

((3R)-6-cyclohexyl-N-hydroxy-3-(1,2,4-oxadiazol-5-y...)Show SMILES NC(=O)c1noc(n1)[C@H](CCCC1CCCCC1)CC(=O)NO |r| Show InChI InChI=1S/C15H24N4O4/c16-13(21)14-17-15(23-19-14)11(9-12(20)18-22)8-4-7-10-5-2-1-3-6-10/h10-11,22H,1-9H2,(H2,16,21)(H,18,20)/t11-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human MMP9 by fluorescence assay |
Bioorg Med Chem Lett 18: 6562-7 (2008)
Article DOI: 10.1016/j.bmcl.2008.10.036
BindingDB Entry DOI: 10.7270/Q2J1031M |
More data for this
Ligand-Target Pair | |
Interstitial collagenase
(Homo sapiens (Human)) | BDBM50216795

((3R)-6-cyclohexyl-N-hydroxy-3-(1,2,4-oxadiazol-5-y...)Show SMILES NC(=O)c1noc(n1)[C@H](CCCC1CCCCC1)CC(=O)NO |r| Show InChI InChI=1S/C15H24N4O4/c16-13(21)14-17-15(23-19-14)11(9-12(20)18-22)8-4-7-10-5-2-1-3-6-10/h10-11,22H,1-9H2,(H2,16,21)(H,18,20)/t11-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of interstitial MMP1 by fluorescence assay |
Bioorg Med Chem Lett 18: 6562-7 (2008)
Article DOI: 10.1016/j.bmcl.2008.10.036
BindingDB Entry DOI: 10.7270/Q2J1031M |
More data for this
Ligand-Target Pair | |
72 kDa type IV collagenase
(Homo sapiens (Human)) | BDBM50256151

((R)-5-(6-cyclohexyl-1-(hydroxyamino)-1-oxohexan-3-...)Show SMILES CCCNC(=O)c1noc(n1)[C@H](CCCC1CCCCC1)CC(=O)NO |r| Show InChI InChI=1S/C18H30N4O4/c1-2-11-19-17(24)16-20-18(26-22-16)14(12-15(23)21-25)10-6-9-13-7-4-3-5-8-13/h13-14,25H,2-12H2,1H3,(H,19,24)(H,21,23)/t14-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.17E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human MMP2 by fluorescence assay |
Bioorg Med Chem Lett 18: 6562-7 (2008)
Article DOI: 10.1016/j.bmcl.2008.10.036
BindingDB Entry DOI: 10.7270/Q2J1031M |
More data for this
Ligand-Target Pair | |
Stromelysin-1
(Homo sapiens (Human)) | BDBM50241177

((R)-5-(6-cyclohexyl-1-(hydroxyamino)-1-oxohexan-3-...)Show SMILES CN(C)C(=O)c1noc(n1)[C@H](CCCC1CCCCC1)CC(=O)NO |r| Show InChI InChI=1S/C17H28N4O4/c1-21(2)17(23)15-18-16(25-20-15)13(11-14(22)19-24)10-6-9-12-7-4-3-5-8-12/h12-13,24H,3-11H2,1-2H3,(H,19,22)/t13-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2.80E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human MMP3 by fluorescence assay |
Bioorg Med Chem Lett 18: 6562-7 (2008)
Article DOI: 10.1016/j.bmcl.2008.10.036
BindingDB Entry DOI: 10.7270/Q2J1031M |
More data for this
Ligand-Target Pair | |
72 kDa type IV collagenase
(Homo sapiens (Human)) | BDBM50256302

((R)-5-(6-cyclohexyl-1-(hydroxyamino)-1-oxohexan-3-...)Show SMILES ONC(=O)C[C@@H](CCCC1CCCCC1)c1nc(no1)C(=O)NCc1ccccn1 |r| Show InChI InChI=1S/C21H29N5O4/c27-18(25-29)13-16(10-6-9-15-7-2-1-3-8-15)21-24-19(26-30-21)20(28)23-14-17-11-4-5-12-22-17/h4-5,11-12,15-16,29H,1-3,6-10,13-14H2,(H,23,28)(H,25,27)/t16-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human MMP2 by fluorescence assay |
Bioorg Med Chem Lett 18: 6562-7 (2008)
Article DOI: 10.1016/j.bmcl.2008.10.036
BindingDB Entry DOI: 10.7270/Q2J1031M |
More data for this
Ligand-Target Pair | |
72 kDa type IV collagenase
(Homo sapiens (Human)) | BDBM50256204

((R)-5-(6-cyclohexyl-1-(hydroxyamino)-1-oxohexan-3-...)Show SMILES ONC(=O)C[C@@H](CCCC1CCCCC1)c1nc(no1)C(=O)NCC1CC1 |r| Show InChI InChI=1S/C19H30N4O4/c24-16(22-26)11-15(8-4-7-13-5-2-1-3-6-13)19-21-17(23-27-19)18(25)20-12-14-9-10-14/h13-15,26H,1-12H2,(H,20,25)(H,22,24)/t15-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human MMP2 by fluorescence assay |
Bioorg Med Chem Lett 18: 6562-7 (2008)
Article DOI: 10.1016/j.bmcl.2008.10.036
BindingDB Entry DOI: 10.7270/Q2J1031M |
More data for this
Ligand-Target Pair | |
72 kDa type IV collagenase
(Homo sapiens (Human)) | BDBM50256150

((R)-5-(6-cyclohexyl-1-(hydroxyamino)-1-oxohexan-3-...)Show SMILES CNC(=O)c1noc(n1)[C@H](CCCC1CCCCC1)CC(=O)NO |r| Show InChI InChI=1S/C16H26N4O4/c1-17-15(22)14-18-16(24-20-14)12(10-13(21)19-23)9-5-8-11-6-3-2-4-7-11/h11-12,23H,2-10H2,1H3,(H,17,22)(H,19,21)/t12-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human MMP2 by fluorescence assay |
Bioorg Med Chem Lett 18: 6562-7 (2008)
Article DOI: 10.1016/j.bmcl.2008.10.036
BindingDB Entry DOI: 10.7270/Q2J1031M |
More data for this
Ligand-Target Pair | |
72 kDa type IV collagenase
(Homo sapiens (Human)) | BDBM50256096

((R)-5-(6-cyclohexyl-1-(hydroxyamino)-1-oxohexan-3-...)Show SMILES CN(Cc1ccccn1)C(=O)c1noc(n1)[C@H](CCCC1CCCCC1)CC(=O)NO |r| Show InChI InChI=1S/C22H31N5O4/c1-27(15-18-12-5-6-13-23-18)22(29)20-24-21(31-26-20)17(14-19(28)25-30)11-7-10-16-8-3-2-4-9-16/h5-6,12-13,16-17,30H,2-4,7-11,14-15H2,1H3,(H,25,28)/t17-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 5.05E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human MMP2 by fluorescence assay |
Bioorg Med Chem Lett 18: 6562-7 (2008)
Article DOI: 10.1016/j.bmcl.2008.10.036
BindingDB Entry DOI: 10.7270/Q2J1031M |
More data for this
Ligand-Target Pair | |
72 kDa type IV collagenase
(Homo sapiens (Human)) | BDBM50216795

((3R)-6-cyclohexyl-N-hydroxy-3-(1,2,4-oxadiazol-5-y...)Show SMILES NC(=O)c1noc(n1)[C@H](CCCC1CCCCC1)CC(=O)NO |r| Show InChI InChI=1S/C15H24N4O4/c16-13(21)14-17-15(23-19-14)11(9-12(20)18-22)8-4-7-10-5-2-1-3-6-10/h10-11,22H,1-9H2,(H2,16,21)(H,18,20)/t11-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >6.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human MMP2 by fluorescence assay |
Bioorg Med Chem Lett 18: 6562-7 (2008)
Article DOI: 10.1016/j.bmcl.2008.10.036
BindingDB Entry DOI: 10.7270/Q2J1031M |
More data for this
Ligand-Target Pair | |
72 kDa type IV collagenase
(Homo sapiens (Human)) | BDBM50256305

((R)-6-cyclohexyl-N-hydroxy-3-(3-(piperidine-1-carb...)Show SMILES ONC(=O)C[C@@H](CCCC1CCCCC1)c1nc(no1)C(=O)N1CCCCC1 |r| Show InChI InChI=1S/C20H32N4O4/c25-17(22-27)14-16(11-7-10-15-8-3-1-4-9-15)19-21-18(23-28-19)20(26)24-12-5-2-6-13-24/h15-16,27H,1-14H2,(H,22,25)/t16-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 7.49E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human MMP2 by fluorescence assay |
Bioorg Med Chem Lett 18: 6562-7 (2008)
Article DOI: 10.1016/j.bmcl.2008.10.036
BindingDB Entry DOI: 10.7270/Q2J1031M |
More data for this
Ligand-Target Pair | |
72 kDa type IV collagenase
(Homo sapiens (Human)) | BDBM50216798

((R)-ethyl 5-(6-cyclohexyl-1-(hydroxyamino)-1-oxohe...)Show SMILES CCOC(=O)c1noc(n1)[C@H](CCCC1CCCCC1)CC(=O)NO |r| Show InChI InChI=1S/C17H27N3O5/c1-2-24-17(22)15-18-16(25-20-15)13(11-14(21)19-23)10-6-9-12-7-4-3-5-8-12/h12-13,23H,2-11H2,1H3,(H,19,21)/t13-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 8.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human MMP2 by fluorescence assay |
Bioorg Med Chem Lett 18: 6562-7 (2008)
Article DOI: 10.1016/j.bmcl.2008.10.036
BindingDB Entry DOI: 10.7270/Q2J1031M |
More data for this
Ligand-Target Pair | |
72 kDa type IV collagenase
(Homo sapiens (Human)) | BDBM50256366

((R)-N-benzyl-5-(6-cyclohexyl-1-(hydroxyamino)-1-ox...)Show SMILES CN(Cc1ccccc1)C(=O)c1noc(n1)[C@H](CCCC1CCCCC1)CC(=O)NO |r| Show InChI InChI=1S/C23H32N4O4/c1-27(16-18-11-6-3-7-12-18)23(29)21-24-22(31-26-21)19(15-20(28)25-30)14-8-13-17-9-4-2-5-10-17/h3,6-7,11-12,17,19,30H,2,4-5,8-10,13-16H2,1H3,(H,25,28)/t19-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 8.18E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human MMP2 by fluorescence assay |
Bioorg Med Chem Lett 18: 6562-7 (2008)
Article DOI: 10.1016/j.bmcl.2008.10.036
BindingDB Entry DOI: 10.7270/Q2J1031M |
More data for this
Ligand-Target Pair | |
72 kDa type IV collagenase
(Homo sapiens (Human)) | BDBM50241177

((R)-5-(6-cyclohexyl-1-(hydroxyamino)-1-oxohexan-3-...)Show SMILES CN(C)C(=O)c1noc(n1)[C@H](CCCC1CCCCC1)CC(=O)NO |r| Show InChI InChI=1S/C17H28N4O4/c1-21(2)17(23)15-18-16(25-20-15)13(11-14(22)19-24)10-6-9-12-7-4-3-5-8-12/h12-13,24H,3-11H2,1-2H3,(H,19,22)/t13-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human MMP2 by fluorescence assay |
Bioorg Med Chem Lett 18: 6562-7 (2008)
Article DOI: 10.1016/j.bmcl.2008.10.036
BindingDB Entry DOI: 10.7270/Q2J1031M |
More data for this
Ligand-Target Pair | |
72 kDa type IV collagenase
(Homo sapiens (Human)) | BDBM50256303

((R)-2-(5-(6-cyclohexyl-1-(hydroxyamino)-1-oxohexan...)Show SMILES ONC(=O)C[C@@H](CCCC1CCCCC1)c1nc(no1)C(=O)NCC(O)=O |r| Show InChI InChI=1S/C17H26N4O6/c22-13(20-26)9-12(8-4-7-11-5-2-1-3-6-11)17-19-15(21-27-17)16(25)18-10-14(23)24/h11-12,26H,1-10H2,(H,18,25)(H,20,22)(H,23,24)/t12-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human MMP2 by fluorescence assay |
Bioorg Med Chem Lett 18: 6562-7 (2008)
Article DOI: 10.1016/j.bmcl.2008.10.036
BindingDB Entry DOI: 10.7270/Q2J1031M |
More data for this
Ligand-Target Pair | |
72 kDa type IV collagenase
(Homo sapiens (Human)) | BDBM50256203

((R)-5-(6-cyclohexyl-1-(hydroxyamino)-1-oxohexan-3-...)Show SMILES CC(C)NC(=O)c1noc(n1)[C@H](CCCC1CCCCC1)CC(=O)NO |r| Show InChI InChI=1S/C18H30N4O4/c1-12(2)19-17(24)16-20-18(26-22-16)14(11-15(23)21-25)10-6-9-13-7-4-3-5-8-13/h12-14,25H,3-11H2,1-2H3,(H,19,24)(H,21,23)/t14-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human MMP2 by fluorescence assay |
Bioorg Med Chem Lett 18: 6562-7 (2008)
Article DOI: 10.1016/j.bmcl.2008.10.036
BindingDB Entry DOI: 10.7270/Q2J1031M |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data