

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cathepsin S (Homo sapiens (Human)) | BDBM33999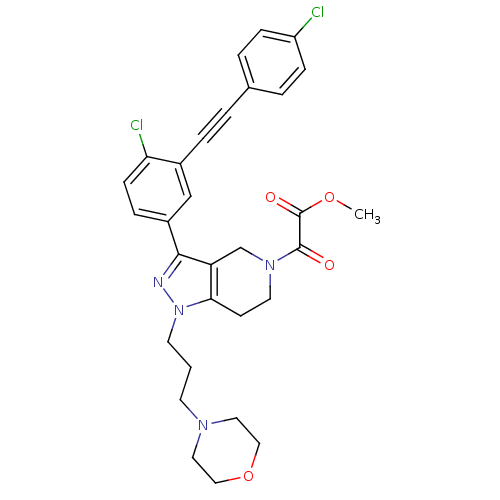 (arylalkyne pyrazole-based compound, 33) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 40 | n/a | n/a | n/a | n/a | 5.0 | 23 |
Johnson & Johnson Pharmaceutical | Assay Description Enzyme assays were run using fluorescence resonance energy transfer-based substrates, (Aedens)EKARVLAEAA(Dabcyl)K-amide and cathepsin S cleaves betwe... | Bioorg Med Chem Lett 19: 6131-4 (2009) Article DOI: 10.1016/j.bmcl.2009.09.014 BindingDB Entry DOI: 10.7270/Q2251GHV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin S (Homo sapiens (Human)) | BDBM34000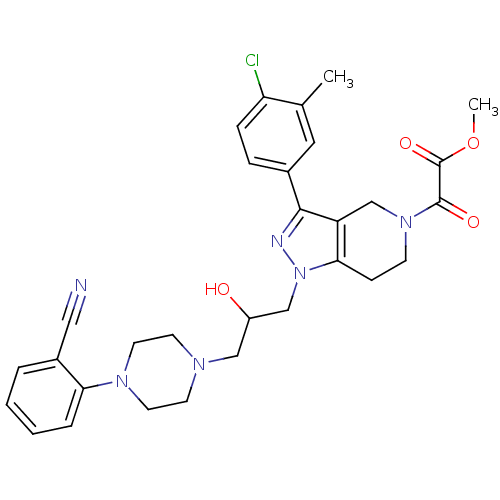 (tetrahydropyrido-pyrazole, 1) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 120 | n/a | n/a | n/a | n/a | 5.0 | 23 |
Johnson & Johnson Pharmaceutical | Assay Description Enzyme assays were run using fluorescence resonance energy transfer-based substrates, (Aedens)EKARVLAEAA(Dabcyl)K-amide and cathepsin S cleaves betwe... | Bioorg Med Chem Lett 19: 6131-4 (2009) Article DOI: 10.1016/j.bmcl.2009.09.014 BindingDB Entry DOI: 10.7270/Q2251GHV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin S (Homo sapiens (Human)) | BDBM33994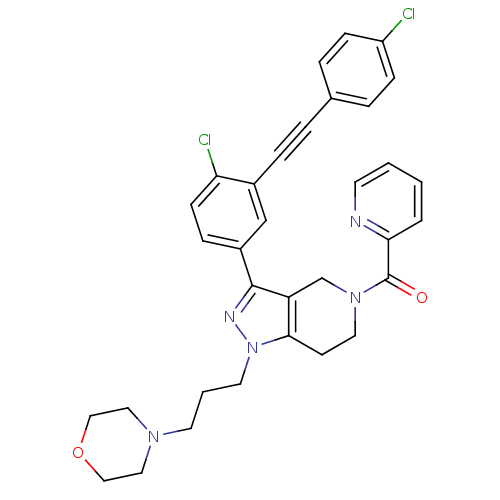 (arylalkyne pyrazole-based compound, 28) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 140 | n/a | n/a | n/a | n/a | 5.0 | 23 |
Johnson & Johnson Pharmaceutical | Assay Description Enzyme assays were run using fluorescence resonance energy transfer-based substrates, (Aedens)EKARVLAEAA(Dabcyl)K-amide and cathepsin S cleaves betwe... | Bioorg Med Chem Lett 19: 6131-4 (2009) Article DOI: 10.1016/j.bmcl.2009.09.014 BindingDB Entry DOI: 10.7270/Q2251GHV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin S (Homo sapiens (Human)) | BDBM33987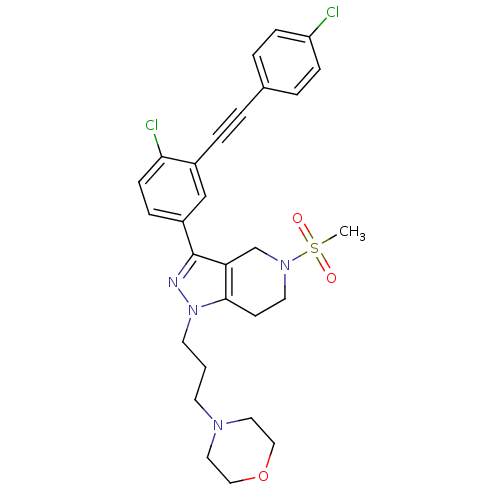 (arylalkyne pyrazole-based compound, 18) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 200 | n/a | n/a | n/a | n/a | 5.0 | 23 |
Johnson & Johnson Pharmaceutical | Assay Description Enzyme assays were run using fluorescence resonance energy transfer-based substrates, (Aedens)EKARVLAEAA(Dabcyl)K-amide and cathepsin S cleaves betwe... | Bioorg Med Chem Lett 19: 6131-4 (2009) Article DOI: 10.1016/j.bmcl.2009.09.014 BindingDB Entry DOI: 10.7270/Q2251GHV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin S (Homo sapiens (Human)) | BDBM33998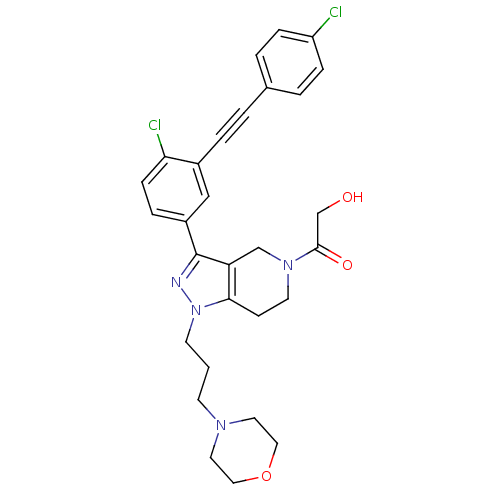 (arylalkyne pyrazole-based compound, 32) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB PDB Article PubMed | n/a | n/a | 220 | n/a | n/a | n/a | n/a | 5.0 | 23 |
Johnson & Johnson Pharmaceutical | Assay Description Enzyme assays were run using fluorescence resonance energy transfer-based substrates, (Aedens)EKARVLAEAA(Dabcyl)K-amide and cathepsin S cleaves betwe... | Bioorg Med Chem Lett 19: 6131-4 (2009) Article DOI: 10.1016/j.bmcl.2009.09.014 BindingDB Entry DOI: 10.7270/Q2251GHV | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Cathepsin S (Homo sapiens (Human)) | BDBM33988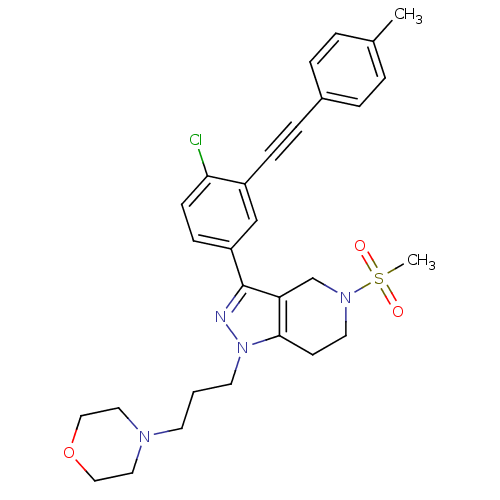 (arylalkyne pyrazole-based compound, 19) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 280 | n/a | n/a | n/a | n/a | 5.0 | 23 |
Johnson & Johnson Pharmaceutical | Assay Description Enzyme assays were run using fluorescence resonance energy transfer-based substrates, (Aedens)EKARVLAEAA(Dabcyl)K-amide and cathepsin S cleaves betwe... | Bioorg Med Chem Lett 19: 6131-4 (2009) Article DOI: 10.1016/j.bmcl.2009.09.014 BindingDB Entry DOI: 10.7270/Q2251GHV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin S (Homo sapiens (Human)) | BDBM33989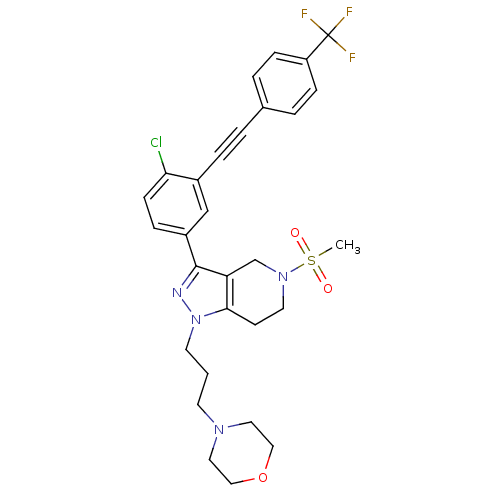 (arylalkyne pyrazole-based compound, 20) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 280 | n/a | n/a | n/a | n/a | 5.0 | 23 |
Johnson & Johnson Pharmaceutical | Assay Description Enzyme assays were run using fluorescence resonance energy transfer-based substrates, (Aedens)EKARVLAEAA(Dabcyl)K-amide and cathepsin S cleaves betwe... | Bioorg Med Chem Lett 19: 6131-4 (2009) Article DOI: 10.1016/j.bmcl.2009.09.014 BindingDB Entry DOI: 10.7270/Q2251GHV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin S (Homo sapiens (Human)) | BDBM33997 (arylalkyne pyrazole-based compound, 31) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 280 | n/a | n/a | n/a | n/a | 5.0 | 23 |
Johnson & Johnson Pharmaceutical | Assay Description Enzyme assays were run using fluorescence resonance energy transfer-based substrates, (Aedens)EKARVLAEAA(Dabcyl)K-amide and cathepsin S cleaves betwe... | Bioorg Med Chem Lett 19: 6131-4 (2009) Article DOI: 10.1016/j.bmcl.2009.09.014 BindingDB Entry DOI: 10.7270/Q2251GHV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin S (Homo sapiens (Human)) | BDBM33996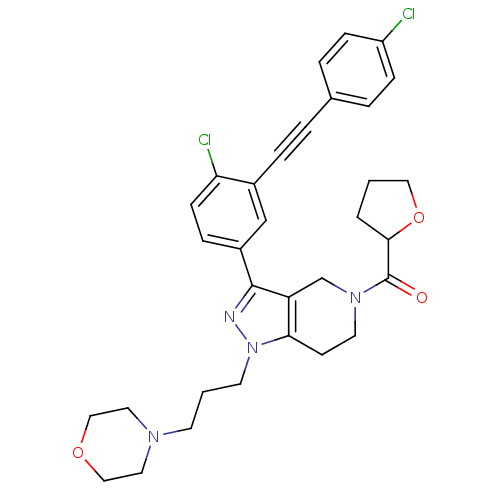 (arylalkyne pyrazole-based compound, 30) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 310 | n/a | n/a | n/a | n/a | 5.0 | 23 |
Johnson & Johnson Pharmaceutical | Assay Description Enzyme assays were run using fluorescence resonance energy transfer-based substrates, (Aedens)EKARVLAEAA(Dabcyl)K-amide and cathepsin S cleaves betwe... | Bioorg Med Chem Lett 19: 6131-4 (2009) Article DOI: 10.1016/j.bmcl.2009.09.014 BindingDB Entry DOI: 10.7270/Q2251GHV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin S (Homo sapiens (Human)) | BDBM33993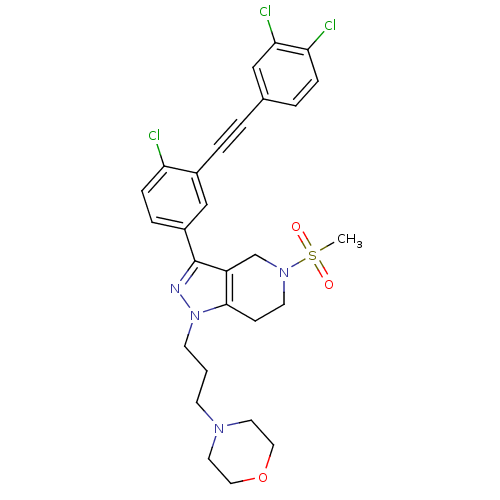 (arylalkyne pyrazole-based compound, 24) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 320 | n/a | n/a | n/a | n/a | 5.0 | 23 |
Johnson & Johnson Pharmaceutical | Assay Description Enzyme assays were run using fluorescence resonance energy transfer-based substrates, (Aedens)EKARVLAEAA(Dabcyl)K-amide and cathepsin S cleaves betwe... | Bioorg Med Chem Lett 19: 6131-4 (2009) Article DOI: 10.1016/j.bmcl.2009.09.014 BindingDB Entry DOI: 10.7270/Q2251GHV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin S (Homo sapiens (Human)) | BDBM33995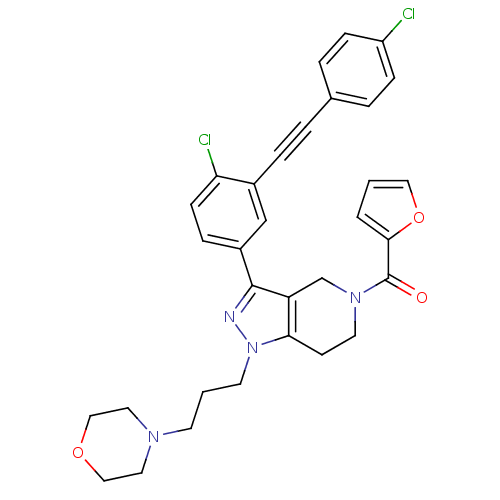 (arylalkyne pyrazole-based compound, 29) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 330 | n/a | n/a | n/a | n/a | 5.0 | 23 |
Johnson & Johnson Pharmaceutical | Assay Description Enzyme assays were run using fluorescence resonance energy transfer-based substrates, (Aedens)EKARVLAEAA(Dabcyl)K-amide and cathepsin S cleaves betwe... | Bioorg Med Chem Lett 19: 6131-4 (2009) Article DOI: 10.1016/j.bmcl.2009.09.014 BindingDB Entry DOI: 10.7270/Q2251GHV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin S (Homo sapiens (Human)) | BDBM33991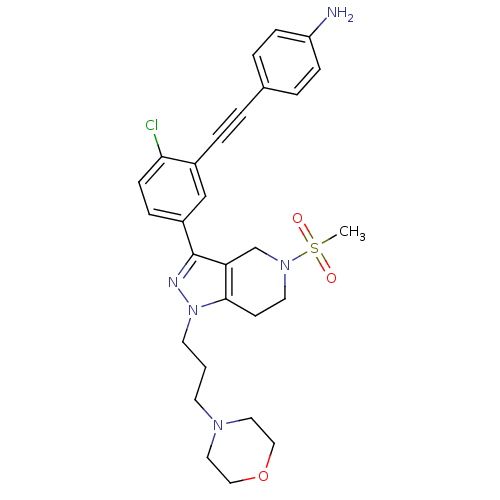 (arylalkyne pyrazole-based compound, 22) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 430 | n/a | n/a | n/a | n/a | 5.0 | 23 |
Johnson & Johnson Pharmaceutical | Assay Description Enzyme assays were run using fluorescence resonance energy transfer-based substrates, (Aedens)EKARVLAEAA(Dabcyl)K-amide and cathepsin S cleaves betwe... | Bioorg Med Chem Lett 19: 6131-4 (2009) Article DOI: 10.1016/j.bmcl.2009.09.014 BindingDB Entry DOI: 10.7270/Q2251GHV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin S (Homo sapiens (Human)) | BDBM33990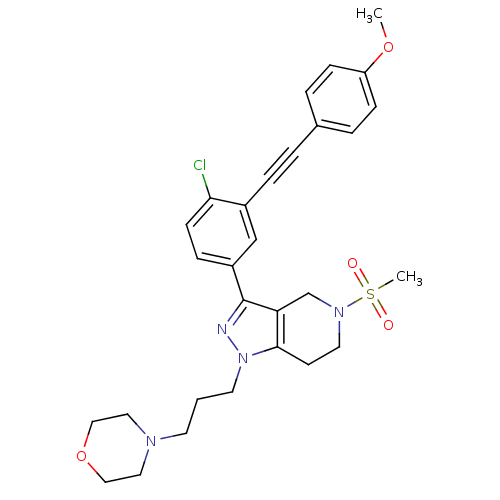 (arylalkyne pyrazole-based compound, 21) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 480 | n/a | n/a | n/a | n/a | 5.0 | 23 |
Johnson & Johnson Pharmaceutical | Assay Description Enzyme assays were run using fluorescence resonance energy transfer-based substrates, (Aedens)EKARVLAEAA(Dabcyl)K-amide and cathepsin S cleaves betwe... | Bioorg Med Chem Lett 19: 6131-4 (2009) Article DOI: 10.1016/j.bmcl.2009.09.014 BindingDB Entry DOI: 10.7270/Q2251GHV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin S (Homo sapiens (Human)) | BDBM33992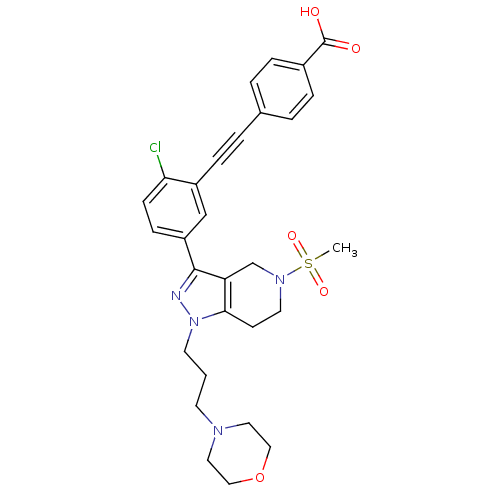 (arylalkyne pyrazole-based compound, 23) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 510 | n/a | n/a | n/a | n/a | 5.0 | 23 |
Johnson & Johnson Pharmaceutical | Assay Description Enzyme assays were run using fluorescence resonance energy transfer-based substrates, (Aedens)EKARVLAEAA(Dabcyl)K-amide and cathepsin S cleaves betwe... | Bioorg Med Chem Lett 19: 6131-4 (2009) Article DOI: 10.1016/j.bmcl.2009.09.014 BindingDB Entry DOI: 10.7270/Q2251GHV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin S (Homo sapiens (Human)) | BDBM33984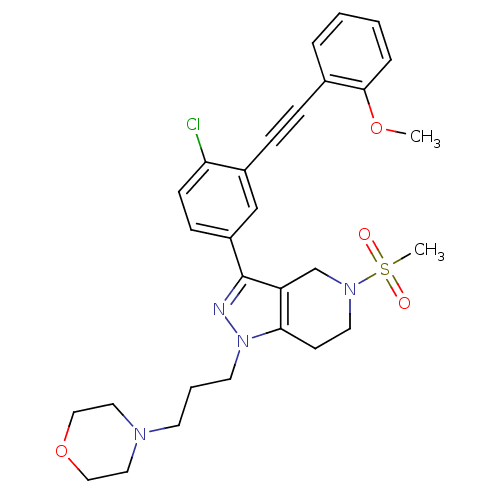 (arylalkyne pyrazole-based compound, 15) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 550 | n/a | n/a | n/a | n/a | 5.0 | 23 |
Johnson & Johnson Pharmaceutical | Assay Description Enzyme assays were run using fluorescence resonance energy transfer-based substrates, (Aedens)EKARVLAEAA(Dabcyl)K-amide and cathepsin S cleaves betwe... | Bioorg Med Chem Lett 19: 6131-4 (2009) Article DOI: 10.1016/j.bmcl.2009.09.014 BindingDB Entry DOI: 10.7270/Q2251GHV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin S (Homo sapiens (Human)) | BDBM33977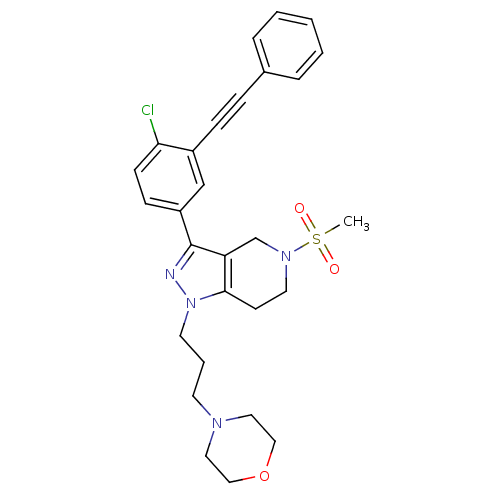 (arylalkyne pyrazole-based compound, 8) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 610 | n/a | n/a | n/a | n/a | 5.0 | 23 |
Johnson & Johnson Pharmaceutical | Assay Description Enzyme assays were run using fluorescence resonance energy transfer-based substrates, (Aedens)EKARVLAEAA(Dabcyl)K-amide and cathepsin S cleaves betwe... | Bioorg Med Chem Lett 19: 6131-4 (2009) Article DOI: 10.1016/j.bmcl.2009.09.014 BindingDB Entry DOI: 10.7270/Q2251GHV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin S (Homo sapiens (Human)) | BDBM33985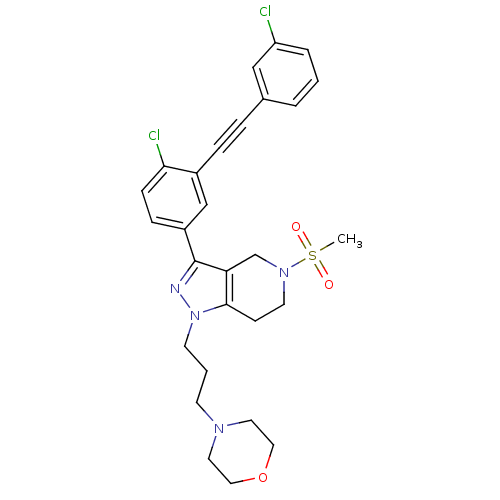 (arylalkyne pyrazole-based compound, 16) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 700 | n/a | n/a | n/a | n/a | 5.0 | 23 |
Johnson & Johnson Pharmaceutical | Assay Description Enzyme assays were run using fluorescence resonance energy transfer-based substrates, (Aedens)EKARVLAEAA(Dabcyl)K-amide and cathepsin S cleaves betwe... | Bioorg Med Chem Lett 19: 6131-4 (2009) Article DOI: 10.1016/j.bmcl.2009.09.014 BindingDB Entry DOI: 10.7270/Q2251GHV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin S (Homo sapiens (Human)) | BDBM33981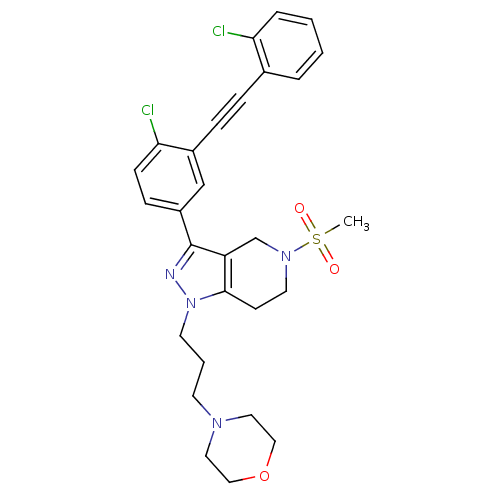 (arylalkyne pyrazole-based compound, 12) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 720 | n/a | n/a | n/a | n/a | 5.0 | 23 |
Johnson & Johnson Pharmaceutical | Assay Description Enzyme assays were run using fluorescence resonance energy transfer-based substrates, (Aedens)EKARVLAEAA(Dabcyl)K-amide and cathepsin S cleaves betwe... | Bioorg Med Chem Lett 19: 6131-4 (2009) Article DOI: 10.1016/j.bmcl.2009.09.014 BindingDB Entry DOI: 10.7270/Q2251GHV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin S (Homo sapiens (Human)) | BDBM33978 (arylalkyne pyrazole-based compound, 9) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 790 | n/a | n/a | n/a | n/a | 5.0 | 23 |
Johnson & Johnson Pharmaceutical | Assay Description Enzyme assays were run using fluorescence resonance energy transfer-based substrates, (Aedens)EKARVLAEAA(Dabcyl)K-amide and cathepsin S cleaves betwe... | Bioorg Med Chem Lett 19: 6131-4 (2009) Article DOI: 10.1016/j.bmcl.2009.09.014 BindingDB Entry DOI: 10.7270/Q2251GHV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin S (Homo sapiens (Human)) | BDBM33983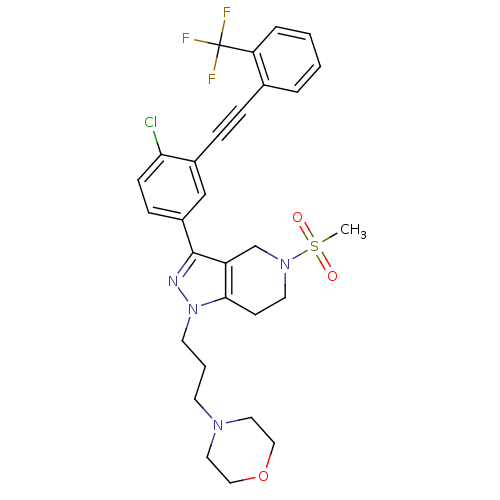 (arylalkyne pyrazole-based compound, 14) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 870 | n/a | n/a | n/a | n/a | 5.0 | 23 |
Johnson & Johnson Pharmaceutical | Assay Description Enzyme assays were run using fluorescence resonance energy transfer-based substrates, (Aedens)EKARVLAEAA(Dabcyl)K-amide and cathepsin S cleaves betwe... | Bioorg Med Chem Lett 19: 6131-4 (2009) Article DOI: 10.1016/j.bmcl.2009.09.014 BindingDB Entry DOI: 10.7270/Q2251GHV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin S (Homo sapiens (Human)) | BDBM33982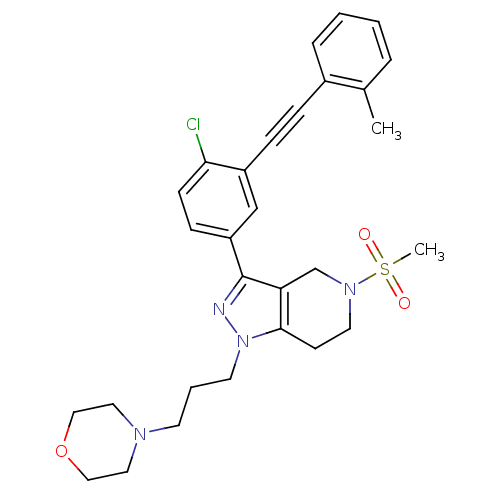 (arylalkyne pyrazole-based compound, 13) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.10E+3 | n/a | n/a | n/a | n/a | 5.0 | 23 |
Johnson & Johnson Pharmaceutical | Assay Description Enzyme assays were run using fluorescence resonance energy transfer-based substrates, (Aedens)EKARVLAEAA(Dabcyl)K-amide and cathepsin S cleaves betwe... | Bioorg Med Chem Lett 19: 6131-4 (2009) Article DOI: 10.1016/j.bmcl.2009.09.014 BindingDB Entry DOI: 10.7270/Q2251GHV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin S (Homo sapiens (Human)) | BDBM33979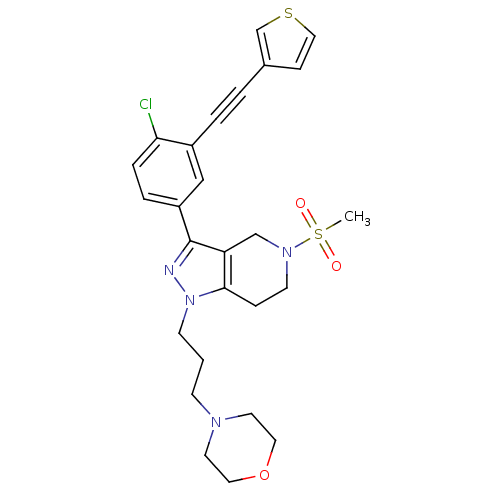 (arylalkyne pyrazole-based compound, 10) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.50E+3 | n/a | n/a | n/a | n/a | 5.0 | 23 |
Johnson & Johnson Pharmaceutical | Assay Description Enzyme assays were run using fluorescence resonance energy transfer-based substrates, (Aedens)EKARVLAEAA(Dabcyl)K-amide and cathepsin S cleaves betwe... | Bioorg Med Chem Lett 19: 6131-4 (2009) Article DOI: 10.1016/j.bmcl.2009.09.014 BindingDB Entry DOI: 10.7270/Q2251GHV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin S (Homo sapiens (Human)) | BDBM33986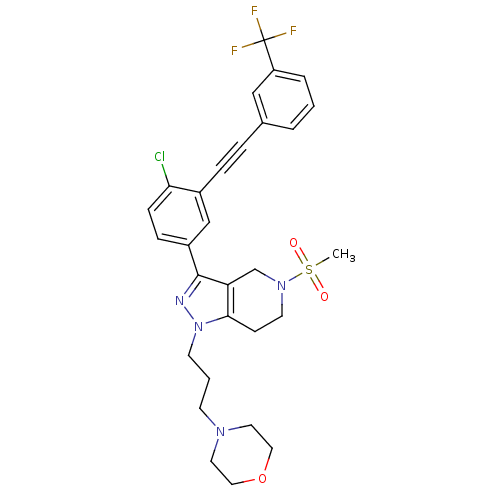 (arylalkyne pyrazole-based compound, 17) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.50E+3 | n/a | n/a | n/a | n/a | 5.0 | 23 |
Johnson & Johnson Pharmaceutical | Assay Description Enzyme assays were run using fluorescence resonance energy transfer-based substrates, (Aedens)EKARVLAEAA(Dabcyl)K-amide and cathepsin S cleaves betwe... | Bioorg Med Chem Lett 19: 6131-4 (2009) Article DOI: 10.1016/j.bmcl.2009.09.014 BindingDB Entry DOI: 10.7270/Q2251GHV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin S (Homo sapiens (Human)) | BDBM33976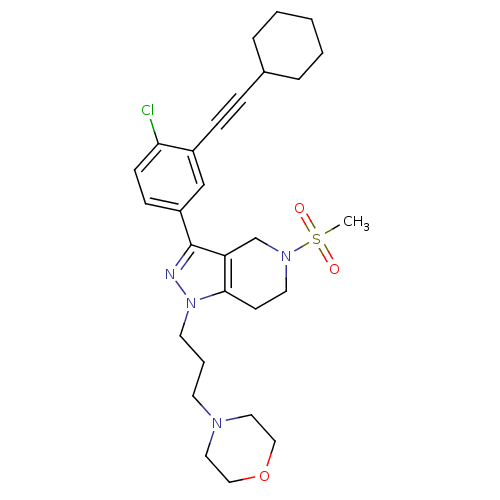 (arylalkyne pyrazole-based compound, 7) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 6.70E+3 | n/a | n/a | n/a | n/a | 5.0 | 23 |
Johnson & Johnson Pharmaceutical | Assay Description Enzyme assays were run using fluorescence resonance energy transfer-based substrates, (Aedens)EKARVLAEAA(Dabcyl)K-amide and cathepsin S cleaves betwe... | Bioorg Med Chem Lett 19: 6131-4 (2009) Article DOI: 10.1016/j.bmcl.2009.09.014 BindingDB Entry DOI: 10.7270/Q2251GHV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin S (Homo sapiens (Human)) | BDBM33980 (arylalkyne pyrazole-based compound, 11) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 7.40E+3 | n/a | n/a | n/a | n/a | 5.0 | 23 |
Johnson & Johnson Pharmaceutical | Assay Description Enzyme assays were run using fluorescence resonance energy transfer-based substrates, (Aedens)EKARVLAEAA(Dabcyl)K-amide and cathepsin S cleaves betwe... | Bioorg Med Chem Lett 19: 6131-4 (2009) Article DOI: 10.1016/j.bmcl.2009.09.014 BindingDB Entry DOI: 10.7270/Q2251GHV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin S (Homo sapiens (Human)) | BDBM33973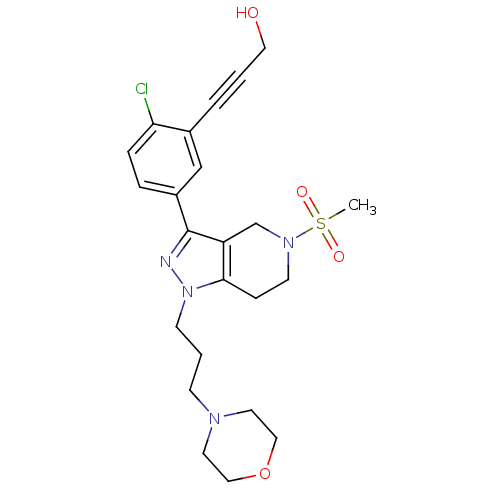 (arylalkyne pyrazole-based compound, 4) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 7.90E+3 | n/a | n/a | n/a | n/a | 5.0 | 23 |
Johnson & Johnson Pharmaceutical | Assay Description Enzyme assays were run using fluorescence resonance energy transfer-based substrates, (Aedens)EKARVLAEAA(Dabcyl)K-amide and cathepsin S cleaves betwe... | Bioorg Med Chem Lett 19: 6131-4 (2009) Article DOI: 10.1016/j.bmcl.2009.09.014 BindingDB Entry DOI: 10.7270/Q2251GHV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin S (Homo sapiens (Human)) | BDBM33975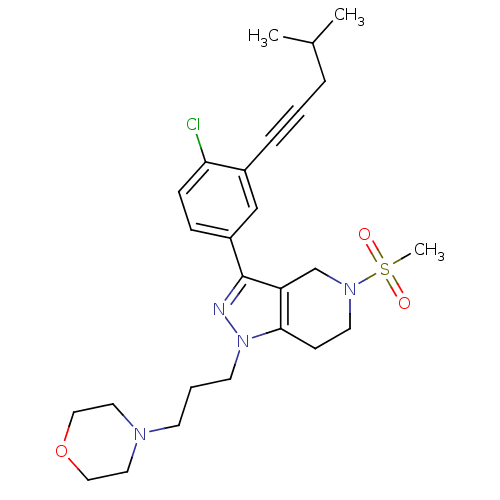 (arylalkyne pyrazole-based compound, 6) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 8.30E+3 | n/a | n/a | n/a | n/a | 5.0 | 23 |
Johnson & Johnson Pharmaceutical | Assay Description Enzyme assays were run using fluorescence resonance energy transfer-based substrates, (Aedens)EKARVLAEAA(Dabcyl)K-amide and cathepsin S cleaves betwe... | Bioorg Med Chem Lett 19: 6131-4 (2009) Article DOI: 10.1016/j.bmcl.2009.09.014 BindingDB Entry DOI: 10.7270/Q2251GHV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin S (Homo sapiens (Human)) | BDBM33974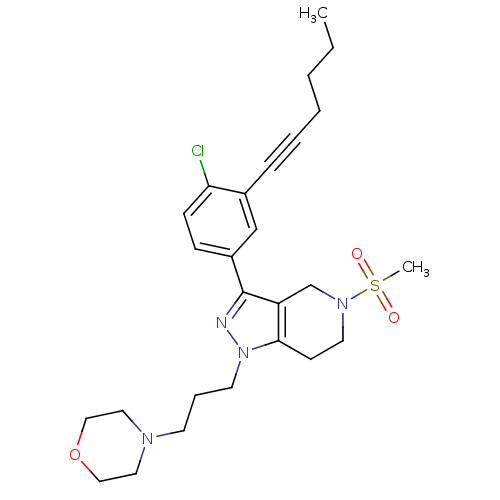 (arylalkyne pyrazole-based compound, 5) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.10E+4 | n/a | n/a | n/a | n/a | 5.0 | 23 |
Johnson & Johnson Pharmaceutical | Assay Description Enzyme assays were run using fluorescence resonance energy transfer-based substrates, (Aedens)EKARVLAEAA(Dabcyl)K-amide and cathepsin S cleaves betwe... | Bioorg Med Chem Lett 19: 6131-4 (2009) Article DOI: 10.1016/j.bmcl.2009.09.014 BindingDB Entry DOI: 10.7270/Q2251GHV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||