

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50041517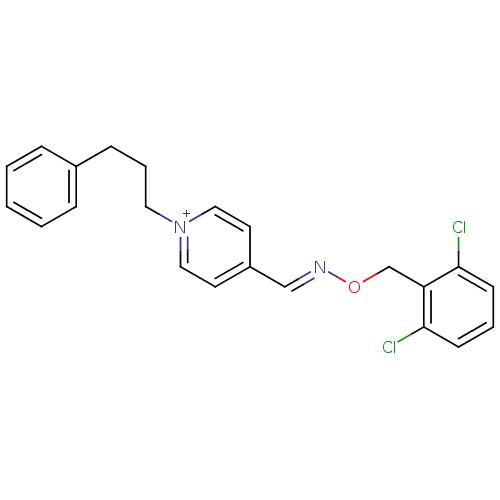 (4-[(2,6-dichloro-benzyloxyimino)-methyl]-1-(3-phen...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 180 | n/a | n/a | n/a | n/a | n/a | n/a |
Ege-University Curated by ChEMBL | Assay Description Inhibition of electric eel AChE by modified Ellman's method | Bioorg Med Chem 18: 2049-59 (2010) Article DOI: 10.1016/j.bmc.2010.01.002 BindingDB Entry DOI: 10.7270/Q2GH9J3H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50307921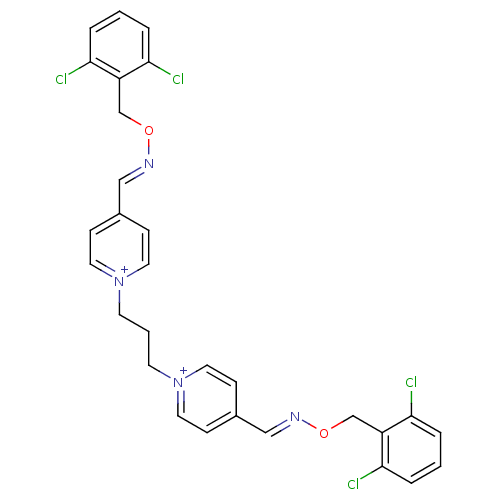 ((E,E)-1,3-bis[4-[[(2,6-dichlorobenzyloxyl)imino]me...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 340 | n/a | n/a | n/a | n/a | n/a | n/a |
Ege-University Curated by ChEMBL | Assay Description Inhibition of electric eel AChE by modified Ellman's method | Bioorg Med Chem 18: 2049-59 (2010) Article DOI: 10.1016/j.bmc.2010.01.002 BindingDB Entry DOI: 10.7270/Q2GH9J3H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50307921 ((E,E)-1,3-bis[4-[[(2,6-dichlorobenzyloxyl)imino]me...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.83E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Ege-University Curated by ChEMBL | Assay Description Inhibition of horse serum BuChE by modified Ellman's method | Bioorg Med Chem 18: 2049-59 (2010) Article DOI: 10.1016/j.bmc.2010.01.002 BindingDB Entry DOI: 10.7270/Q2GH9J3H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50041517 (4-[(2,6-dichloro-benzyloxyimino)-methyl]-1-(3-phen...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.78E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Ege-University Curated by ChEMBL | Assay Description Inhibition of horse serum BuChE by modified Ellman's method | Bioorg Med Chem 18: 2049-59 (2010) Article DOI: 10.1016/j.bmc.2010.01.002 BindingDB Entry DOI: 10.7270/Q2GH9J3H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50307927 (4-((2,6-dichlorobenzylidene)hydrazono)-1-(3-phenyl...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.66E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Ege-University Curated by ChEMBL | Assay Description Inhibition of electric eel AChE by modified Ellman's method | Bioorg Med Chem 18: 2049-59 (2010) Article DOI: 10.1016/j.bmc.2010.01.002 BindingDB Entry DOI: 10.7270/Q2GH9J3H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50307928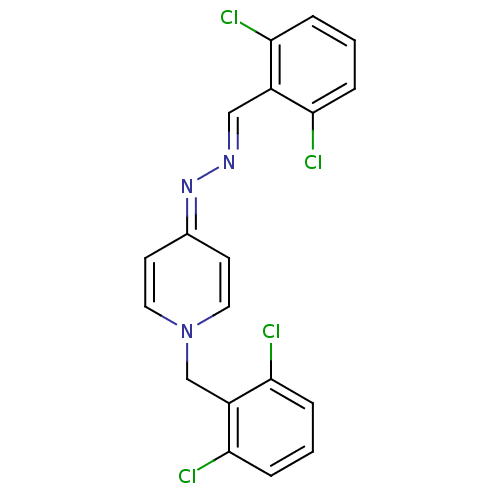 (1-(2,6-dichlorobenzyl)-4-((2,6-dichlorobenzylidene...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Ege-University Curated by ChEMBL | Assay Description Inhibition of electric eel AChE by modified Ellman's method | Bioorg Med Chem 18: 2049-59 (2010) Article DOI: 10.1016/j.bmc.2010.01.002 BindingDB Entry DOI: 10.7270/Q2GH9J3H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50307925 (1-benzyl-4-((2,6-dichlorobenzylidene)hydrazono)-1,...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 8.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Ege-University Curated by ChEMBL | Assay Description Inhibition of horse serum BuChE by modified Ellman's method | Bioorg Med Chem 18: 2049-59 (2010) Article DOI: 10.1016/j.bmc.2010.01.002 BindingDB Entry DOI: 10.7270/Q2GH9J3H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50307926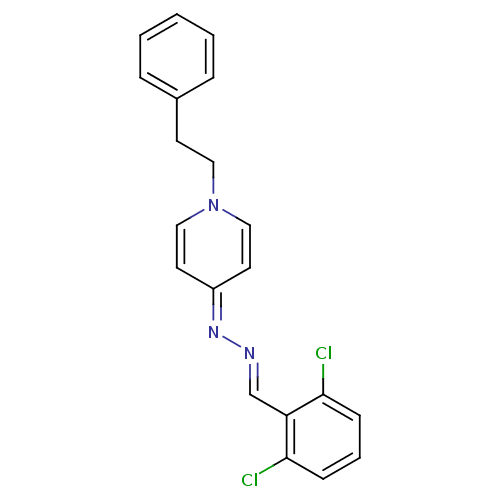 (4-((2,6-dichlorobenzylidene)hydrazono)-1-phenethyl...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 9.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Ege-University Curated by ChEMBL | Assay Description Inhibition of horse serum BuChE by modified Ellman's method | Bioorg Med Chem 18: 2049-59 (2010) Article DOI: 10.1016/j.bmc.2010.01.002 BindingDB Entry DOI: 10.7270/Q2GH9J3H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50307927 (4-((2,6-dichlorobenzylidene)hydrazono)-1-(3-phenyl...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Ege-University Curated by ChEMBL | Assay Description Inhibition of horse serum BuChE by modified Ellman's method | Bioorg Med Chem 18: 2049-59 (2010) Article DOI: 10.1016/j.bmc.2010.01.002 BindingDB Entry DOI: 10.7270/Q2GH9J3H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50307924 (4-((2,6-dichlorobenzylidene)hydrazono)-1-methyl-1,...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.10E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Ege-University Curated by ChEMBL | Assay Description Inhibition of horse serum BuChE by modified Ellman's method | Bioorg Med Chem 18: 2049-59 (2010) Article DOI: 10.1016/j.bmc.2010.01.002 BindingDB Entry DOI: 10.7270/Q2GH9J3H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50231232 (1-(2-phenylpropyl)-4-oxopiperidine O-(2,6-Dichloro...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.21E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Ege-University Curated by ChEMBL | Assay Description Inhibition of electric eel AChE by modified Ellman's method | Bioorg Med Chem 18: 2049-59 (2010) Article DOI: 10.1016/j.bmc.2010.01.002 BindingDB Entry DOI: 10.7270/Q2GH9J3H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50307925 (1-benzyl-4-((2,6-dichlorobenzylidene)hydrazono)-1,...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.74E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Ege-University Curated by ChEMBL | Assay Description Inhibition of electric eel AChE by modified Ellman's method | Bioorg Med Chem 18: 2049-59 (2010) Article DOI: 10.1016/j.bmc.2010.01.002 BindingDB Entry DOI: 10.7270/Q2GH9J3H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50231234 (1-(3-phenylpropyl)piperidine-4-carbaldehyde O-2,6-...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.91E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Ege-University Curated by ChEMBL | Assay Description Inhibition of electric eel AChE by modified Ellman's method | Bioorg Med Chem 18: 2049-59 (2010) Article DOI: 10.1016/j.bmc.2010.01.002 BindingDB Entry DOI: 10.7270/Q2GH9J3H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50307926 (4-((2,6-dichlorobenzylidene)hydrazono)-1-phenethyl...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.67E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Ege-University Curated by ChEMBL | Assay Description Inhibition of electric eel AChE by modified Ellman's method | Bioorg Med Chem 18: 2049-59 (2010) Article DOI: 10.1016/j.bmc.2010.01.002 BindingDB Entry DOI: 10.7270/Q2GH9J3H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50231232 (1-(2-phenylpropyl)-4-oxopiperidine O-(2,6-Dichloro...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Ege-University Curated by ChEMBL | Assay Description Inhibition of horse serum BuChE by modified Ellman's method | Bioorg Med Chem 18: 2049-59 (2010) Article DOI: 10.1016/j.bmc.2010.01.002 BindingDB Entry DOI: 10.7270/Q2GH9J3H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50307923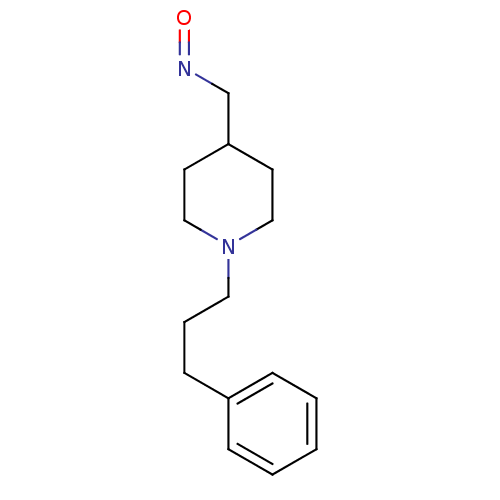 (1-(3-phenylpropyl)piperidine-4-carbaldehyde oxime ...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.78E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Ege-University Curated by ChEMBL | Assay Description Inhibition of electric eel AChE by modified Ellman's method | Bioorg Med Chem 18: 2049-59 (2010) Article DOI: 10.1016/j.bmc.2010.01.002 BindingDB Entry DOI: 10.7270/Q2GH9J3H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50307924 (4-((2,6-dichlorobenzylidene)hydrazono)-1-methyl-1,...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 7.39E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Ege-University Curated by ChEMBL | Assay Description Inhibition of electric eel AChE by modified Ellman's method | Bioorg Med Chem 18: 2049-59 (2010) Article DOI: 10.1016/j.bmc.2010.01.002 BindingDB Entry DOI: 10.7270/Q2GH9J3H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||