 Found 49 hits of Enzyme Inhibition Constant Data
Found 49 hits of Enzyme Inhibition Constant Data Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Transporter
(Rattus norvegicus (rat)) | BDBM50308250
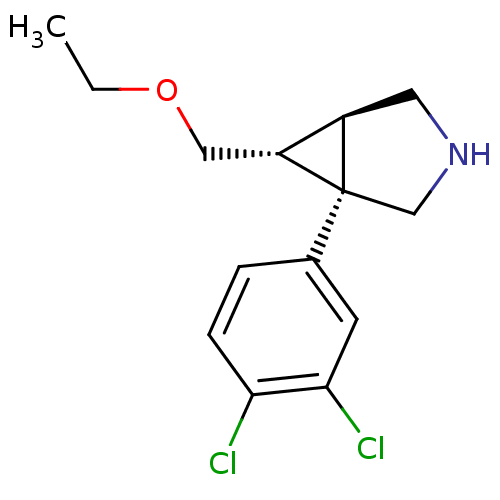
((1R,5R,6R)-1-(3,4-dichlorophenyl)-6-(ethoxymethyl)...)Show SMILES CCOC[C@@H]1[C@H]2CNC[C@@]12c1ccc(Cl)c(Cl)c1 |r| Show InChI InChI=1S/C14H17Cl2NO/c1-2-18-7-11-10-6-17-8-14(10,11)9-3-4-12(15)13(16)5-9/h3-5,10-11,17H,2,6-8H2,1H3/t10-,11-,14+/m1/s1 | Reactome pathway
UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.135 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Medicine Research Centre
Curated by ChEMBL
| Assay Description
Displacement of [N-methyl-3H]nisoxetine from rat hippocampus NET by filtration binding assay |
J Med Chem 53: 2534-51 (2010)
Article DOI: 10.1021/jm901818u
BindingDB Entry DOI: 10.7270/Q2ST7PX2 |
More data for this
Ligand-Target Pair | |
Sodium-dependent serotonin transporter
(MOUSE) | BDBM50308250

((1R,5R,6R)-1-(3,4-dichlorophenyl)-6-(ethoxymethyl)...)Show SMILES CCOC[C@@H]1[C@H]2CNC[C@@]12c1ccc(Cl)c(Cl)c1 |r| Show InChI InChI=1S/C14H17Cl2NO/c1-2-18-7-11-10-6-17-8-14(10,11)9-3-4-12(15)13(16)5-9/h3-5,10-11,17H,2,6-8H2,1H3/t10-,11-,14+/m1/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.851 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Medicine Research Centre
Curated by ChEMBL
| Assay Description
Displacement of [3H]citalopram from mouse cortex SERT by filtration binding assay |
J Med Chem 53: 2534-51 (2010)
Article DOI: 10.1021/jm901818u
BindingDB Entry DOI: 10.7270/Q2ST7PX2 |
More data for this
Ligand-Target Pair | |
Histamine H1 receptor
(Homo sapiens (Human)) | BDBM50308250

((1R,5R,6R)-1-(3,4-dichlorophenyl)-6-(ethoxymethyl)...)Show SMILES CCOC[C@@H]1[C@H]2CNC[C@@]12c1ccc(Cl)c(Cl)c1 |r| Show InChI InChI=1S/C14H17Cl2NO/c1-2-18-7-11-10-6-17-8-14(10,11)9-3-4-12(15)13(16)5-9/h3-5,10-11,17H,2,6-8H2,1H3/t10-,11-,14+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Medicine Research Centre
Curated by ChEMBL
| Assay Description
Inhibition of histamine H1 receptor |
J Med Chem 53: 2534-51 (2010)
Article DOI: 10.1021/jm901818u
BindingDB Entry DOI: 10.7270/Q2ST7PX2 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50308250

((1R,5R,6R)-1-(3,4-dichlorophenyl)-6-(ethoxymethyl)...)Show SMILES CCOC[C@@H]1[C@H]2CNC[C@@]12c1ccc(Cl)c(Cl)c1 |r| Show InChI InChI=1S/C14H17Cl2NO/c1-2-18-7-11-10-6-17-8-14(10,11)9-3-4-12(15)13(16)5-9/h3-5,10-11,17H,2,6-8H2,1H3/t10-,11-,14+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.26E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Medicine Research Centre
Curated by ChEMBL
| Assay Description
from Dfrom ifrom sfrom pfrom lfrom afrom cfrom efrom mfrom efrom nfrom tfrom from ofrom ffrom from [from 3from Hfrom ]from dfrom ofrom ffrom efrom tf... |
J Med Chem 53: 2534-51 (2010)
Article DOI: 10.1021/jm901818u
BindingDB Entry DOI: 10.7270/Q2ST7PX2 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2D6
(Homo sapiens (Human)) | BDBM50308254

(CHEMBL610220 | exo-6-(3,4-Dichlorophenyl)-6-{[(2,2...)Show SMILES FC(F)(F)COC[C@]1([C@H]2CNC[C@@H]12)c1ccc(Cl)c(Cl)c1 |r| Show InChI InChI=1S/C14H14Cl2F3NO/c15-11-2-1-8(3-12(11)16)13(6-21-7-14(17,18)19)9-4-20-5-10(9)13/h1-3,9-10,20H,4-7H2/t9-,10+,13+ | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 300 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Medicine Research Centre
Curated by ChEMBL
| Assay Description
Inhibition of human CYP2CD6 by fluorescence assay |
J Med Chem 53: 2534-51 (2010)
Article DOI: 10.1021/jm901818u
BindingDB Entry DOI: 10.7270/Q2ST7PX2 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 1A2
(Homo sapiens (Human)) | BDBM50308248
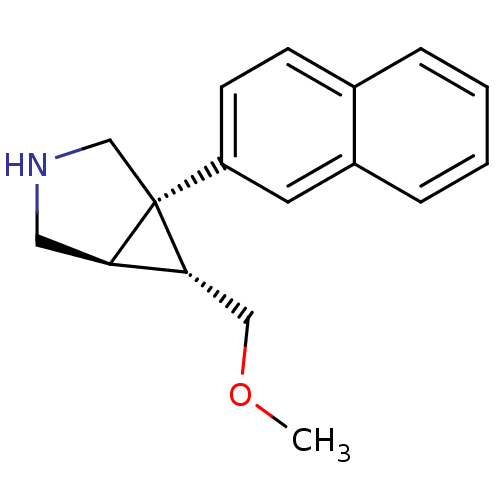
(CHEMBL598215 | exo-6-[(Methyloxy)methyl]-1-(2-naph...)Show SMILES COC[C@@H]1[C@H]2CNC[C@@]12c1ccc2ccccc2c1 |r| Show InChI InChI=1S/C17H19NO/c1-19-10-16-15-9-18-11-17(15,16)14-7-6-12-4-2-3-5-13(12)8-14/h2-8,15-16,18H,9-11H2,1H3/t15-,16-,17+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 900 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Medicine Research Centre
Curated by ChEMBL
| Assay Description
Inhibition of human CYP1A2 by fluorescence assay |
J Med Chem 53: 2534-51 (2010)
Article DOI: 10.1021/jm901818u
BindingDB Entry DOI: 10.7270/Q2ST7PX2 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2D6
(Homo sapiens (Human)) | BDBM50308246

(CHEMBL598644 | exo-6-(3,4-Dichlorophenyl)-3-azabic...)Show SMILES Clc1ccc(cc1Cl)[C@]1(COCC2CC2)[C@H]2CNC[C@@H]12 |r| Show InChI InChI=1S/C16H19Cl2NO/c17-14-4-3-11(5-15(14)18)16(9-20-8-10-1-2-10)12-6-19-7-13(12)16/h3-5,10,12-13,19H,1-2,6-9H2/t12-,13+,16+ | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Medicine Research Centre
Curated by ChEMBL
| Assay Description
Inhibition of human CYP2CD6 by fluorescence assay |
J Med Chem 53: 2534-51 (2010)
Article DOI: 10.1021/jm901818u
BindingDB Entry DOI: 10.7270/Q2ST7PX2 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2D6
(Homo sapiens (Human)) | BDBM50308253
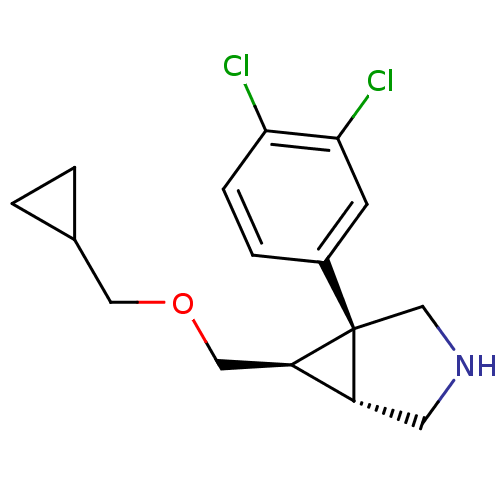
(CHEMBL610795 | exo-6-{[(Cyclopropylmethyl)oxy]meth...)Show SMILES Clc1ccc(cc1Cl)[C@@]12CNC[C@@H]1[C@H]2COCC1CC1 |r| Show InChI InChI=1S/C16H19Cl2NO/c17-14-4-3-11(5-15(14)18)16-9-19-6-12(16)13(16)8-20-7-10-1-2-10/h3-5,10,12-13,19H,1-2,6-9H2/t12-,13-,16+/m1/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Medicine Research Centre
Curated by ChEMBL
| Assay Description
Inhibition of human CYP2CD6 by fluorescence assay |
J Med Chem 53: 2534-51 (2010)
Article DOI: 10.1021/jm901818u
BindingDB Entry DOI: 10.7270/Q2ST7PX2 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50308245
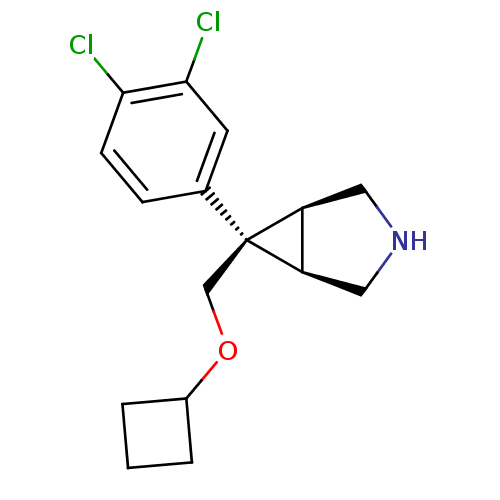
(CHEMBL590472 | exo-6-[(Cyclobutyloxy)methyl]-6-(3,...)Show SMILES Clc1ccc(cc1Cl)[C@]1(COC2CCC2)[C@H]2CNC[C@@H]12 |r| Show InChI InChI=1S/C16H19Cl2NO/c17-14-5-4-10(6-15(14)18)16(9-20-11-2-1-3-11)12-7-19-8-13(12)16/h4-6,11-13,19H,1-3,7-9H2/t12-,13+,16+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Medicine Research Centre
Curated by ChEMBL
| Assay Description
Inhibition of human CYP3A4 by fluorescence assay |
J Med Chem 53: 2534-51 (2010)
Article DOI: 10.1021/jm901818u
BindingDB Entry DOI: 10.7270/Q2ST7PX2 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C19
(Homo sapiens (Human)) | BDBM50308253

(CHEMBL610795 | exo-6-{[(Cyclopropylmethyl)oxy]meth...)Show SMILES Clc1ccc(cc1Cl)[C@@]12CNC[C@@H]1[C@H]2COCC1CC1 |r| Show InChI InChI=1S/C16H19Cl2NO/c17-14-4-3-11(5-15(14)18)16-9-19-6-12(16)13(16)8-20-7-10-1-2-10/h3-5,10,12-13,19H,1-2,6-9H2/t12-,13-,16+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Medicine Research Centre
Curated by ChEMBL
| Assay Description
Inhibition of human CYP2C19 by fluorescence assay |
J Med Chem 53: 2534-51 (2010)
Article DOI: 10.1021/jm901818u
BindingDB Entry DOI: 10.7270/Q2ST7PX2 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50308248

(CHEMBL598215 | exo-6-[(Methyloxy)methyl]-1-(2-naph...)Show SMILES COC[C@@H]1[C@H]2CNC[C@@]12c1ccc2ccccc2c1 |r| Show InChI InChI=1S/C17H19NO/c1-19-10-16-15-9-18-11-17(15,16)14-7-6-12-4-2-3-5-13(12)8-14/h2-8,15-16,18H,9-11H2,1H3/t15-,16-,17+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >4.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Medicine Research Centre
Curated by ChEMBL
| Assay Description
Inhibition of human CYP3A4 by fluorescence assay |
J Med Chem 53: 2534-51 (2010)
Article DOI: 10.1021/jm901818u
BindingDB Entry DOI: 10.7270/Q2ST7PX2 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50308246

(CHEMBL598644 | exo-6-(3,4-Dichlorophenyl)-3-azabic...)Show SMILES Clc1ccc(cc1Cl)[C@]1(COCC2CC2)[C@H]2CNC[C@@H]12 |r| Show InChI InChI=1S/C16H19Cl2NO/c17-14-4-3-11(5-15(14)18)16(9-20-8-10-1-2-10)12-6-19-7-13(12)16/h3-5,10,12-13,19H,1-2,6-9H2/t12-,13+,16+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Medicine Research Centre
Curated by ChEMBL
| Assay Description
Inhibition of human CYP3A4 by fluorescence assay |
J Med Chem 53: 2534-51 (2010)
Article DOI: 10.1021/jm901818u
BindingDB Entry DOI: 10.7270/Q2ST7PX2 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2D6
(Homo sapiens (Human)) | BDBM50415478

(CHEMBL2021577)Show InChI InChI=1S/C9H17NO/c1-3-11-5-8-7-4-10-6-9(7,8)2/h7-8,10H,3-6H2,1-2H3/t7-,8-,9-/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 4.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Medicine Research Centre
Curated by ChEMBL
| Assay Description
Inhibition of human CYP2CD6 by fluorescence assay |
J Med Chem 53: 2534-51 (2010)
Article DOI: 10.1021/jm901818u
BindingDB Entry DOI: 10.7270/Q2ST7PX2 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C19
(Homo sapiens (Human)) | BDBM50308248

(CHEMBL598215 | exo-6-[(Methyloxy)methyl]-1-(2-naph...)Show SMILES COC[C@@H]1[C@H]2CNC[C@@]12c1ccc2ccccc2c1 |r| Show InChI InChI=1S/C17H19NO/c1-19-10-16-15-9-18-11-17(15,16)14-7-6-12-4-2-3-5-13(12)8-14/h2-8,15-16,18H,9-11H2,1H3/t15-,16-,17+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >4.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Medicine Research Centre
Curated by ChEMBL
| Assay Description
Inhibition of human CYP2C19 by fluorescence assay |
J Med Chem 53: 2534-51 (2010)
Article DOI: 10.1021/jm901818u
BindingDB Entry DOI: 10.7270/Q2ST7PX2 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C9
(Homo sapiens (Human)) | BDBM50308248

(CHEMBL598215 | exo-6-[(Methyloxy)methyl]-1-(2-naph...)Show SMILES COC[C@@H]1[C@H]2CNC[C@@]12c1ccc2ccccc2c1 |r| Show InChI InChI=1S/C17H19NO/c1-19-10-16-15-9-18-11-17(15,16)14-7-6-12-4-2-3-5-13(12)8-14/h2-8,15-16,18H,9-11H2,1H3/t15-,16-,17+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >4.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Medicine Research Centre
Curated by ChEMBL
| Assay Description
Inhibition of human CYP2C9 by fluorescence assay |
J Med Chem 53: 2534-51 (2010)
Article DOI: 10.1021/jm901818u
BindingDB Entry DOI: 10.7270/Q2ST7PX2 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2D6
(Homo sapiens (Human)) | BDBM50308251
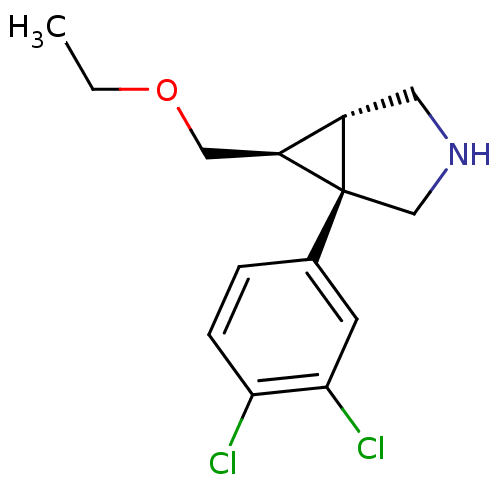
((1S,5S,6S)-1-(3,4-Dichlorophenyl)-6-[(ethyloxy)met...)Show SMILES CCOC[C@H]1[C@@H]2CNC[C@]12c1ccc(Cl)c(Cl)c1 |r| Show InChI InChI=1S/C14H17Cl2NO/c1-2-18-7-11-10-6-17-8-14(10,11)9-3-4-12(15)13(16)5-9/h3-5,10-11,17H,2,6-8H2,1H3/t10-,11-,14+/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 4.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Medicine Research Centre
Curated by ChEMBL
| Assay Description
Inhibition of human CYP2CD6 by fluorescence assay |
J Med Chem 53: 2534-51 (2010)
Article DOI: 10.1021/jm901818u
BindingDB Entry DOI: 10.7270/Q2ST7PX2 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2D6
(Homo sapiens (Human)) | BDBM50308248

(CHEMBL598215 | exo-6-[(Methyloxy)methyl]-1-(2-naph...)Show SMILES COC[C@@H]1[C@H]2CNC[C@@]12c1ccc2ccccc2c1 |r| Show InChI InChI=1S/C17H19NO/c1-19-10-16-15-9-18-11-17(15,16)14-7-6-12-4-2-3-5-13(12)8-14/h2-8,15-16,18H,9-11H2,1H3/t15-,16-,17+/m1/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >4.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Medicine Research Centre
Curated by ChEMBL
| Assay Description
Inhibition of human CYP2CD6 by fluorescence assay |
J Med Chem 53: 2534-51 (2010)
Article DOI: 10.1021/jm901818u
BindingDB Entry DOI: 10.7270/Q2ST7PX2 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50308251

((1S,5S,6S)-1-(3,4-Dichlorophenyl)-6-[(ethyloxy)met...)Show SMILES CCOC[C@H]1[C@@H]2CNC[C@]12c1ccc(Cl)c(Cl)c1 |r| Show InChI InChI=1S/C14H17Cl2NO/c1-2-18-7-11-10-6-17-8-14(10,11)9-3-4-12(15)13(16)5-9/h3-5,10-11,17H,2,6-8H2,1H3/t10-,11-,14+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 4.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Medicine Research Centre
Curated by ChEMBL
| Assay Description
Inhibition of human ERG assessed as blockade of channel current by electrophysiology assay |
J Med Chem 53: 2534-51 (2010)
Article DOI: 10.1021/jm901818u
BindingDB Entry DOI: 10.7270/Q2ST7PX2 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2D6
(Homo sapiens (Human)) | BDBM50415477

(CHEMBL2021582)Show SMILES CCOC[C@]1([C@H]2CCC[C@@H]12)c1ccc(Cl)c(Cl)c1 |r| Show InChI InChI=1S/C15H18Cl2O/c1-2-18-9-15(11-4-3-5-12(11)15)10-6-7-13(16)14(17)8-10/h6-8,11-12H,2-5,9H2,1H3/t11-,12+,15+ | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >5.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Medicine Research Centre
Curated by ChEMBL
| Assay Description
Inhibition of human CYP2CD6 by fluorescence assay |
J Med Chem 53: 2534-51 (2010)
Article DOI: 10.1021/jm901818u
BindingDB Entry DOI: 10.7270/Q2ST7PX2 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C19
(Homo sapiens (Human)) | BDBM50415477

(CHEMBL2021582)Show SMILES CCOC[C@]1([C@H]2CCC[C@@H]12)c1ccc(Cl)c(Cl)c1 |r| Show InChI InChI=1S/C15H18Cl2O/c1-2-18-9-15(11-4-3-5-12(11)15)10-6-7-13(16)14(17)8-10/h6-8,11-12H,2-5,9H2,1H3/t11-,12+,15+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >5.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Medicine Research Centre
Curated by ChEMBL
| Assay Description
Inhibition of human CYP2C19 by fluorescence assay |
J Med Chem 53: 2534-51 (2010)
Article DOI: 10.1021/jm901818u
BindingDB Entry DOI: 10.7270/Q2ST7PX2 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C9
(Homo sapiens (Human)) | BDBM50415477

(CHEMBL2021582)Show SMILES CCOC[C@]1([C@H]2CCC[C@@H]12)c1ccc(Cl)c(Cl)c1 |r| Show InChI InChI=1S/C15H18Cl2O/c1-2-18-9-15(11-4-3-5-12(11)15)10-6-7-13(16)14(17)8-10/h6-8,11-12H,2-5,9H2,1H3/t11-,12+,15+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >5.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Medicine Research Centre
Curated by ChEMBL
| Assay Description
Inhibition of human CYP2C9 by fluorescence assay |
J Med Chem 53: 2534-51 (2010)
Article DOI: 10.1021/jm901818u
BindingDB Entry DOI: 10.7270/Q2ST7PX2 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 1A2
(Homo sapiens (Human)) | BDBM50415477

(CHEMBL2021582)Show SMILES CCOC[C@]1([C@H]2CCC[C@@H]12)c1ccc(Cl)c(Cl)c1 |r| Show InChI InChI=1S/C15H18Cl2O/c1-2-18-9-15(11-4-3-5-12(11)15)10-6-7-13(16)14(17)8-10/h6-8,11-12H,2-5,9H2,1H3/t11-,12+,15+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >5.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Medicine Research Centre
Curated by ChEMBL
| Assay Description
Inhibition of human CYP1A2 by fluorescence assay |
J Med Chem 53: 2534-51 (2010)
Article DOI: 10.1021/jm901818u
BindingDB Entry DOI: 10.7270/Q2ST7PX2 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50308252
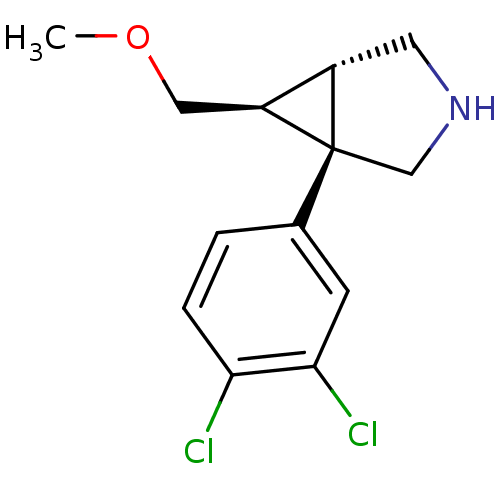
(CHEMBL596809 | exo-1-(3,4-dichlorophenyl)-6-(metho...)Show SMILES COC[C@H]1[C@@H]2CNC[C@]12c1ccc(Cl)c(Cl)c1 |r| Show InChI InChI=1S/C13H15Cl2NO/c1-17-6-10-9-5-16-7-13(9,10)8-2-3-11(14)12(15)4-8/h2-4,9-10,16H,5-7H2,1H3/t9-,10-,13+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >6.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Medicine Research Centre
Curated by ChEMBL
| Assay Description
Inhibition of human CYP3A4 by fluorescence assay |
J Med Chem 53: 2534-51 (2010)
Article DOI: 10.1021/jm901818u
BindingDB Entry DOI: 10.7270/Q2ST7PX2 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C19
(Homo sapiens (Human)) | BDBM50308252

(CHEMBL596809 | exo-1-(3,4-dichlorophenyl)-6-(metho...)Show SMILES COC[C@H]1[C@@H]2CNC[C@]12c1ccc(Cl)c(Cl)c1 |r| Show InChI InChI=1S/C13H15Cl2NO/c1-17-6-10-9-5-16-7-13(9,10)8-2-3-11(14)12(15)4-8/h2-4,9-10,16H,5-7H2,1H3/t9-,10-,13+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >6.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Medicine Research Centre
Curated by ChEMBL
| Assay Description
Inhibition of human CYP2C19 by fluorescence assay |
J Med Chem 53: 2534-51 (2010)
Article DOI: 10.1021/jm901818u
BindingDB Entry DOI: 10.7270/Q2ST7PX2 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2D6
(Homo sapiens (Human)) | BDBM50308252

(CHEMBL596809 | exo-1-(3,4-dichlorophenyl)-6-(metho...)Show SMILES COC[C@H]1[C@@H]2CNC[C@]12c1ccc(Cl)c(Cl)c1 |r| Show InChI InChI=1S/C13H15Cl2NO/c1-17-6-10-9-5-16-7-13(9,10)8-2-3-11(14)12(15)4-8/h2-4,9-10,16H,5-7H2,1H3/t9-,10-,13+/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >6.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Medicine Research Centre
Curated by ChEMBL
| Assay Description
Inhibition of human CYP2CD6 by fluorescence assay |
J Med Chem 53: 2534-51 (2010)
Article DOI: 10.1021/jm901818u
BindingDB Entry DOI: 10.7270/Q2ST7PX2 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C9
(Homo sapiens (Human)) | BDBM50308252

(CHEMBL596809 | exo-1-(3,4-dichlorophenyl)-6-(metho...)Show SMILES COC[C@H]1[C@@H]2CNC[C@]12c1ccc(Cl)c(Cl)c1 |r| Show InChI InChI=1S/C13H15Cl2NO/c1-17-6-10-9-5-16-7-13(9,10)8-2-3-11(14)12(15)4-8/h2-4,9-10,16H,5-7H2,1H3/t9-,10-,13+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >6.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Medicine Research Centre
Curated by ChEMBL
| Assay Description
Inhibition of human CYP2C9 by fluorescence assay |
J Med Chem 53: 2534-51 (2010)
Article DOI: 10.1021/jm901818u
BindingDB Entry DOI: 10.7270/Q2ST7PX2 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 1A2
(Homo sapiens (Human)) | BDBM50308252

(CHEMBL596809 | exo-1-(3,4-dichlorophenyl)-6-(metho...)Show SMILES COC[C@H]1[C@@H]2CNC[C@]12c1ccc(Cl)c(Cl)c1 |r| Show InChI InChI=1S/C13H15Cl2NO/c1-17-6-10-9-5-16-7-13(9,10)8-2-3-11(14)12(15)4-8/h2-4,9-10,16H,5-7H2,1H3/t9-,10-,13+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >6.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Medicine Research Centre
Curated by ChEMBL
| Assay Description
Inhibition of human CYP1A2 by fluorescence assay |
J Med Chem 53: 2534-51 (2010)
Article DOI: 10.1021/jm901818u
BindingDB Entry DOI: 10.7270/Q2ST7PX2 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C19
(Homo sapiens (Human)) | BDBM50308249
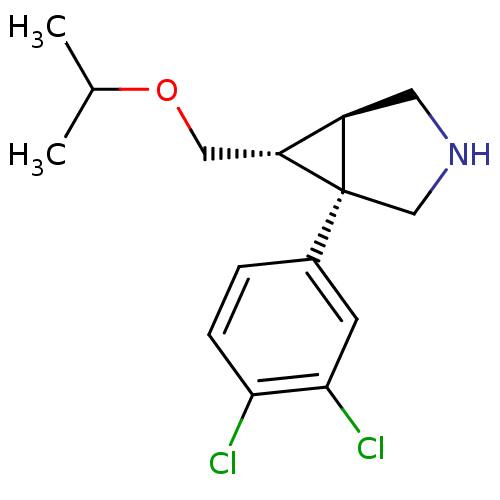
(CHEMBL605209 | exo-1-(3,4-Dichlorophenyl)-6-{[(1-m...)Show SMILES CC(C)OC[C@@H]1[C@H]2CNC[C@@]12c1ccc(Cl)c(Cl)c1 |r| Show InChI InChI=1S/C15H19Cl2NO/c1-9(2)19-7-12-11-6-18-8-15(11,12)10-3-4-13(16)14(17)5-10/h3-5,9,11-12,18H,6-8H2,1-2H3/t11-,12-,15+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >8.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Medicine Research Centre
Curated by ChEMBL
| Assay Description
Inhibition of human CYP2C19 by fluorescence assay |
J Med Chem 53: 2534-51 (2010)
Article DOI: 10.1021/jm901818u
BindingDB Entry DOI: 10.7270/Q2ST7PX2 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 1A2
(Homo sapiens (Human)) | BDBM50308249

(CHEMBL605209 | exo-1-(3,4-Dichlorophenyl)-6-{[(1-m...)Show SMILES CC(C)OC[C@@H]1[C@H]2CNC[C@@]12c1ccc(Cl)c(Cl)c1 |r| Show InChI InChI=1S/C15H19Cl2NO/c1-9(2)19-7-12-11-6-18-8-15(11,12)10-3-4-13(16)14(17)5-10/h3-5,9,11-12,18H,6-8H2,1-2H3/t11-,12-,15+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >8.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Medicine Research Centre
Curated by ChEMBL
| Assay Description
Inhibition of human CYP1A2 by fluorescence assay |
J Med Chem 53: 2534-51 (2010)
Article DOI: 10.1021/jm901818u
BindingDB Entry DOI: 10.7270/Q2ST7PX2 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2D6
(Homo sapiens (Human)) | BDBM50308249

(CHEMBL605209 | exo-1-(3,4-Dichlorophenyl)-6-{[(1-m...)Show SMILES CC(C)OC[C@@H]1[C@H]2CNC[C@@]12c1ccc(Cl)c(Cl)c1 |r| Show InChI InChI=1S/C15H19Cl2NO/c1-9(2)19-7-12-11-6-18-8-15(11,12)10-3-4-13(16)14(17)5-10/h3-5,9,11-12,18H,6-8H2,1-2H3/t11-,12-,15+/m1/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >8.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Medicine Research Centre
Curated by ChEMBL
| Assay Description
Inhibition of human CYP2CD6 by fluorescence assay |
J Med Chem 53: 2534-51 (2010)
Article DOI: 10.1021/jm901818u
BindingDB Entry DOI: 10.7270/Q2ST7PX2 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C9
(Homo sapiens (Human)) | BDBM50308249

(CHEMBL605209 | exo-1-(3,4-Dichlorophenyl)-6-{[(1-m...)Show SMILES CC(C)OC[C@@H]1[C@H]2CNC[C@@]12c1ccc(Cl)c(Cl)c1 |r| Show InChI InChI=1S/C15H19Cl2NO/c1-9(2)19-7-12-11-6-18-8-15(11,12)10-3-4-13(16)14(17)5-10/h3-5,9,11-12,18H,6-8H2,1-2H3/t11-,12-,15+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >8.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Medicine Research Centre
Curated by ChEMBL
| Assay Description
Inhibition of human CYP2C9 by fluorescence assay |
J Med Chem 53: 2534-51 (2010)
Article DOI: 10.1021/jm901818u
BindingDB Entry DOI: 10.7270/Q2ST7PX2 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50308249

(CHEMBL605209 | exo-1-(3,4-Dichlorophenyl)-6-{[(1-m...)Show SMILES CC(C)OC[C@@H]1[C@H]2CNC[C@@]12c1ccc(Cl)c(Cl)c1 |r| Show InChI InChI=1S/C15H19Cl2NO/c1-9(2)19-7-12-11-6-18-8-15(11,12)10-3-4-13(16)14(17)5-10/h3-5,9,11-12,18H,6-8H2,1-2H3/t11-,12-,15+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >8.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Medicine Research Centre
Curated by ChEMBL
| Assay Description
Inhibition of human CYP3A4 by fluorescence assay |
J Med Chem 53: 2534-51 (2010)
Article DOI: 10.1021/jm901818u
BindingDB Entry DOI: 10.7270/Q2ST7PX2 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C19
(Homo sapiens (Human)) | BDBM50415478

(CHEMBL2021577)Show InChI InChI=1S/C9H17NO/c1-3-11-5-8-7-4-10-6-9(7,8)2/h7-8,10H,3-6H2,1-2H3/t7-,8-,9-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >9.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Medicine Research Centre
Curated by ChEMBL
| Assay Description
Inhibition of human CYP2C19 by fluorescence assay |
J Med Chem 53: 2534-51 (2010)
Article DOI: 10.1021/jm901818u
BindingDB Entry DOI: 10.7270/Q2ST7PX2 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50308251

((1S,5S,6S)-1-(3,4-Dichlorophenyl)-6-[(ethyloxy)met...)Show SMILES CCOC[C@H]1[C@@H]2CNC[C@]12c1ccc(Cl)c(Cl)c1 |r| Show InChI InChI=1S/C14H17Cl2NO/c1-2-18-7-11-10-6-17-8-14(10,11)9-3-4-12(15)13(16)5-9/h3-5,10-11,17H,2,6-8H2,1H3/t10-,11-,14+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >9.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Medicine Research Centre
Curated by ChEMBL
| Assay Description
Inhibition of human CYP3A4 by fluorescence assay |
J Med Chem 53: 2534-51 (2010)
Article DOI: 10.1021/jm901818u
BindingDB Entry DOI: 10.7270/Q2ST7PX2 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 1A2
(Homo sapiens (Human)) | BDBM50415478

(CHEMBL2021577)Show InChI InChI=1S/C9H17NO/c1-3-11-5-8-7-4-10-6-9(7,8)2/h7-8,10H,3-6H2,1-2H3/t7-,8-,9-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >9.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Medicine Research Centre
Curated by ChEMBL
| Assay Description
Inhibition of human CYP1A2 by fluorescence assay |
J Med Chem 53: 2534-51 (2010)
Article DOI: 10.1021/jm901818u
BindingDB Entry DOI: 10.7270/Q2ST7PX2 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C19
(Homo sapiens (Human)) | BDBM50308251

((1S,5S,6S)-1-(3,4-Dichlorophenyl)-6-[(ethyloxy)met...)Show SMILES CCOC[C@H]1[C@@H]2CNC[C@]12c1ccc(Cl)c(Cl)c1 |r| Show InChI InChI=1S/C14H17Cl2NO/c1-2-18-7-11-10-6-17-8-14(10,11)9-3-4-12(15)13(16)5-9/h3-5,10-11,17H,2,6-8H2,1H3/t10-,11-,14+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >9.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Medicine Research Centre
Curated by ChEMBL
| Assay Description
Inhibition of human CYP2C19 by fluorescence assay |
J Med Chem 53: 2534-51 (2010)
Article DOI: 10.1021/jm901818u
BindingDB Entry DOI: 10.7270/Q2ST7PX2 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C9
(Homo sapiens (Human)) | BDBM50308251

((1S,5S,6S)-1-(3,4-Dichlorophenyl)-6-[(ethyloxy)met...)Show SMILES CCOC[C@H]1[C@@H]2CNC[C@]12c1ccc(Cl)c(Cl)c1 |r| Show InChI InChI=1S/C14H17Cl2NO/c1-2-18-7-11-10-6-17-8-14(10,11)9-3-4-12(15)13(16)5-9/h3-5,10-11,17H,2,6-8H2,1H3/t10-,11-,14+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >9.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Medicine Research Centre
Curated by ChEMBL
| Assay Description
Inhibition of human CYP2C9 by fluorescence assay |
J Med Chem 53: 2534-51 (2010)
Article DOI: 10.1021/jm901818u
BindingDB Entry DOI: 10.7270/Q2ST7PX2 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C9
(Homo sapiens (Human)) | BDBM50415478

(CHEMBL2021577)Show InChI InChI=1S/C9H17NO/c1-3-11-5-8-7-4-10-6-9(7,8)2/h7-8,10H,3-6H2,1-2H3/t7-,8-,9-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >9.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Medicine Research Centre
Curated by ChEMBL
| Assay Description
Inhibition of human CYP2C9 by fluorescence assay |
J Med Chem 53: 2534-51 (2010)
Article DOI: 10.1021/jm901818u
BindingDB Entry DOI: 10.7270/Q2ST7PX2 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 1A2
(Homo sapiens (Human)) | BDBM50308251

((1S,5S,6S)-1-(3,4-Dichlorophenyl)-6-[(ethyloxy)met...)Show SMILES CCOC[C@H]1[C@@H]2CNC[C@]12c1ccc(Cl)c(Cl)c1 |r| Show InChI InChI=1S/C14H17Cl2NO/c1-2-18-7-11-10-6-17-8-14(10,11)9-3-4-12(15)13(16)5-9/h3-5,10-11,17H,2,6-8H2,1H3/t10-,11-,14+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >9.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Medicine Research Centre
Curated by ChEMBL
| Assay Description
Inhibition of human CYP1A2 by fluorescence assay |
J Med Chem 53: 2534-51 (2010)
Article DOI: 10.1021/jm901818u
BindingDB Entry DOI: 10.7270/Q2ST7PX2 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C19
(Homo sapiens (Human)) | BDBM50308245

(CHEMBL590472 | exo-6-[(Cyclobutyloxy)methyl]-6-(3,...)Show SMILES Clc1ccc(cc1Cl)[C@]1(COC2CCC2)[C@H]2CNC[C@@H]12 |r| Show InChI InChI=1S/C16H19Cl2NO/c17-14-5-4-10(6-15(14)18)16(9-20-11-2-1-3-11)12-7-19-8-13(12)16/h4-6,11-13,19H,1-3,7-9H2/t12-,13+,16+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Medicine Research Centre
Curated by ChEMBL
| Assay Description
Inhibition of human CYP2C19 by fluorescence assay |
J Med Chem 53: 2534-51 (2010)
Article DOI: 10.1021/jm901818u
BindingDB Entry DOI: 10.7270/Q2ST7PX2 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2D6
(Homo sapiens (Human)) | BDBM50308245

(CHEMBL590472 | exo-6-[(Cyclobutyloxy)methyl]-6-(3,...)Show SMILES Clc1ccc(cc1Cl)[C@]1(COC2CCC2)[C@H]2CNC[C@@H]12 |r| Show InChI InChI=1S/C16H19Cl2NO/c17-14-5-4-10(6-15(14)18)16(9-20-11-2-1-3-11)12-7-19-8-13(12)16/h4-6,11-13,19H,1-3,7-9H2/t12-,13+,16+ | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Medicine Research Centre
Curated by ChEMBL
| Assay Description
Inhibition of human CYP2CD6 by fluorescence assay |
J Med Chem 53: 2534-51 (2010)
Article DOI: 10.1021/jm901818u
BindingDB Entry DOI: 10.7270/Q2ST7PX2 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C9
(Homo sapiens (Human)) | BDBM50308246

(CHEMBL598644 | exo-6-(3,4-Dichlorophenyl)-3-azabic...)Show SMILES Clc1ccc(cc1Cl)[C@]1(COCC2CC2)[C@H]2CNC[C@@H]12 |r| Show InChI InChI=1S/C16H19Cl2NO/c17-14-4-3-11(5-15(14)18)16(9-20-8-10-1-2-10)12-6-19-7-13(12)16/h3-5,10,12-13,19H,1-2,6-9H2/t12-,13+,16+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Medicine Research Centre
Curated by ChEMBL
| Assay Description
Inhibition of human CYP2C9 by fluorescence assay |
J Med Chem 53: 2534-51 (2010)
Article DOI: 10.1021/jm901818u
BindingDB Entry DOI: 10.7270/Q2ST7PX2 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 1A2
(Homo sapiens (Human)) | BDBM50308253

(CHEMBL610795 | exo-6-{[(Cyclopropylmethyl)oxy]meth...)Show SMILES Clc1ccc(cc1Cl)[C@@]12CNC[C@@H]1[C@H]2COCC1CC1 |r| Show InChI InChI=1S/C16H19Cl2NO/c17-14-4-3-11(5-15(14)18)16-9-19-6-12(16)13(16)8-20-7-10-1-2-10/h3-5,10,12-13,19H,1-2,6-9H2/t12-,13-,16+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Medicine Research Centre
Curated by ChEMBL
| Assay Description
Inhibition of human CYP1A2 by fluorescence assay |
J Med Chem 53: 2534-51 (2010)
Article DOI: 10.1021/jm901818u
BindingDB Entry DOI: 10.7270/Q2ST7PX2 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 1A2
(Homo sapiens (Human)) | BDBM50308245

(CHEMBL590472 | exo-6-[(Cyclobutyloxy)methyl]-6-(3,...)Show SMILES Clc1ccc(cc1Cl)[C@]1(COC2CCC2)[C@H]2CNC[C@@H]12 |r| Show InChI InChI=1S/C16H19Cl2NO/c17-14-5-4-10(6-15(14)18)16(9-20-11-2-1-3-11)12-7-19-8-13(12)16/h4-6,11-13,19H,1-3,7-9H2/t12-,13+,16+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Medicine Research Centre
Curated by ChEMBL
| Assay Description
Inhibition of human CYP1A2 by fluorescence assay |
J Med Chem 53: 2534-51 (2010)
Article DOI: 10.1021/jm901818u
BindingDB Entry DOI: 10.7270/Q2ST7PX2 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C9
(Homo sapiens (Human)) | BDBM50308253

(CHEMBL610795 | exo-6-{[(Cyclopropylmethyl)oxy]meth...)Show SMILES Clc1ccc(cc1Cl)[C@@]12CNC[C@@H]1[C@H]2COCC1CC1 |r| Show InChI InChI=1S/C16H19Cl2NO/c17-14-4-3-11(5-15(14)18)16-9-19-6-12(16)13(16)8-20-7-10-1-2-10/h3-5,10,12-13,19H,1-2,6-9H2/t12-,13-,16+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Medicine Research Centre
Curated by ChEMBL
| Assay Description
Inhibition of human CYP2C9 by fluorescence assay |
J Med Chem 53: 2534-51 (2010)
Article DOI: 10.1021/jm901818u
BindingDB Entry DOI: 10.7270/Q2ST7PX2 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C9
(Homo sapiens (Human)) | BDBM50308245

(CHEMBL590472 | exo-6-[(Cyclobutyloxy)methyl]-6-(3,...)Show SMILES Clc1ccc(cc1Cl)[C@]1(COC2CCC2)[C@H]2CNC[C@@H]12 |r| Show InChI InChI=1S/C16H19Cl2NO/c17-14-5-4-10(6-15(14)18)16(9-20-11-2-1-3-11)12-7-19-8-13(12)16/h4-6,11-13,19H,1-3,7-9H2/t12-,13+,16+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Medicine Research Centre
Curated by ChEMBL
| Assay Description
Inhibition of human CYP2C9 by fluorescence assay |
J Med Chem 53: 2534-51 (2010)
Article DOI: 10.1021/jm901818u
BindingDB Entry DOI: 10.7270/Q2ST7PX2 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C19
(Homo sapiens (Human)) | BDBM50308246

(CHEMBL598644 | exo-6-(3,4-Dichlorophenyl)-3-azabic...)Show SMILES Clc1ccc(cc1Cl)[C@]1(COCC2CC2)[C@H]2CNC[C@@H]12 |r| Show InChI InChI=1S/C16H19Cl2NO/c17-14-4-3-11(5-15(14)18)16(9-20-8-10-1-2-10)12-6-19-7-13(12)16/h3-5,10,12-13,19H,1-2,6-9H2/t12-,13+,16+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Medicine Research Centre
Curated by ChEMBL
| Assay Description
Inhibition of human CYP2C19 by fluorescence assay |
J Med Chem 53: 2534-51 (2010)
Article DOI: 10.1021/jm901818u
BindingDB Entry DOI: 10.7270/Q2ST7PX2 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 1A2
(Homo sapiens (Human)) | BDBM50308246

(CHEMBL598644 | exo-6-(3,4-Dichlorophenyl)-3-azabic...)Show SMILES Clc1ccc(cc1Cl)[C@]1(COCC2CC2)[C@H]2CNC[C@@H]12 |r| Show InChI InChI=1S/C16H19Cl2NO/c17-14-4-3-11(5-15(14)18)16(9-20-8-10-1-2-10)12-6-19-7-13(12)16/h3-5,10,12-13,19H,1-2,6-9H2/t12-,13+,16+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Medicine Research Centre
Curated by ChEMBL
| Assay Description
Inhibition of human CYP1A2 by fluorescence assay |
J Med Chem 53: 2534-51 (2010)
Article DOI: 10.1021/jm901818u
BindingDB Entry DOI: 10.7270/Q2ST7PX2 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50308253

(CHEMBL610795 | exo-6-{[(Cyclopropylmethyl)oxy]meth...)Show SMILES Clc1ccc(cc1Cl)[C@@]12CNC[C@@H]1[C@H]2COCC1CC1 |r| Show InChI InChI=1S/C16H19Cl2NO/c17-14-4-3-11(5-15(14)18)16-9-19-6-12(16)13(16)8-20-7-10-1-2-10/h3-5,10,12-13,19H,1-2,6-9H2/t12-,13-,16+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Medicine Research Centre
Curated by ChEMBL
| Assay Description
Inhibition of human CYP3A4 by fluorescence assay |
J Med Chem 53: 2534-51 (2010)
Article DOI: 10.1021/jm901818u
BindingDB Entry DOI: 10.7270/Q2ST7PX2 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data