 Found 41 hits of Enzyme Inhibition Constant Data
Found 41 hits of Enzyme Inhibition Constant Data Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Insulin-like growth factor 1 receptor
(Rattus norvegicus) | BDBM50318112

((S)-4-(2-(3-chlorophenyl)-2-hydroxyethylamino)-3-(...)Show SMILES COCCN1CCN(CC1)c1cc(C)c2nc([nH]c2c1)-c1c(NC[C@@H](O)c2cccc(Cl)c2)cc[nH]c1=O |r| Show InChI InChI=1S/C28H33ClN6O3/c1-18-14-21(35-10-8-34(9-11-35)12-13-38-2)16-23-26(18)33-27(32-23)25-22(6-7-30-28(25)37)31-17-24(36)19-4-3-5-20(29)15-19/h3-7,14-16,24,36H,8-13,17H2,1-2H3,(H,32,33)(H2,30,31,37)/t24-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 28 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company
Curated by ChEMBL
| Assay Description
Inhibition of rat IGF1R |
Bioorg Med Chem Lett 20: 3182-5 (2010)
Article DOI: 10.1016/j.bmcl.2010.03.057
BindingDB Entry DOI: 10.7270/Q2GX4BQB |
More data for this
Ligand-Target Pair | |
Insulin-like growth factor 1 receptor
(Homo sapiens (Human)) | BDBM50318122
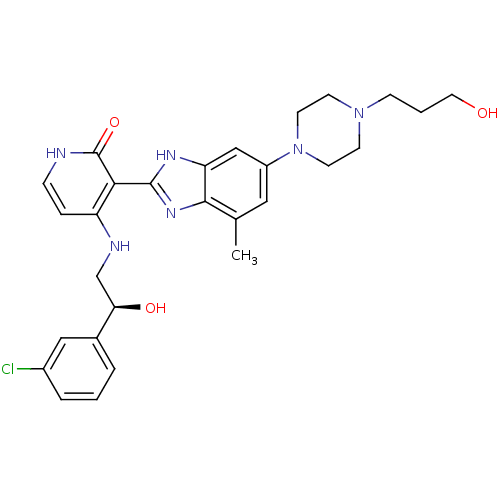
((S)-4-(2-(3-chlorophenyl)-2-hydroxyethylamino)-3-(...)Show SMILES Cc1cc(cc2[nH]c(nc12)-c1c(NC[C@@H](O)c2cccc(Cl)c2)cc[nH]c1=O)N1CCN(CCCO)CC1 |r| Show InChI InChI=1S/C28H33ClN6O3/c1-18-14-21(35-11-9-34(10-12-35)8-3-13-36)16-23-26(18)33-27(32-23)25-22(6-7-30-28(25)38)31-17-24(37)19-4-2-5-20(29)15-19/h2,4-7,14-16,24,36-37H,3,8-13,17H2,1H3,(H,32,33)(H2,30,31,38)/t24-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company
Curated by ChEMBL
| Assay Description
Inhibition of IGF1R |
Bioorg Med Chem Lett 20: 3182-5 (2010)
Article DOI: 10.1016/j.bmcl.2010.03.057
BindingDB Entry DOI: 10.7270/Q2GX4BQB |
More data for this
Ligand-Target Pair | |
Insulin-like growth factor 1 receptor
(Homo sapiens (Human)) | BDBM50318112

((S)-4-(2-(3-chlorophenyl)-2-hydroxyethylamino)-3-(...)Show SMILES COCCN1CCN(CC1)c1cc(C)c2nc([nH]c2c1)-c1c(NC[C@@H](O)c2cccc(Cl)c2)cc[nH]c1=O |r| Show InChI InChI=1S/C28H33ClN6O3/c1-18-14-21(35-10-8-34(9-11-35)12-13-38-2)16-23-26(18)33-27(32-23)25-22(6-7-30-28(25)37)31-17-24(36)19-4-3-5-20(29)15-19/h3-7,14-16,24,36H,8-13,17H2,1-2H3,(H,32,33)(H2,30,31,37)/t24-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company
Curated by ChEMBL
| Assay Description
Inhibition of IGF1R |
Bioorg Med Chem Lett 20: 3182-5 (2010)
Article DOI: 10.1016/j.bmcl.2010.03.057
BindingDB Entry DOI: 10.7270/Q2GX4BQB |
More data for this
Ligand-Target Pair | |
Insulin-like growth factor 1 receptor
(Homo sapiens (Human)) | BDBM50318113
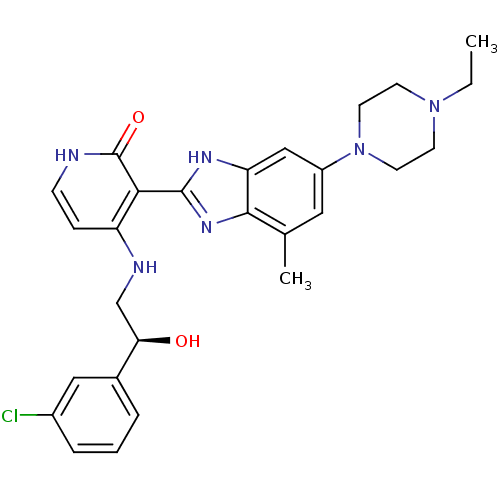
((S)-4-(2-(3-chlorophenyl)-2-hydroxyethylamino)-3-(...)Show SMILES CCN1CCN(CC1)c1cc(C)c2nc([nH]c2c1)-c1c(NC[C@@H](O)c2cccc(Cl)c2)cc[nH]c1=O |r| Show InChI InChI=1S/C27H31ClN6O2/c1-3-33-9-11-34(12-10-33)20-13-17(2)25-22(15-20)31-26(32-25)24-21(7-8-29-27(24)36)30-16-23(35)18-5-4-6-19(28)14-18/h4-8,13-15,23,35H,3,9-12,16H2,1-2H3,(H,31,32)(H2,29,30,36)/t23-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 17 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company
Curated by ChEMBL
| Assay Description
Inhibition of IGF1R |
Bioorg Med Chem Lett 20: 3182-5 (2010)
Article DOI: 10.1016/j.bmcl.2010.03.057
BindingDB Entry DOI: 10.7270/Q2GX4BQB |
More data for this
Ligand-Target Pair | |
Insulin-like growth factor 1 receptor
(Homo sapiens (Human)) | BDBM50313558
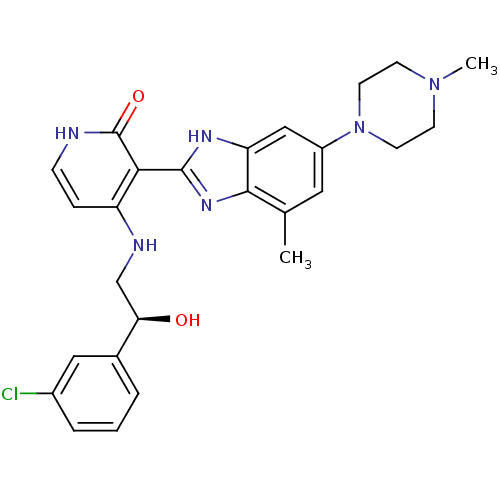
((S)-4-(2-(3-chlorophenyl)-2-hydroxyethylamino)-3-(...)Show SMILES CN1CCN(CC1)c1cc(C)c2nc([nH]c2c1)-c1c(NC[C@@H](O)c2cccc(Cl)c2)cc[nH]c1=O |r| Show InChI InChI=1S/C26H29ClN6O2/c1-16-12-19(33-10-8-32(2)9-11-33)14-21-24(16)31-25(30-21)23-20(6-7-28-26(23)35)29-15-22(34)17-4-3-5-18(27)13-17/h3-7,12-14,22,34H,8-11,15H2,1-2H3,(H,30,31)(H2,28,29,35)/t22-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 18 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company
Curated by ChEMBL
| Assay Description
Inhibition of IGF1R |
Bioorg Med Chem Lett 20: 3182-5 (2010)
Article DOI: 10.1016/j.bmcl.2010.03.057
BindingDB Entry DOI: 10.7270/Q2GX4BQB |
More data for this
Ligand-Target Pair | |
Insulin-like growth factor 1 receptor
(Homo sapiens (Human)) | BDBM50318121
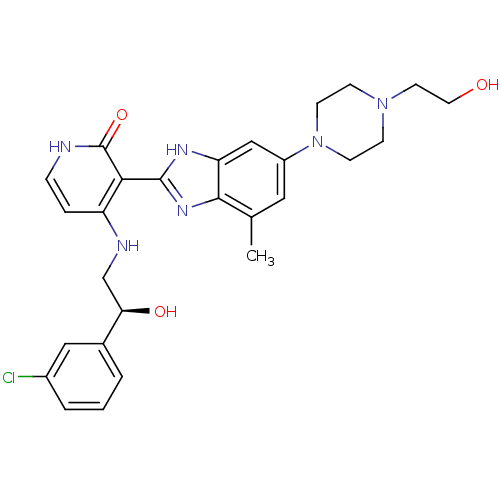
((S)-4-(2-(3-chlorophenyl)-2-hydroxyethylamino)-3-(...)Show SMILES Cc1cc(cc2[nH]c(nc12)-c1c(NC[C@@H](O)c2cccc(Cl)c2)cc[nH]c1=O)N1CCN(CCO)CC1 |r| Show InChI InChI=1S/C27H31ClN6O3/c1-17-13-20(34-9-7-33(8-10-34)11-12-35)15-22-25(17)32-26(31-22)24-21(5-6-29-27(24)37)30-16-23(36)18-3-2-4-19(28)14-18/h2-6,13-15,23,35-36H,7-12,16H2,1H3,(H,31,32)(H2,29,30,37)/t23-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 26 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company
Curated by ChEMBL
| Assay Description
Inhibition of IGF1R |
Bioorg Med Chem Lett 20: 3182-5 (2010)
Article DOI: 10.1016/j.bmcl.2010.03.057
BindingDB Entry DOI: 10.7270/Q2GX4BQB |
More data for this
Ligand-Target Pair | |
Insulin-like growth factor 1 receptor
(Homo sapiens (Human)) | BDBM50318120
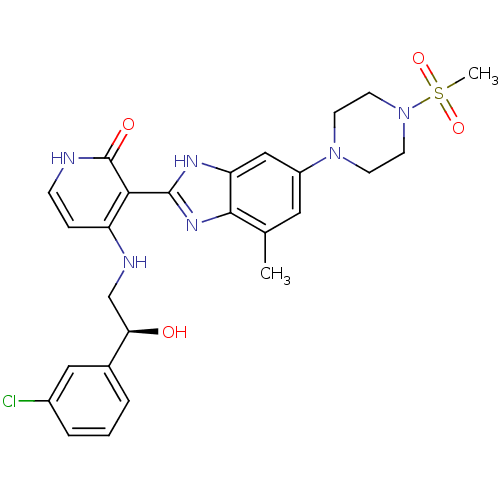
((S)-4-(2-(3-chlorophenyl)-2-hydroxyethylamino)-3-(...)Show SMILES Cc1cc(cc2[nH]c(nc12)-c1c(NC[C@@H](O)c2cccc(Cl)c2)cc[nH]c1=O)N1CCN(CC1)S(C)(=O)=O |r| Show InChI InChI=1S/C26H29ClN6O4S/c1-16-12-19(32-8-10-33(11-9-32)38(2,36)37)14-21-24(16)31-25(30-21)23-20(6-7-28-26(23)35)29-15-22(34)17-4-3-5-18(27)13-17/h3-7,12-14,22,34H,8-11,15H2,1-2H3,(H,30,31)(H2,28,29,35)/t22-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 27 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company
Curated by ChEMBL
| Assay Description
Inhibition of IGF1R |
Bioorg Med Chem Lett 20: 3182-5 (2010)
Article DOI: 10.1016/j.bmcl.2010.03.057
BindingDB Entry DOI: 10.7270/Q2GX4BQB |
More data for this
Ligand-Target Pair | |
Insulin-like growth factor 1 receptor
(Homo sapiens (Human)) | BDBM50318115
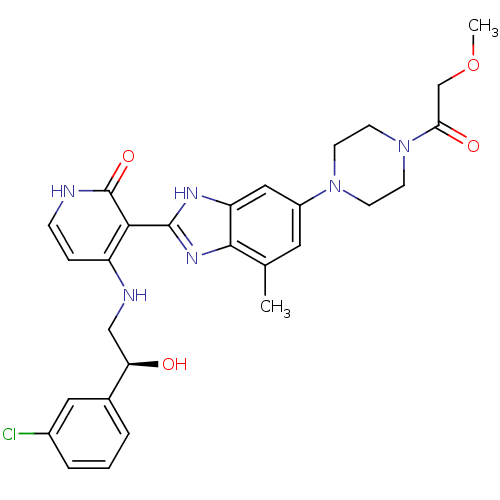
((S)-4-(2-(3-chlorophenyl)-2-hydroxyethylamino)-3-(...)Show SMILES COCC(=O)N1CCN(CC1)c1cc(C)c2nc([nH]c2c1)-c1c(NC[C@@H](O)c2cccc(Cl)c2)cc[nH]c1=O |r| Show InChI InChI=1S/C28H31ClN6O4/c1-17-12-20(34-8-10-35(11-9-34)24(37)16-39-2)14-22-26(17)33-27(32-22)25-21(6-7-30-28(25)38)31-15-23(36)18-4-3-5-19(29)13-18/h3-7,12-14,23,36H,8-11,15-16H2,1-2H3,(H,32,33)(H2,30,31,38)/t23-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 28 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company
Curated by ChEMBL
| Assay Description
Inhibition of IGF1R |
Bioorg Med Chem Lett 20: 3182-5 (2010)
Article DOI: 10.1016/j.bmcl.2010.03.057
BindingDB Entry DOI: 10.7270/Q2GX4BQB |
More data for this
Ligand-Target Pair | |
Insulin-like growth factor 1 receptor
(Homo sapiens (Human)) | BDBM50318109

((S)-4-(2-(3-chlorophenyl)-2-hydroxyethylamino)-3-(...)Show SMILES Cc1cc(cc2[nH]c(nc12)-c1c(NC[C@@H](O)c2cccc(Cl)c2)cc[nH]c1=O)N1CCN(CCCF)CC1 |r| Show InChI InChI=1S/C28H32ClFN6O2/c1-18-14-21(36-12-10-35(11-13-36)9-3-7-30)16-23-26(18)34-27(33-23)25-22(6-8-31-28(25)38)32-17-24(37)19-4-2-5-20(29)15-19/h2,4-6,8,14-16,24,37H,3,7,9-13,17H2,1H3,(H,33,34)(H2,31,32,38)/t24-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company
Curated by ChEMBL
| Assay Description
Inhibition of IGF1R |
Bioorg Med Chem Lett 20: 3182-5 (2010)
Article DOI: 10.1016/j.bmcl.2010.03.057
BindingDB Entry DOI: 10.7270/Q2GX4BQB |
More data for this
Ligand-Target Pair | |
Insulin-like growth factor 1 receptor
(Homo sapiens (Human)) | BDBM50313557
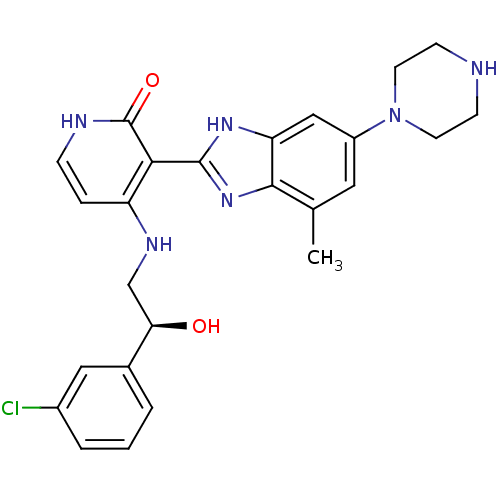
((S)-4-(2-(3-chlorophenyl)-2-hydroxyethylamino)-3-(...)Show SMILES Cc1cc(cc2[nH]c(nc12)-c1c(NC[C@@H](O)c2cccc(Cl)c2)cc[nH]c1=O)N1CCNCC1 |r| Show InChI InChI=1S/C25H27ClN6O2/c1-15-11-18(32-9-7-27-8-10-32)13-20-23(15)31-24(30-20)22-19(5-6-28-25(22)34)29-14-21(33)16-3-2-4-17(26)12-16/h2-6,11-13,21,27,33H,7-10,14H2,1H3,(H,30,31)(H2,28,29,34)/t21-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 32 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company
Curated by ChEMBL
| Assay Description
Inhibition of IGF1R |
Bioorg Med Chem Lett 20: 3182-5 (2010)
Article DOI: 10.1016/j.bmcl.2010.03.057
BindingDB Entry DOI: 10.7270/Q2GX4BQB |
More data for this
Ligand-Target Pair | |
Insulin-like growth factor 1 receptor
(Homo sapiens (Human)) | BDBM50318107

((S)-2-(4-(2-(4-(2-(3-chlorophenyl)-2-hydroxyethyla...)Show SMILES CNC(=O)CN1CCN(CC1)c1cc(C)c2nc([nH]c2c1)-c1c(NC[C@@H](O)c2cccc(Cl)c2)cc[nH]c1=O |r| Show InChI InChI=1S/C28H32ClN7O3/c1-17-12-20(36-10-8-35(9-11-36)16-24(38)30-2)14-22-26(17)34-27(33-22)25-21(6-7-31-28(25)39)32-15-23(37)18-4-3-5-19(29)13-18/h3-7,12-14,23,37H,8-11,15-16H2,1-2H3,(H,30,38)(H,33,34)(H2,31,32,39)/t23-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 38 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company
Curated by ChEMBL
| Assay Description
Inhibition of IGF1R |
Bioorg Med Chem Lett 20: 3182-5 (2010)
Article DOI: 10.1016/j.bmcl.2010.03.057
BindingDB Entry DOI: 10.7270/Q2GX4BQB |
More data for this
Ligand-Target Pair | |
Insulin-like growth factor 1 receptor
(Homo sapiens (Human)) | BDBM50318110
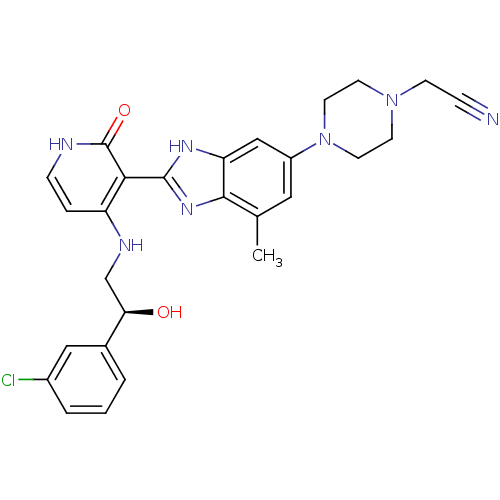
((S)-2-(4-(2-(4-(2-(3-chlorophenyl)-2-hydroxyethyla...)Show SMILES Cc1cc(cc2[nH]c(nc12)-c1c(NC[C@@H](O)c2cccc(Cl)c2)cc[nH]c1=O)N1CCN(CC#N)CC1 |r| Show InChI InChI=1S/C27H28ClN7O2/c1-17-13-20(35-11-9-34(8-6-29)10-12-35)15-22-25(17)33-26(32-22)24-21(5-7-30-27(24)37)31-16-23(36)18-3-2-4-19(28)14-18/h2-5,7,13-15,23,36H,8-12,16H2,1H3,(H,32,33)(H2,30,31,37)/t23-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 38 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company
Curated by ChEMBL
| Assay Description
Inhibition of IGF1R |
Bioorg Med Chem Lett 20: 3182-5 (2010)
Article DOI: 10.1016/j.bmcl.2010.03.057
BindingDB Entry DOI: 10.7270/Q2GX4BQB |
More data for this
Ligand-Target Pair | |
Insulin-like growth factor 1 receptor
(Homo sapiens (Human)) | BDBM50318108
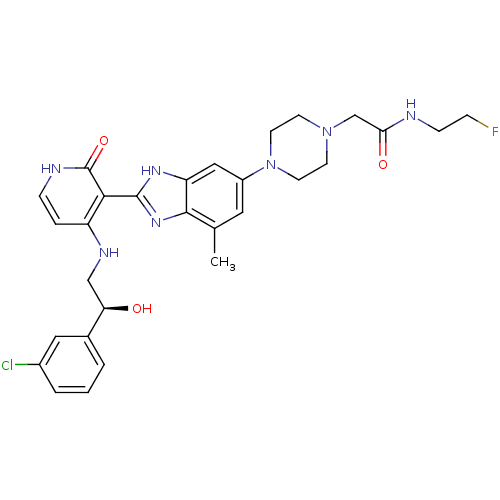
((S)-2-(4-(2-(4-(2-(3-chlorophenyl)-2-hydroxyethyla...)Show SMILES Cc1cc(cc2[nH]c(nc12)-c1c(NC[C@@H](O)c2cccc(Cl)c2)cc[nH]c1=O)N1CCN(CC(=O)NCCF)CC1 |r| Show InChI InChI=1S/C29H33ClFN7O3/c1-18-13-21(38-11-9-37(10-12-38)17-25(40)32-8-6-31)15-23-27(18)36-28(35-23)26-22(5-7-33-29(26)41)34-16-24(39)19-3-2-4-20(30)14-19/h2-5,7,13-15,24,39H,6,8-12,16-17H2,1H3,(H,32,40)(H,35,36)(H2,33,34,41)/t24-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 41 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company
Curated by ChEMBL
| Assay Description
Inhibition of IGF1R |
Bioorg Med Chem Lett 20: 3182-5 (2010)
Article DOI: 10.1016/j.bmcl.2010.03.057
BindingDB Entry DOI: 10.7270/Q2GX4BQB |
More data for this
Ligand-Target Pair | |
Insulin-like growth factor 1 receptor
(Homo sapiens (Human)) | BDBM50318111

((S)-3-(4-(2-(4-(2-(3-chlorophenyl)-2-hydroxyethyla...)Show SMILES Cc1cc(cc2[nH]c(nc12)-c1c(NC[C@@H](O)c2cccc(Cl)c2)cc[nH]c1=O)N1CCN(CCC#N)CC1 |r| Show InChI InChI=1S/C28H30ClN7O2/c1-18-14-21(36-12-10-35(11-13-36)9-3-7-30)16-23-26(18)34-27(33-23)25-22(6-8-31-28(25)38)32-17-24(37)19-4-2-5-20(29)15-19/h2,4-6,8,14-16,24,37H,3,9-13,17H2,1H3,(H,33,34)(H2,31,32,38)/t24-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 61 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company
Curated by ChEMBL
| Assay Description
Inhibition of IGF1R |
Bioorg Med Chem Lett 20: 3182-5 (2010)
Article DOI: 10.1016/j.bmcl.2010.03.057
BindingDB Entry DOI: 10.7270/Q2GX4BQB |
More data for this
Ligand-Target Pair | |
Insulin-like growth factor 1 receptor
(Homo sapiens (Human)) | BDBM50318116

((S)-4-(2-(3-chlorophenyl)-2-hydroxyethylamino)-3-(...)Show SMILES Cc1cc(cc2[nH]c(nc12)-c1c(NC[C@@H](O)c2cccc(Cl)c2)cc[nH]c1=O)N1CCN(CC1)C(=O)CCCF |r| Show InChI InChI=1S/C29H32ClFN6O3/c1-18-14-21(36-10-12-37(13-11-36)25(39)6-3-8-31)16-23-27(18)35-28(34-23)26-22(7-9-32-29(26)40)33-17-24(38)19-4-2-5-20(30)15-19/h2,4-5,7,9,14-16,24,38H,3,6,8,10-13,17H2,1H3,(H,34,35)(H2,32,33,40)/t24-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 62 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company
Curated by ChEMBL
| Assay Description
Inhibition of IGF1R |
Bioorg Med Chem Lett 20: 3182-5 (2010)
Article DOI: 10.1016/j.bmcl.2010.03.057
BindingDB Entry DOI: 10.7270/Q2GX4BQB |
More data for this
Ligand-Target Pair | |
Insulin-like growth factor 1 receptor
(Homo sapiens (Human)) | BDBM50318114

((S)-3-(6-(4-acetylpiperazin-1-yl)-4-methyl-1H-benz...)Show SMILES CC(=O)N1CCN(CC1)c1cc(C)c2nc([nH]c2c1)-c1c(NC[C@@H](O)c2cccc(Cl)c2)cc[nH]c1=O |r| Show InChI InChI=1S/C27H29ClN6O3/c1-16-12-20(34-10-8-33(9-11-34)17(2)35)14-22-25(16)32-26(31-22)24-21(6-7-29-27(24)37)30-15-23(36)18-4-3-5-19(28)13-18/h3-7,12-14,23,36H,8-11,15H2,1-2H3,(H,31,32)(H2,29,30,37)/t23-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company
Curated by ChEMBL
| Assay Description
Inhibition of IGF1R |
Bioorg Med Chem Lett 20: 3182-5 (2010)
Article DOI: 10.1016/j.bmcl.2010.03.057
BindingDB Entry DOI: 10.7270/Q2GX4BQB |
More data for this
Ligand-Target Pair | |
Insulin-like growth factor 1 receptor
(Homo sapiens (Human)) | BDBM27879

(4-{[(2S)-2-(3-chlorophenyl)-2-hydroxyethyl]amino}-...)Show SMILES Cc1cc(cc2[nH]c(nc12)-c1c(NC[C@@H](O)c2cccc(Cl)c2)cc[nH]c1=O)N1CCOCC1 |r| Show InChI InChI=1S/C25H26ClN5O3/c1-15-11-18(31-7-9-34-10-8-31)13-20-23(15)30-24(29-20)22-19(5-6-27-25(22)33)28-14-21(32)16-3-2-4-17(26)12-16/h2-6,11-13,21,32H,7-10,14H2,1H3,(H,29,30)(H2,27,28,33)/t21-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company
Curated by ChEMBL
| Assay Description
Inhibition of IGF1R |
Bioorg Med Chem Lett 20: 3182-5 (2010)
Article DOI: 10.1016/j.bmcl.2010.03.057
BindingDB Entry DOI: 10.7270/Q2GX4BQB |
More data for this
Ligand-Target Pair | |
Insulin-like growth factor 1 receptor
(Homo sapiens (Human)) | BDBM50313559
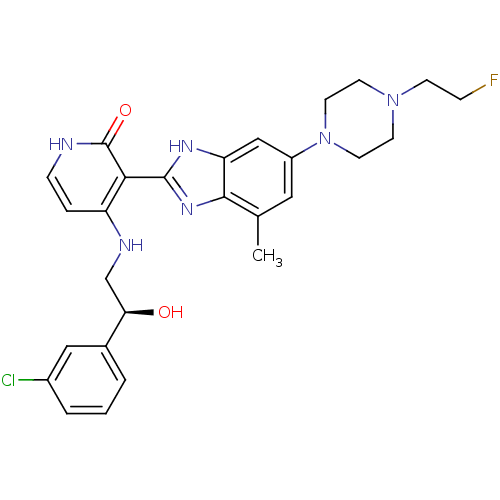
((S)-4-(2-(3-chlorophenyl)-2-hydroxyethylamino)-3-(...)Show SMILES Cc1cc(cc2[nH]c(nc12)-c1c(NC[C@@H](O)c2cccc(Cl)c2)cc[nH]c1=O)N1CCN(CCF)CC1 |r| Show InChI InChI=1S/C27H30ClFN6O2/c1-17-13-20(35-11-9-34(8-6-29)10-12-35)15-22-25(17)33-26(32-22)24-21(5-7-30-27(24)37)31-16-23(36)18-3-2-4-19(28)14-18/h2-5,7,13-15,23,36H,6,8-12,16H2,1H3,(H,32,33)(H2,30,31,37)/t23-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company
Curated by ChEMBL
| Assay Description
Inhibition of IGF1R |
Bioorg Med Chem Lett 20: 3182-5 (2010)
Article DOI: 10.1016/j.bmcl.2010.03.057
BindingDB Entry DOI: 10.7270/Q2GX4BQB |
More data for this
Ligand-Target Pair | |
Insulin-like growth factor 1 receptor
(Homo sapiens (Human)) | BDBM50318118

((S)-methyl 4-(2-(4-(2-(3-chlorophenyl)-2-hydroxyet...)Show SMILES COC(=O)N1CCN(CC1)c1cc(C)c2nc([nH]c2c1)-c1c(NC[C@@H](O)c2cccc(Cl)c2)cc[nH]c1=O |r| Show InChI InChI=1S/C27H29ClN6O4/c1-16-12-19(33-8-10-34(11-9-33)27(37)38-2)14-21-24(16)32-25(31-21)23-20(6-7-29-26(23)36)30-15-22(35)17-4-3-5-18(28)13-17/h3-7,12-14,22,35H,8-11,15H2,1-2H3,(H,31,32)(H2,29,30,36)/t22-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 175 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company
Curated by ChEMBL
| Assay Description
Inhibition of IGF1R |
Bioorg Med Chem Lett 20: 3182-5 (2010)
Article DOI: 10.1016/j.bmcl.2010.03.057
BindingDB Entry DOI: 10.7270/Q2GX4BQB |
More data for this
Ligand-Target Pair | |
Insulin-like growth factor 1 receptor
(Homo sapiens (Human)) | BDBM50318117

((S)-tert-butyl 4-(2-(4-(2-(3-chlorophenyl)-2-hydro...)Show SMILES Cc1cc(cc2[nH]c(nc12)-c1c(NC[C@@H](O)c2cccc(Cl)c2)cc[nH]c1=O)N1CCN(CC1)C(=O)OC(C)(C)C |r| Show InChI InChI=1S/C30H35ClN6O4/c1-18-14-21(36-10-12-37(13-11-36)29(40)41-30(2,3)4)16-23-26(18)35-27(34-23)25-22(8-9-32-28(25)39)33-17-24(38)19-6-5-7-20(31)15-19/h5-9,14-16,24,38H,10-13,17H2,1-4H3,(H,34,35)(H2,32,33,39)/t24-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 210 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company
Curated by ChEMBL
| Assay Description
Inhibition of IGF1R |
Bioorg Med Chem Lett 20: 3182-5 (2010)
Article DOI: 10.1016/j.bmcl.2010.03.057
BindingDB Entry DOI: 10.7270/Q2GX4BQB |
More data for this
Ligand-Target Pair | |
Insulin-like growth factor 1 receptor
(Homo sapiens (Human)) | BDBM50318119

((S)-ethyl 4-(2-(4-(2-(3-chlorophenyl)-2-hydroxyeth...)Show SMILES CCOC(=O)N1CCN(CC1)c1cc(C)c2nc([nH]c2c1)-c1c(NC[C@@H](O)c2cccc(Cl)c2)cc[nH]c1=O |r| Show InChI InChI=1S/C28H31ClN6O4/c1-3-39-28(38)35-11-9-34(10-12-35)20-13-17(2)25-22(15-20)32-26(33-25)24-21(7-8-30-27(24)37)31-16-23(36)18-5-4-6-19(29)14-18/h4-8,13-15,23,36H,3,9-12,16H2,1-2H3,(H,32,33)(H2,30,31,37)/t23-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 270 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company
Curated by ChEMBL
| Assay Description
Inhibition of IGF1R |
Bioorg Med Chem Lett 20: 3182-5 (2010)
Article DOI: 10.1016/j.bmcl.2010.03.057
BindingDB Entry DOI: 10.7270/Q2GX4BQB |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50318109

((S)-4-(2-(3-chlorophenyl)-2-hydroxyethylamino)-3-(...)Show SMILES Cc1cc(cc2[nH]c(nc12)-c1c(NC[C@@H](O)c2cccc(Cl)c2)cc[nH]c1=O)N1CCN(CCCF)CC1 |r| Show InChI InChI=1S/C28H32ClFN6O2/c1-18-14-21(36-12-10-35(11-13-36)9-3-7-30)16-23-26(18)34-27(33-23)25-22(6-8-31-28(25)38)32-17-24(37)19-4-2-5-20(29)15-19/h2,4-6,8,14-16,24,37H,3,7,9-13,17H2,1H3,(H,33,34)(H2,31,32,38)/t24-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 450 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company
Curated by ChEMBL
| Assay Description
Inhibition of CYP3A4 assessed as dealkylation of 7-benzyloxy-4-trifluoromethylcoumarin |
Bioorg Med Chem Lett 20: 3182-5 (2010)
Article DOI: 10.1016/j.bmcl.2010.03.057
BindingDB Entry DOI: 10.7270/Q2GX4BQB |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM27879

(4-{[(2S)-2-(3-chlorophenyl)-2-hydroxyethyl]amino}-...)Show SMILES Cc1cc(cc2[nH]c(nc12)-c1c(NC[C@@H](O)c2cccc(Cl)c2)cc[nH]c1=O)N1CCOCC1 |r| Show InChI InChI=1S/C25H26ClN5O3/c1-15-11-18(31-7-9-34-10-8-31)13-20-23(15)30-24(29-20)22-19(5-6-27-25(22)33)28-14-21(32)16-3-2-4-17(26)12-16/h2-6,11-13,21,32H,7-10,14H2,1H3,(H,29,30)(H2,27,28,33)/t21-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 500 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company
Curated by ChEMBL
| Assay Description
Inhibition of CYP3A4 assessed as dealkylation of 7-benzyloxy-4-trifluoromethylcoumarin |
Bioorg Med Chem Lett 20: 3182-5 (2010)
Article DOI: 10.1016/j.bmcl.2010.03.057
BindingDB Entry DOI: 10.7270/Q2GX4BQB |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50318120

((S)-4-(2-(3-chlorophenyl)-2-hydroxyethylamino)-3-(...)Show SMILES Cc1cc(cc2[nH]c(nc12)-c1c(NC[C@@H](O)c2cccc(Cl)c2)cc[nH]c1=O)N1CCN(CC1)S(C)(=O)=O |r| Show InChI InChI=1S/C26H29ClN6O4S/c1-16-12-19(32-8-10-33(11-9-32)38(2,36)37)14-21-24(16)31-25(30-21)23-20(6-7-28-26(23)35)29-15-22(34)17-4-3-5-18(27)13-17/h3-7,12-14,22,34H,8-11,15H2,1-2H3,(H,30,31)(H2,28,29,35)/t22-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 600 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company
Curated by ChEMBL
| Assay Description
Inhibition of CYP3A4 assessed as dealkylation of 7-benzyloxy-4-trifluoromethylcoumarin |
Bioorg Med Chem Lett 20: 3182-5 (2010)
Article DOI: 10.1016/j.bmcl.2010.03.057
BindingDB Entry DOI: 10.7270/Q2GX4BQB |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50318118

((S)-methyl 4-(2-(4-(2-(3-chlorophenyl)-2-hydroxyet...)Show SMILES COC(=O)N1CCN(CC1)c1cc(C)c2nc([nH]c2c1)-c1c(NC[C@@H](O)c2cccc(Cl)c2)cc[nH]c1=O |r| Show InChI InChI=1S/C27H29ClN6O4/c1-16-12-19(33-8-10-34(11-9-33)27(37)38-2)14-21-24(16)32-25(31-21)23-20(6-7-29-26(23)36)30-15-22(35)17-4-3-5-18(28)13-17/h3-7,12-14,22,35H,8-11,15H2,1-2H3,(H,31,32)(H2,29,30,36)/t22-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 700 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company
Curated by ChEMBL
| Assay Description
Inhibition of CYP3A4 assessed as dealkylation of 7-benzyloxy-4-trifluoromethylcoumarin |
Bioorg Med Chem Lett 20: 3182-5 (2010)
Article DOI: 10.1016/j.bmcl.2010.03.057
BindingDB Entry DOI: 10.7270/Q2GX4BQB |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50318115

((S)-4-(2-(3-chlorophenyl)-2-hydroxyethylamino)-3-(...)Show SMILES COCC(=O)N1CCN(CC1)c1cc(C)c2nc([nH]c2c1)-c1c(NC[C@@H](O)c2cccc(Cl)c2)cc[nH]c1=O |r| Show InChI InChI=1S/C28H31ClN6O4/c1-17-12-20(34-8-10-35(11-9-34)24(37)16-39-2)14-22-26(17)33-27(32-22)25-21(6-7-30-28(25)38)31-15-23(36)18-4-3-5-19(29)13-18/h3-7,12-14,23,36H,8-11,15-16H2,1-2H3,(H,32,33)(H2,30,31,38)/t23-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 800 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company
Curated by ChEMBL
| Assay Description
Inhibition of CYP3A4 assessed as dealkylation of 7-benzyloxy-4-trifluoromethylcoumarin |
Bioorg Med Chem Lett 20: 3182-5 (2010)
Article DOI: 10.1016/j.bmcl.2010.03.057
BindingDB Entry DOI: 10.7270/Q2GX4BQB |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50318116

((S)-4-(2-(3-chlorophenyl)-2-hydroxyethylamino)-3-(...)Show SMILES Cc1cc(cc2[nH]c(nc12)-c1c(NC[C@@H](O)c2cccc(Cl)c2)cc[nH]c1=O)N1CCN(CC1)C(=O)CCCF |r| Show InChI InChI=1S/C29H32ClFN6O3/c1-18-14-21(36-10-12-37(13-11-36)25(39)6-3-8-31)16-23-27(18)35-28(34-23)26-22(7-9-32-29(26)40)33-17-24(38)19-4-2-5-20(30)15-19/h2,4-5,7,9,14-16,24,38H,3,6,8,10-13,17H2,1H3,(H,34,35)(H2,32,33,40)/t24-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 800 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company
Curated by ChEMBL
| Assay Description
Inhibition of CYP3A4 assessed as dealkylation of 7-benzyloxy-4-trifluoromethylcoumarin |
Bioorg Med Chem Lett 20: 3182-5 (2010)
Article DOI: 10.1016/j.bmcl.2010.03.057
BindingDB Entry DOI: 10.7270/Q2GX4BQB |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50318119

((S)-ethyl 4-(2-(4-(2-(3-chlorophenyl)-2-hydroxyeth...)Show SMILES CCOC(=O)N1CCN(CC1)c1cc(C)c2nc([nH]c2c1)-c1c(NC[C@@H](O)c2cccc(Cl)c2)cc[nH]c1=O |r| Show InChI InChI=1S/C28H31ClN6O4/c1-3-39-28(38)35-11-9-34(10-12-35)20-13-17(2)25-22(15-20)32-26(33-25)24-21(7-8-30-27(24)37)31-16-23(36)18-5-4-6-19(29)14-18/h4-8,13-15,23,36H,3,9-12,16H2,1-2H3,(H,32,33)(H2,30,31,37)/t23-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company
Curated by ChEMBL
| Assay Description
Inhibition of CYP3A4 assessed as dealkylation of 7-benzyloxy-4-trifluoromethylcoumarin |
Bioorg Med Chem Lett 20: 3182-5 (2010)
Article DOI: 10.1016/j.bmcl.2010.03.057
BindingDB Entry DOI: 10.7270/Q2GX4BQB |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50318114

((S)-3-(6-(4-acetylpiperazin-1-yl)-4-methyl-1H-benz...)Show SMILES CC(=O)N1CCN(CC1)c1cc(C)c2nc([nH]c2c1)-c1c(NC[C@@H](O)c2cccc(Cl)c2)cc[nH]c1=O |r| Show InChI InChI=1S/C27H29ClN6O3/c1-16-12-20(34-10-8-33(9-11-34)17(2)35)14-22-25(16)32-26(31-22)24-21(6-7-29-27(24)37)30-15-23(36)18-4-3-5-19(28)13-18/h3-7,12-14,23,36H,8-11,15H2,1-2H3,(H,31,32)(H2,29,30,37)/t23-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company
Curated by ChEMBL
| Assay Description
Inhibition of CYP3A4 assessed as dealkylation of 7-benzyloxy-4-trifluoromethylcoumarin |
Bioorg Med Chem Lett 20: 3182-5 (2010)
Article DOI: 10.1016/j.bmcl.2010.03.057
BindingDB Entry DOI: 10.7270/Q2GX4BQB |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50318111

((S)-3-(4-(2-(4-(2-(3-chlorophenyl)-2-hydroxyethyla...)Show SMILES Cc1cc(cc2[nH]c(nc12)-c1c(NC[C@@H](O)c2cccc(Cl)c2)cc[nH]c1=O)N1CCN(CCC#N)CC1 |r| Show InChI InChI=1S/C28H30ClN7O2/c1-18-14-21(36-12-10-35(11-13-36)9-3-7-30)16-23-26(18)34-27(33-23)25-22(6-8-31-28(25)38)32-17-24(37)19-4-2-5-20(29)15-19/h2,4-6,8,14-16,24,37H,3,9-13,17H2,1H3,(H,33,34)(H2,31,32,38)/t24-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company
Curated by ChEMBL
| Assay Description
Inhibition of CYP3A4 assessed as dealkylation of 7-benzyloxy-4-trifluoromethylcoumarin |
Bioorg Med Chem Lett 20: 3182-5 (2010)
Article DOI: 10.1016/j.bmcl.2010.03.057
BindingDB Entry DOI: 10.7270/Q2GX4BQB |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50318110

((S)-2-(4-(2-(4-(2-(3-chlorophenyl)-2-hydroxyethyla...)Show SMILES Cc1cc(cc2[nH]c(nc12)-c1c(NC[C@@H](O)c2cccc(Cl)c2)cc[nH]c1=O)N1CCN(CC#N)CC1 |r| Show InChI InChI=1S/C27H28ClN7O2/c1-17-13-20(35-11-9-34(8-6-29)10-12-35)15-22-25(17)33-26(32-22)24-21(5-7-30-27(24)37)31-16-23(36)18-3-2-4-19(28)14-18/h2-5,7,13-15,23,36H,8-12,16H2,1H3,(H,32,33)(H2,30,31,37)/t23-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company
Curated by ChEMBL
| Assay Description
Inhibition of CYP3A4 assessed as dealkylation of 7-benzyloxy-4-trifluoromethylcoumarin |
Bioorg Med Chem Lett 20: 3182-5 (2010)
Article DOI: 10.1016/j.bmcl.2010.03.057
BindingDB Entry DOI: 10.7270/Q2GX4BQB |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50318117

((S)-tert-butyl 4-(2-(4-(2-(3-chlorophenyl)-2-hydro...)Show SMILES Cc1cc(cc2[nH]c(nc12)-c1c(NC[C@@H](O)c2cccc(Cl)c2)cc[nH]c1=O)N1CCN(CC1)C(=O)OC(C)(C)C |r| Show InChI InChI=1S/C30H35ClN6O4/c1-18-14-21(36-10-12-37(13-11-36)29(40)41-30(2,3)4)16-23-26(18)35-27(34-23)25-22(8-9-32-28(25)39)33-17-24(38)19-6-5-7-20(31)15-19/h5-9,14-16,24,38H,10-13,17H2,1-4H3,(H,34,35)(H2,32,33,39)/t24-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company
Curated by ChEMBL
| Assay Description
Inhibition of CYP3A4 assessed as dealkylation of 7-benzyloxy-4-trifluoromethylcoumarin |
Bioorg Med Chem Lett 20: 3182-5 (2010)
Article DOI: 10.1016/j.bmcl.2010.03.057
BindingDB Entry DOI: 10.7270/Q2GX4BQB |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50318122

((S)-4-(2-(3-chlorophenyl)-2-hydroxyethylamino)-3-(...)Show SMILES Cc1cc(cc2[nH]c(nc12)-c1c(NC[C@@H](O)c2cccc(Cl)c2)cc[nH]c1=O)N1CCN(CCCO)CC1 |r| Show InChI InChI=1S/C28H33ClN6O3/c1-18-14-21(35-11-9-34(10-12-35)8-3-13-36)16-23-26(18)33-27(32-23)25-22(6-7-30-28(25)38)31-17-24(37)19-4-2-5-20(29)15-19/h2,4-7,14-16,24,36-37H,3,8-13,17H2,1H3,(H,32,33)(H2,30,31,38)/t24-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company
Curated by ChEMBL
| Assay Description
Inhibition of CYP3A4 assessed as dealkylation of 7-benzyloxy-4-trifluoromethylcoumarin |
Bioorg Med Chem Lett 20: 3182-5 (2010)
Article DOI: 10.1016/j.bmcl.2010.03.057
BindingDB Entry DOI: 10.7270/Q2GX4BQB |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50318113

((S)-4-(2-(3-chlorophenyl)-2-hydroxyethylamino)-3-(...)Show SMILES CCN1CCN(CC1)c1cc(C)c2nc([nH]c2c1)-c1c(NC[C@@H](O)c2cccc(Cl)c2)cc[nH]c1=O |r| Show InChI InChI=1S/C27H31ClN6O2/c1-3-33-9-11-34(12-10-33)20-13-17(2)25-22(15-20)31-26(32-25)24-21(7-8-29-27(24)36)30-16-23(35)18-5-4-6-19(28)14-18/h4-8,13-15,23,35H,3,9-12,16H2,1-2H3,(H,31,32)(H2,29,30,36)/t23-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company
Curated by ChEMBL
| Assay Description
Inhibition of CYP3A4 assessed as dealkylation of 7-benzyloxy-4-trifluoromethylcoumarin |
Bioorg Med Chem Lett 20: 3182-5 (2010)
Article DOI: 10.1016/j.bmcl.2010.03.057
BindingDB Entry DOI: 10.7270/Q2GX4BQB |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50313559

((S)-4-(2-(3-chlorophenyl)-2-hydroxyethylamino)-3-(...)Show SMILES Cc1cc(cc2[nH]c(nc12)-c1c(NC[C@@H](O)c2cccc(Cl)c2)cc[nH]c1=O)N1CCN(CCF)CC1 |r| Show InChI InChI=1S/C27H30ClFN6O2/c1-17-13-20(35-11-9-34(8-6-29)10-12-35)15-22-25(17)33-26(32-22)24-21(5-7-30-27(24)37)31-16-23(36)18-3-2-4-19(28)14-18/h2-5,7,13-15,23,36H,6,8-12,16H2,1H3,(H,32,33)(H2,30,31,37)/t23-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company
Curated by ChEMBL
| Assay Description
Inhibition of CYP3A4 assessed as dealkylation of 7-benzyloxy-4-trifluoromethylcoumarin |
Bioorg Med Chem Lett 20: 3182-5 (2010)
Article DOI: 10.1016/j.bmcl.2010.03.057
BindingDB Entry DOI: 10.7270/Q2GX4BQB |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50318108

((S)-2-(4-(2-(4-(2-(3-chlorophenyl)-2-hydroxyethyla...)Show SMILES Cc1cc(cc2[nH]c(nc12)-c1c(NC[C@@H](O)c2cccc(Cl)c2)cc[nH]c1=O)N1CCN(CC(=O)NCCF)CC1 |r| Show InChI InChI=1S/C29H33ClFN7O3/c1-18-13-21(38-11-9-37(10-12-38)17-25(40)32-8-6-31)15-23-27(18)36-28(35-23)26-22(5-7-33-29(26)41)34-16-24(39)19-3-2-4-20(30)14-19/h2-5,7,13-15,24,39H,6,8-12,16-17H2,1H3,(H,32,40)(H,35,36)(H2,33,34,41)/t24-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company
Curated by ChEMBL
| Assay Description
Inhibition of CYP3A4 assessed as dealkylation of 7-benzyloxy-4-trifluoromethylcoumarin |
Bioorg Med Chem Lett 20: 3182-5 (2010)
Article DOI: 10.1016/j.bmcl.2010.03.057
BindingDB Entry DOI: 10.7270/Q2GX4BQB |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50318107

((S)-2-(4-(2-(4-(2-(3-chlorophenyl)-2-hydroxyethyla...)Show SMILES CNC(=O)CN1CCN(CC1)c1cc(C)c2nc([nH]c2c1)-c1c(NC[C@@H](O)c2cccc(Cl)c2)cc[nH]c1=O |r| Show InChI InChI=1S/C28H32ClN7O3/c1-17-12-20(36-10-8-35(9-11-36)16-24(38)30-2)14-22-26(17)34-27(33-22)25-21(6-7-31-28(25)39)32-15-23(37)18-4-3-5-19(29)13-18/h3-7,12-14,23,37H,8-11,15-16H2,1-2H3,(H,30,38)(H,33,34)(H2,31,32,39)/t23-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company
Curated by ChEMBL
| Assay Description
Inhibition of CYP3A4 assessed as dealkylation of 7-benzyloxy-4-trifluoromethylcoumarin |
Bioorg Med Chem Lett 20: 3182-5 (2010)
Article DOI: 10.1016/j.bmcl.2010.03.057
BindingDB Entry DOI: 10.7270/Q2GX4BQB |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50318112

((S)-4-(2-(3-chlorophenyl)-2-hydroxyethylamino)-3-(...)Show SMILES COCCN1CCN(CC1)c1cc(C)c2nc([nH]c2c1)-c1c(NC[C@@H](O)c2cccc(Cl)c2)cc[nH]c1=O |r| Show InChI InChI=1S/C28H33ClN6O3/c1-18-14-21(35-10-8-34(9-11-35)12-13-38-2)16-23-26(18)33-27(32-23)25-22(6-7-30-28(25)37)31-17-24(36)19-4-3-5-20(29)15-19/h3-7,14-16,24,36H,8-13,17H2,1-2H3,(H,32,33)(H2,30,31,37)/t24-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company
Curated by ChEMBL
| Assay Description
Inhibition of CYP3A4 assessed as dealkylation of 7-benzyloxy-4-trifluoromethylcoumarin |
Bioorg Med Chem Lett 20: 3182-5 (2010)
Article DOI: 10.1016/j.bmcl.2010.03.057
BindingDB Entry DOI: 10.7270/Q2GX4BQB |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50313557

((S)-4-(2-(3-chlorophenyl)-2-hydroxyethylamino)-3-(...)Show SMILES Cc1cc(cc2[nH]c(nc12)-c1c(NC[C@@H](O)c2cccc(Cl)c2)cc[nH]c1=O)N1CCNCC1 |r| Show InChI InChI=1S/C25H27ClN6O2/c1-15-11-18(32-9-7-27-8-10-32)13-20-23(15)31-24(30-20)22-19(5-6-28-25(22)34)29-14-21(33)16-3-2-4-17(26)12-16/h2-6,11-13,21,27,33H,7-10,14H2,1H3,(H,30,31)(H2,28,29,34)/t21-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 3.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company
Curated by ChEMBL
| Assay Description
Inhibition of CYP3A4 assessed as dealkylation of 7-benzyloxy-4-trifluoromethylcoumarin |
Bioorg Med Chem Lett 20: 3182-5 (2010)
Article DOI: 10.1016/j.bmcl.2010.03.057
BindingDB Entry DOI: 10.7270/Q2GX4BQB |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50318121

((S)-4-(2-(3-chlorophenyl)-2-hydroxyethylamino)-3-(...)Show SMILES Cc1cc(cc2[nH]c(nc12)-c1c(NC[C@@H](O)c2cccc(Cl)c2)cc[nH]c1=O)N1CCN(CCO)CC1 |r| Show InChI InChI=1S/C27H31ClN6O3/c1-17-13-20(34-9-7-33(8-10-34)11-12-35)15-22-25(17)32-26(31-22)24-21(5-6-29-27(24)37)30-16-23(36)18-3-2-4-19(28)14-18/h2-6,13-15,23,35-36H,7-12,16H2,1H3,(H,31,32)(H2,29,30,37)/t23-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 3.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company
Curated by ChEMBL
| Assay Description
Inhibition of CYP3A4 assessed as dealkylation of 7-benzyloxy-4-trifluoromethylcoumarin |
Bioorg Med Chem Lett 20: 3182-5 (2010)
Article DOI: 10.1016/j.bmcl.2010.03.057
BindingDB Entry DOI: 10.7270/Q2GX4BQB |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50313558

((S)-4-(2-(3-chlorophenyl)-2-hydroxyethylamino)-3-(...)Show SMILES CN1CCN(CC1)c1cc(C)c2nc([nH]c2c1)-c1c(NC[C@@H](O)c2cccc(Cl)c2)cc[nH]c1=O |r| Show InChI InChI=1S/C26H29ClN6O2/c1-16-12-19(33-10-8-32(2)9-11-33)14-21-24(16)31-25(30-21)23-20(6-7-28-26(23)35)29-15-22(34)17-4-3-5-18(27)13-17/h3-7,12-14,22,34H,8-11,15H2,1-2H3,(H,30,31)(H2,28,29,35)/t22-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 3.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company
Curated by ChEMBL
| Assay Description
Inhibition of CYP3A4 assessed as dealkylation of 7-benzyloxy-4-trifluoromethylcoumarin |
Bioorg Med Chem Lett 20: 3182-5 (2010)
Article DOI: 10.1016/j.bmcl.2010.03.057
BindingDB Entry DOI: 10.7270/Q2GX4BQB |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data