 Found 29 hits of Enzyme Inhibition Constant Data
Found 29 hits of Enzyme Inhibition Constant Data Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Sodium-dependent serotonin transporter
(MOUSE) | BDBM50322698
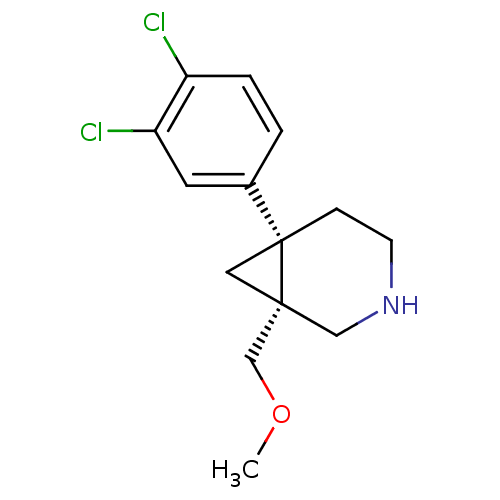
(rel-1-(3,4-dichlorophenyl)-6-(methoxymethyl)-3-aza...)Show InChI InChI=1S/C14H17Cl2NO/c1-18-9-13-7-14(13,4-5-17-8-13)10-2-3-11(15)12(16)6-10/h2-3,6,17H,4-5,7-9H2,1H3/t13-,14-/m1/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.58 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Medicines Research Centre
Curated by ChEMBL
| Assay Description
Displacement of [3H]-citalopram from SERT in mouse cortex after 2 hrs by liquid scintillation counting |
J Med Chem 53: 4989-5001 (2010)
Article DOI: 10.1021/jm100481d
BindingDB Entry DOI: 10.7270/Q20V8DR3 |
More data for this
Ligand-Target Pair | |
Sodium-dependent noradrenaline transporter
(MOUSE) | BDBM50322698

(rel-1-(3,4-dichlorophenyl)-6-(methoxymethyl)-3-aza...)Show InChI InChI=1S/C14H17Cl2NO/c1-18-9-13-7-14(13,4-5-17-8-13)10-2-3-11(15)12(16)6-10/h2-3,6,17H,4-5,7-9H2,1H3/t13-,14-/m1/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.58 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Medicines Research Centre
Curated by ChEMBL
| Assay Description
Displacement of [3H]nisoxetine from NET in mouse brain |
J Med Chem 53: 4989-5001 (2010)
Article DOI: 10.1021/jm100481d
BindingDB Entry DOI: 10.7270/Q20V8DR3 |
More data for this
Ligand-Target Pair | |
Transporter
(Rattus norvegicus (rat)) | BDBM50322698

(rel-1-(3,4-dichlorophenyl)-6-(methoxymethyl)-3-aza...)Show InChI InChI=1S/C14H17Cl2NO/c1-18-9-13-7-14(13,4-5-17-8-13)10-2-3-11(15)12(16)6-10/h2-3,6,17H,4-5,7-9H2,1H3/t13-,14-/m1/s1 | Reactome pathway
UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 2.51 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Medicines Research Centre
Curated by ChEMBL
| Assay Description
Displacement of [3H]nisoxetine from NET in rat hippocampus after 2 hrs by liquid scintillation counting |
J Med Chem 53: 4989-5001 (2010)
Article DOI: 10.1021/jm100481d
BindingDB Entry DOI: 10.7270/Q20V8DR3 |
More data for this
Ligand-Target Pair | |
Sodium-dependent dopamine transporter
(MOUSE) | BDBM50322698

(rel-1-(3,4-dichlorophenyl)-6-(methoxymethyl)-3-aza...)Show InChI InChI=1S/C14H17Cl2NO/c1-18-9-13-7-14(13,4-5-17-8-13)10-2-3-11(15)12(16)6-10/h2-3,6,17H,4-5,7-9H2,1H3/t13-,14-/m1/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Medicines Research Centre
Curated by ChEMBL
| Assay Description
Displacement of [3H]WIN-35428 from DAT in mouse brain |
J Med Chem 53: 4989-5001 (2010)
Article DOI: 10.1021/jm100481d
BindingDB Entry DOI: 10.7270/Q20V8DR3 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 1A2
(Homo sapiens (Human)) | BDBM50322696
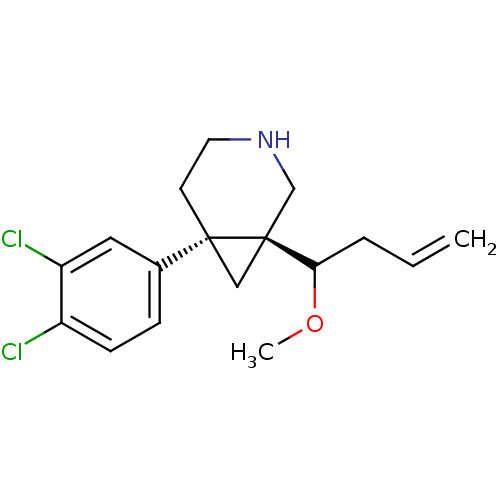
(6-(3,4-DICHLOROPHENYL)-1-[1-(METHYLOXY)-3-BUTEN-1-...)Show SMILES COC(CC=C)[C@@]12C[C@]1(CCNC2)c1ccc(Cl)c(Cl)c1 |r| Show InChI InChI=1S/C17H21Cl2NO/c1-3-4-15(21-2)17-10-16(17,7-8-20-11-17)12-5-6-13(18)14(19)9-12/h3,5-6,9,15,20H,1,4,7-8,10-11H2,2H3/t15?,16-,17+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Medicines Research Centre
Curated by ChEMBL
| Assay Description
Inhibition of human CYP1A2 after 10 mins |
J Med Chem 53: 4989-5001 (2010)
Article DOI: 10.1021/jm100481d
BindingDB Entry DOI: 10.7270/Q20V8DR3 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C9
(Homo sapiens (Human)) | BDBM50322696

(6-(3,4-DICHLOROPHENYL)-1-[1-(METHYLOXY)-3-BUTEN-1-...)Show SMILES COC(CC=C)[C@@]12C[C@]1(CCNC2)c1ccc(Cl)c(Cl)c1 |r| Show InChI InChI=1S/C17H21Cl2NO/c1-3-4-15(21-2)17-10-16(17,7-8-20-11-17)12-5-6-13(18)14(19)9-12/h3,5-6,9,15,20H,1,4,7-8,10-11H2,2H3/t15?,16-,17+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Medicines Research Centre
Curated by ChEMBL
| Assay Description
Inhibition of human CYP2C9 after 10 mins |
J Med Chem 53: 4989-5001 (2010)
Article DOI: 10.1021/jm100481d
BindingDB Entry DOI: 10.7270/Q20V8DR3 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C19
(Homo sapiens (Human)) | BDBM50322696

(6-(3,4-DICHLOROPHENYL)-1-[1-(METHYLOXY)-3-BUTEN-1-...)Show SMILES COC(CC=C)[C@@]12C[C@]1(CCNC2)c1ccc(Cl)c(Cl)c1 |r| Show InChI InChI=1S/C17H21Cl2NO/c1-3-4-15(21-2)17-10-16(17,7-8-20-11-17)12-5-6-13(18)14(19)9-12/h3,5-6,9,15,20H,1,4,7-8,10-11H2,2H3/t15?,16-,17+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Medicines Research Centre
Curated by ChEMBL
| Assay Description
Inhibition of human CYP2C19 after 10 mins |
J Med Chem 53: 4989-5001 (2010)
Article DOI: 10.1021/jm100481d
BindingDB Entry DOI: 10.7270/Q20V8DR3 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50322696

(6-(3,4-DICHLOROPHENYL)-1-[1-(METHYLOXY)-3-BUTEN-1-...)Show SMILES COC(CC=C)[C@@]12C[C@]1(CCNC2)c1ccc(Cl)c(Cl)c1 |r| Show InChI InChI=1S/C17H21Cl2NO/c1-3-4-15(21-2)17-10-16(17,7-8-20-11-17)12-5-6-13(18)14(19)9-12/h3,5-6,9,15,20H,1,4,7-8,10-11H2,2H3/t15?,16-,17+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Medicines Research Centre
Curated by ChEMBL
| Assay Description
Inhibition of human CYP3A4 after 10 mins |
J Med Chem 53: 4989-5001 (2010)
Article DOI: 10.1021/jm100481d
BindingDB Entry DOI: 10.7270/Q20V8DR3 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2D6
(Homo sapiens (Human)) | BDBM50322698

(rel-1-(3,4-dichlorophenyl)-6-(methoxymethyl)-3-aza...)Show InChI InChI=1S/C14H17Cl2NO/c1-18-9-13-7-14(13,4-5-17-8-13)10-2-3-11(15)12(16)6-10/h2-3,6,17H,4-5,7-9H2,1H3/t13-,14-/m1/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 3.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Medicines Research Centre
Curated by ChEMBL
| Assay Description
Inhibition of human CYP2D6 after 10 mins |
J Med Chem 53: 4989-5001 (2010)
Article DOI: 10.1021/jm100481d
BindingDB Entry DOI: 10.7270/Q20V8DR3 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2D6
(Homo sapiens (Human)) | BDBM50322698

(rel-1-(3,4-dichlorophenyl)-6-(methoxymethyl)-3-aza...)Show InChI InChI=1S/C14H17Cl2NO/c1-18-9-13-7-14(13,4-5-17-8-13)10-2-3-11(15)12(16)6-10/h2-3,6,17H,4-5,7-9H2,1H3/t13-,14-/m1/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 3.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Medicines Research Centre
Curated by ChEMBL
| Assay Description
Inhibition of human CYP2D6 after 10 mins |
J Med Chem 53: 4989-5001 (2010)
Article DOI: 10.1021/jm100481d
BindingDB Entry DOI: 10.7270/Q20V8DR3 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2D6
(Homo sapiens (Human)) | BDBM50322696

(6-(3,4-DICHLOROPHENYL)-1-[1-(METHYLOXY)-3-BUTEN-1-...)Show SMILES COC(CC=C)[C@@]12C[C@]1(CCNC2)c1ccc(Cl)c(Cl)c1 |r| Show InChI InChI=1S/C17H21Cl2NO/c1-3-4-15(21-2)17-10-16(17,7-8-20-11-17)12-5-6-13(18)14(19)9-12/h3,5-6,9,15,20H,1,4,7-8,10-11H2,2H3/t15?,16-,17+/m1/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Medicines Research Centre
Curated by ChEMBL
| Assay Description
Inhibition of human CYP2D6 after 10 mins |
J Med Chem 53: 4989-5001 (2010)
Article DOI: 10.1021/jm100481d
BindingDB Entry DOI: 10.7270/Q20V8DR3 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2D6
(Homo sapiens (Human)) | BDBM50322698

(rel-1-(3,4-dichlorophenyl)-6-(methoxymethyl)-3-aza...)Show InChI InChI=1S/C14H17Cl2NO/c1-18-9-13-7-14(13,4-5-17-8-13)10-2-3-11(15)12(16)6-10/h2-3,6,17H,4-5,7-9H2,1H3/t13-,14-/m1/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 3.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Medicines Research Centre
Curated by ChEMBL
| Assay Description
Inhibition of human CYP2D6 after 10 mins |
J Med Chem 53: 4989-5001 (2010)
Article DOI: 10.1021/jm100481d
BindingDB Entry DOI: 10.7270/Q20V8DR3 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C9
(Homo sapiens (Human)) | BDBM50322697
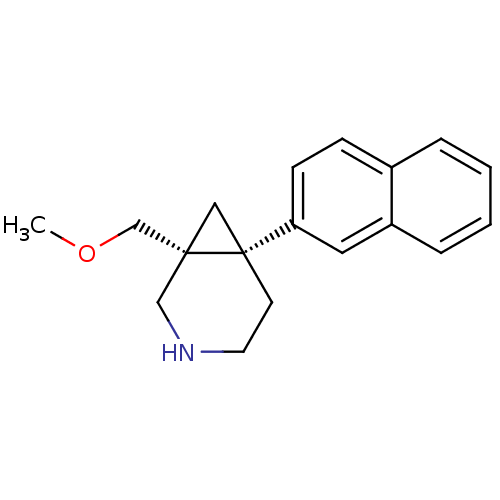
(1-(methoxymethyl)-6-(naphthalen-2-yl)-3-azabicyclo...)Show InChI InChI=1S/C18H21NO/c1-20-13-17-11-18(17,8-9-19-12-17)16-7-6-14-4-2-3-5-15(14)10-16/h2-7,10,19H,8-9,11-13H2,1H3/t17-,18-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | 8.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Medicines Research Centre
Curated by ChEMBL
| Assay Description
Inhibition of human CYP2C9 after 10 mins |
J Med Chem 53: 4989-5001 (2010)
Article DOI: 10.1021/jm100481d
BindingDB Entry DOI: 10.7270/Q20V8DR3 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 1A2
(Homo sapiens (Human)) | BDBM50322697

(1-(methoxymethyl)-6-(naphthalen-2-yl)-3-azabicyclo...)Show InChI InChI=1S/C18H21NO/c1-20-13-17-11-18(17,8-9-19-12-17)16-7-6-14-4-2-3-5-15(14)10-16/h2-7,10,19H,8-9,11-13H2,1H3/t17-,18-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | 8.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Medicines Research Centre
Curated by ChEMBL
| Assay Description
Inhibition of human CYP1A2 after 10 mins |
J Med Chem 53: 4989-5001 (2010)
Article DOI: 10.1021/jm100481d
BindingDB Entry DOI: 10.7270/Q20V8DR3 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2D6
(Homo sapiens (Human)) | BDBM50322697

(1-(methoxymethyl)-6-(naphthalen-2-yl)-3-azabicyclo...)Show InChI InChI=1S/C18H21NO/c1-20-13-17-11-18(17,8-9-19-12-17)16-7-6-14-4-2-3-5-15(14)10-16/h2-7,10,19H,8-9,11-13H2,1H3/t17-,18-/m1/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | 8.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Medicines Research Centre
Curated by ChEMBL
| Assay Description
Inhibition of human CYP2D6 after 10 mins |
J Med Chem 53: 4989-5001 (2010)
Article DOI: 10.1021/jm100481d
BindingDB Entry DOI: 10.7270/Q20V8DR3 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50322697

(1-(methoxymethyl)-6-(naphthalen-2-yl)-3-azabicyclo...)Show InChI InChI=1S/C18H21NO/c1-20-13-17-11-18(17,8-9-19-12-17)16-7-6-14-4-2-3-5-15(14)10-16/h2-7,10,19H,8-9,11-13H2,1H3/t17-,18-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | 8.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Medicines Research Centre
Curated by ChEMBL
| Assay Description
Inhibition of human CYP3A4 after 10 mins |
J Med Chem 53: 4989-5001 (2010)
Article DOI: 10.1021/jm100481d
BindingDB Entry DOI: 10.7270/Q20V8DR3 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C19
(Homo sapiens (Human)) | BDBM50322697

(1-(methoxymethyl)-6-(naphthalen-2-yl)-3-azabicyclo...)Show InChI InChI=1S/C18H21NO/c1-20-13-17-11-18(17,8-9-19-12-17)16-7-6-14-4-2-3-5-15(14)10-16/h2-7,10,19H,8-9,11-13H2,1H3/t17-,18-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | 8.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Medicines Research Centre
Curated by ChEMBL
| Assay Description
Inhibition of human CYP2C19 after 10 mins |
J Med Chem 53: 4989-5001 (2010)
Article DOI: 10.1021/jm100481d
BindingDB Entry DOI: 10.7270/Q20V8DR3 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 1A2
(Homo sapiens (Human)) | BDBM50322698

(rel-1-(3,4-dichlorophenyl)-6-(methoxymethyl)-3-aza...)Show InChI InChI=1S/C14H17Cl2NO/c1-18-9-13-7-14(13,4-5-17-8-13)10-2-3-11(15)12(16)6-10/h2-3,6,17H,4-5,7-9H2,1H3/t13-,14-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Medicines Research Centre
Curated by ChEMBL
| Assay Description
Inhibition of human CYP1A2 after 10 mins |
J Med Chem 53: 4989-5001 (2010)
Article DOI: 10.1021/jm100481d
BindingDB Entry DOI: 10.7270/Q20V8DR3 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C19
(Homo sapiens (Human)) | BDBM50322698

(rel-1-(3,4-dichlorophenyl)-6-(methoxymethyl)-3-aza...)Show InChI InChI=1S/C14H17Cl2NO/c1-18-9-13-7-14(13,4-5-17-8-13)10-2-3-11(15)12(16)6-10/h2-3,6,17H,4-5,7-9H2,1H3/t13-,14-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Medicines Research Centre
Curated by ChEMBL
| Assay Description
Inhibition of human CYP2C19 after 10 mins |
J Med Chem 53: 4989-5001 (2010)
Article DOI: 10.1021/jm100481d
BindingDB Entry DOI: 10.7270/Q20V8DR3 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 1A2
(Homo sapiens (Human)) | BDBM50322698

(rel-1-(3,4-dichlorophenyl)-6-(methoxymethyl)-3-aza...)Show InChI InChI=1S/C14H17Cl2NO/c1-18-9-13-7-14(13,4-5-17-8-13)10-2-3-11(15)12(16)6-10/h2-3,6,17H,4-5,7-9H2,1H3/t13-,14-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Medicines Research Centre
Curated by ChEMBL
| Assay Description
Inhibition of human CYP1A2 after 10 mins |
J Med Chem 53: 4989-5001 (2010)
Article DOI: 10.1021/jm100481d
BindingDB Entry DOI: 10.7270/Q20V8DR3 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50322698

(rel-1-(3,4-dichlorophenyl)-6-(methoxymethyl)-3-aza...)Show InChI InChI=1S/C14H17Cl2NO/c1-18-9-13-7-14(13,4-5-17-8-13)10-2-3-11(15)12(16)6-10/h2-3,6,17H,4-5,7-9H2,1H3/t13-,14-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Medicines Research Centre
Curated by ChEMBL
| Assay Description
Inhibition of human CYP3A4 after 10 mins |
J Med Chem 53: 4989-5001 (2010)
Article DOI: 10.1021/jm100481d
BindingDB Entry DOI: 10.7270/Q20V8DR3 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C9
(Homo sapiens (Human)) | BDBM50322698

(rel-1-(3,4-dichlorophenyl)-6-(methoxymethyl)-3-aza...)Show InChI InChI=1S/C14H17Cl2NO/c1-18-9-13-7-14(13,4-5-17-8-13)10-2-3-11(15)12(16)6-10/h2-3,6,17H,4-5,7-9H2,1H3/t13-,14-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Medicines Research Centre
Curated by ChEMBL
| Assay Description
Inhibition of human CYP2C9 after 10 mins |
J Med Chem 53: 4989-5001 (2010)
Article DOI: 10.1021/jm100481d
BindingDB Entry DOI: 10.7270/Q20V8DR3 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 1A2
(Homo sapiens (Human)) | BDBM50322698

(rel-1-(3,4-dichlorophenyl)-6-(methoxymethyl)-3-aza...)Show InChI InChI=1S/C14H17Cl2NO/c1-18-9-13-7-14(13,4-5-17-8-13)10-2-3-11(15)12(16)6-10/h2-3,6,17H,4-5,7-9H2,1H3/t13-,14-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Medicines Research Centre
Curated by ChEMBL
| Assay Description
Inhibition of human CYP1A2 after 10 mins |
J Med Chem 53: 4989-5001 (2010)
Article DOI: 10.1021/jm100481d
BindingDB Entry DOI: 10.7270/Q20V8DR3 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50322698

(rel-1-(3,4-dichlorophenyl)-6-(methoxymethyl)-3-aza...)Show InChI InChI=1S/C14H17Cl2NO/c1-18-9-13-7-14(13,4-5-17-8-13)10-2-3-11(15)12(16)6-10/h2-3,6,17H,4-5,7-9H2,1H3/t13-,14-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Medicines Research Centre
Curated by ChEMBL
| Assay Description
Inhibition of human CYP3A4 after 10 mins |
J Med Chem 53: 4989-5001 (2010)
Article DOI: 10.1021/jm100481d
BindingDB Entry DOI: 10.7270/Q20V8DR3 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C9
(Homo sapiens (Human)) | BDBM50322698

(rel-1-(3,4-dichlorophenyl)-6-(methoxymethyl)-3-aza...)Show InChI InChI=1S/C14H17Cl2NO/c1-18-9-13-7-14(13,4-5-17-8-13)10-2-3-11(15)12(16)6-10/h2-3,6,17H,4-5,7-9H2,1H3/t13-,14-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Medicines Research Centre
Curated by ChEMBL
| Assay Description
Inhibition of human CYP2C9 after 10 mins |
J Med Chem 53: 4989-5001 (2010)
Article DOI: 10.1021/jm100481d
BindingDB Entry DOI: 10.7270/Q20V8DR3 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C19
(Homo sapiens (Human)) | BDBM50322698

(rel-1-(3,4-dichlorophenyl)-6-(methoxymethyl)-3-aza...)Show InChI InChI=1S/C14H17Cl2NO/c1-18-9-13-7-14(13,4-5-17-8-13)10-2-3-11(15)12(16)6-10/h2-3,6,17H,4-5,7-9H2,1H3/t13-,14-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Medicines Research Centre
Curated by ChEMBL
| Assay Description
Inhibition of human CYP2C19 after 10 mins |
J Med Chem 53: 4989-5001 (2010)
Article DOI: 10.1021/jm100481d
BindingDB Entry DOI: 10.7270/Q20V8DR3 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50322698

(rel-1-(3,4-dichlorophenyl)-6-(methoxymethyl)-3-aza...)Show InChI InChI=1S/C14H17Cl2NO/c1-18-9-13-7-14(13,4-5-17-8-13)10-2-3-11(15)12(16)6-10/h2-3,6,17H,4-5,7-9H2,1H3/t13-,14-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Medicines Research Centre
Curated by ChEMBL
| Assay Description
Inhibition of human CYP3A4 after 10 mins |
J Med Chem 53: 4989-5001 (2010)
Article DOI: 10.1021/jm100481d
BindingDB Entry DOI: 10.7270/Q20V8DR3 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C19
(Homo sapiens (Human)) | BDBM50322698

(rel-1-(3,4-dichlorophenyl)-6-(methoxymethyl)-3-aza...)Show InChI InChI=1S/C14H17Cl2NO/c1-18-9-13-7-14(13,4-5-17-8-13)10-2-3-11(15)12(16)6-10/h2-3,6,17H,4-5,7-9H2,1H3/t13-,14-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Medicines Research Centre
Curated by ChEMBL
| Assay Description
Inhibition of human CYP2C19 after 10 mins |
J Med Chem 53: 4989-5001 (2010)
Article DOI: 10.1021/jm100481d
BindingDB Entry DOI: 10.7270/Q20V8DR3 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C9
(Homo sapiens (Human)) | BDBM50322698

(rel-1-(3,4-dichlorophenyl)-6-(methoxymethyl)-3-aza...)Show InChI InChI=1S/C14H17Cl2NO/c1-18-9-13-7-14(13,4-5-17-8-13)10-2-3-11(15)12(16)6-10/h2-3,6,17H,4-5,7-9H2,1H3/t13-,14-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Medicines Research Centre
Curated by ChEMBL
| Assay Description
Inhibition of human CYP2C9 after 10 mins |
J Med Chem 53: 4989-5001 (2010)
Article DOI: 10.1021/jm100481d
BindingDB Entry DOI: 10.7270/Q20V8DR3 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data