 Found 6 hits of Enzyme Inhibition Constant Data
Found 6 hits of Enzyme Inhibition Constant Data Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Delta-type opioid receptor/Kappa-type opioid receptor/Mu-type opioid receptor/Sigma non-opioid intracellular receptor 1
(Rattus norvegicus (rat)-RAT) | BDBM82427
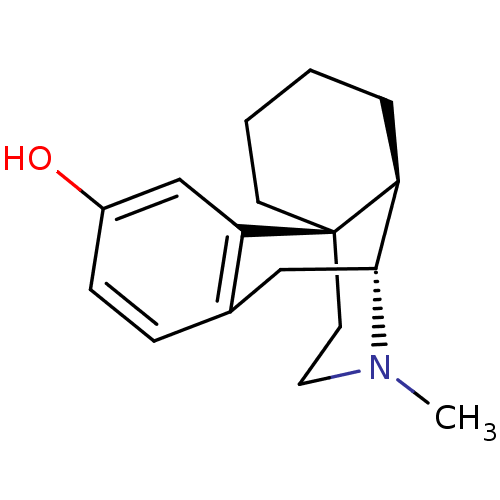
(CAS_5985-38-6 | LEVORPHANOL-tartarate)Show SMILES CN1CC[C@]23CCCC[C@H]2[C@H]1Cc1ccc(O)cc31 |r| Show InChI InChI=1S/C17H23NO/c1-18-9-8-17-7-3-2-4-14(17)16(18)10-12-5-6-13(19)11-15(12)17/h5-6,11,14,16,19H,2-4,7-10H2,1H3/t14-,16+,17+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
DrugBank
KEGG
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 4.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Creighton University
Curated by ChEMBL
| Assay Description
Concentration that produces 50% inhibition of stereospecific [3H]naloxone binding to opioid receptors in rat brain in absence of sodium. |
J Med Chem 33: 245-8 (1990)
BindingDB Entry DOI: 10.7270/Q2H997FH |
More data for this
Ligand-Target Pair | |
Delta-type opioid receptor/Kappa-type opioid receptor/Mu-type opioid receptor/Sigma non-opioid intracellular receptor 1
(Rattus norvegicus (rat)-RAT) | BDBM82427

(CAS_5985-38-6 | LEVORPHANOL-tartarate)Show SMILES CN1CC[C@]23CCCC[C@H]2[C@H]1Cc1ccc(O)cc31 |r| Show InChI InChI=1S/C17H23NO/c1-18-9-8-17-7-3-2-4-14(17)16(18)10-12-5-6-13(19)11-15(12)17/h5-6,11,14,16,19H,2-4,7-10H2,1H3/t14-,16+,17+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
DrugBank
KEGG
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 57 | n/a | n/a | n/a | n/a | n/a | n/a |
Creighton University
Curated by ChEMBL
| Assay Description
Concentration that produces 50% inhibition of stereospecific [3H]naloxone binding to opioid receptors in rat brain in presence of sodium. |
J Med Chem 33: 245-8 (1990)
BindingDB Entry DOI: 10.7270/Q2H997FH |
More data for this
Ligand-Target Pair | |
Delta-type opioid receptor/Kappa-type opioid receptor/Mu-type opioid receptor/Sigma non-opioid intracellular receptor 1
(Rattus norvegicus (rat)-RAT) | BDBM50000092

((-)-(etorphine) | (-)-morphine | (1S,5R,13R,14S)-1...)Show SMILES CN1CC[C@@]23[C@H]4Oc5c2c(C[C@@H]1[C@@H]3C=C[C@@H]4O)ccc5O |r,c:16,TLB:13:12:8.9.10:3.2.1| Show InChI InChI=1S/C17H19NO3/c1-18-7-6-17-10-3-5-13(20)16(17)21-15-12(19)4-2-9(14(15)17)8-11(10)18/h2-5,10-11,13,16,19-20H,6-8H2,1H3/t10-,11+,13-,16-,17-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
PubMed
| n/a | n/a | 226 | n/a | n/a | n/a | n/a | n/a | n/a |
Creighton University
Curated by ChEMBL
| Assay Description
Concentration that produces 50% inhibition of stereospecific [3H]naloxone binding to opioid receptors in rat brain in absence of sodium. |
J Med Chem 33: 245-8 (1990)
BindingDB Entry DOI: 10.7270/Q2H997FH |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Delta-type opioid receptor/Kappa-type opioid receptor/Mu-type opioid receptor/Sigma non-opioid intracellular receptor 1
(Rattus norvegicus (rat)-RAT) | BDBM50228352
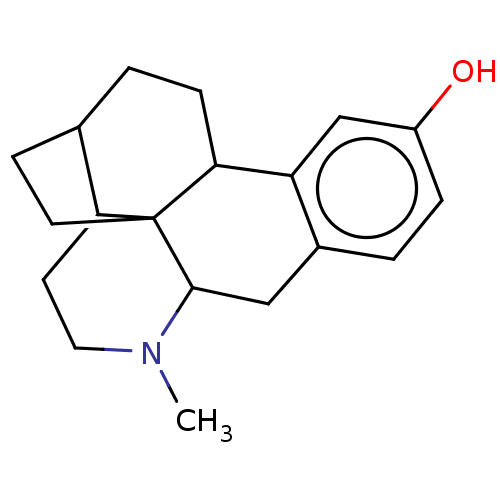
(CHEMBL81058)Show InChI InChI=1S/C19H25NO.ClH/c1-20-9-7-16-12-3-5-17-15-11-14(21)4-2-13(15)10-18(20)19(16,17)8-6-12;/h2,4,11-12,16-18,21H,3,5-10H2,1H3;1H | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 472 | n/a | n/a | n/a | n/a | n/a | n/a |
Creighton University
Curated by ChEMBL
| Assay Description
Concentration that produces 50% inhibition of stereospecific [3H]naloxone binding to opioid receptors in rat brain in absence of sodium. |
J Med Chem 33: 245-8 (1990)
BindingDB Entry DOI: 10.7270/Q2H997FH |
More data for this
Ligand-Target Pair | |
Delta-type opioid receptor/Kappa-type opioid receptor/Mu-type opioid receptor/Sigma non-opioid intracellular receptor 1
(Rattus norvegicus (rat)-RAT) | BDBM50000092

((-)-(etorphine) | (-)-morphine | (1S,5R,13R,14S)-1...)Show SMILES CN1CC[C@@]23[C@H]4Oc5c2c(C[C@@H]1[C@@H]3C=C[C@@H]4O)ccc5O |r,c:16,TLB:13:12:8.9.10:3.2.1| Show InChI InChI=1S/C17H19NO3/c1-18-7-6-17-10-3-5-13(20)16(17)21-15-12(19)4-2-9(14(15)17)8-11(10)18/h2-5,10-11,13,16,19-20H,6-8H2,1H3/t10-,11+,13-,16-,17-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
PubMed
| n/a | n/a | 487 | n/a | n/a | n/a | n/a | n/a | n/a |
Creighton University
Curated by ChEMBL
| Assay Description
Concentration that produces 50% inhibition of stereospecific [3H]naloxone binding to opioid receptors in rat brain in presence of sodium. |
J Med Chem 33: 245-8 (1990)
BindingDB Entry DOI: 10.7270/Q2H997FH |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Delta-type opioid receptor/Kappa-type opioid receptor/Mu-type opioid receptor/Sigma non-opioid intracellular receptor 1
(Rattus norvegicus (rat)-RAT) | BDBM50228352

(CHEMBL81058)Show InChI InChI=1S/C19H25NO.ClH/c1-20-9-7-16-12-3-5-17-15-11-14(21)4-2-13(15)10-18(20)19(16,17)8-6-12;/h2,4,11-12,16-18,21H,3,5-10H2,1H3;1H | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 5.59E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Creighton University
Curated by ChEMBL
| Assay Description
Concentration that produces 50% inhibition of stereospecific [3H]naloxone binding to opioid receptors in rat brain in presence of sodium. |
J Med Chem 33: 245-8 (1990)
BindingDB Entry DOI: 10.7270/Q2H997FH |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data