

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Acetylcholinesterase (Rattus norvegicus (rat)) | BDBM8960 ((+/-)-2-[(1-benzylpiperidin-4-yl)methyl]-5,6-dimet...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Zhejiang University Curated by ChEMBL | Assay Description Inhibition of AChE in rat cortex homogenates using acetylthiocholine iodide as substrate after 15 mins by Ellman's method | Bioorg Med Chem 19: 7158-67 (2011) Article DOI: 10.1016/j.bmc.2011.09.061 BindingDB Entry DOI: 10.7270/Q24F1R52 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM50359378 (CHEMBL1929417) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
Zhejiang University Curated by ChEMBL | Assay Description Inhibition of BACE1 | Bioorg Med Chem 19: 7158-67 (2011) Article DOI: 10.1016/j.bmc.2011.09.061 BindingDB Entry DOI: 10.7270/Q24F1R52 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50169850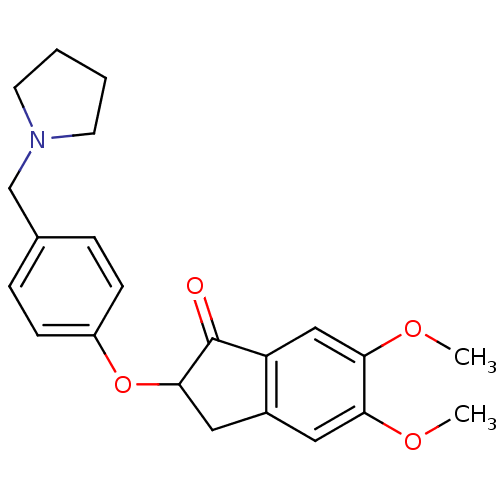 (5,6-Dimethoxy-2-(4-pyrrolidin-1-ylmethyl-phenoxy)-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
Zhejiang University Curated by ChEMBL | Assay Description Inhibition of AChE | Bioorg Med Chem 19: 7158-67 (2011) Article DOI: 10.1016/j.bmc.2011.09.061 BindingDB Entry DOI: 10.7270/Q24F1R52 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50359382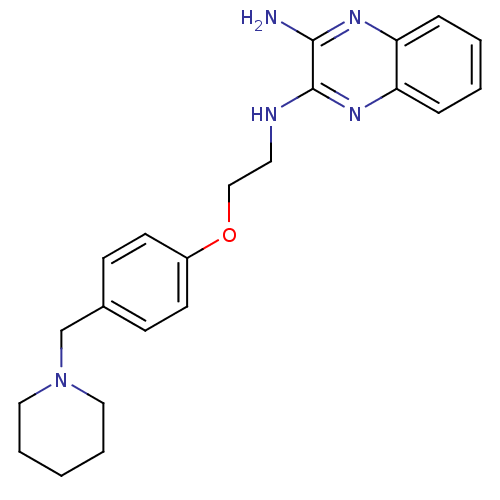 (CHEMBL1929406) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 52 | n/a | n/a | n/a | n/a | n/a | n/a |
Zhejiang University Curated by ChEMBL | Assay Description Antagonist activity at histamine H3 receptor expressed in HEK293 cells co-transfected with pCRE-Luc gene assessed as inhibition of forskolin/histamin... | Bioorg Med Chem 19: 7158-67 (2011) Article DOI: 10.1016/j.bmc.2011.09.061 BindingDB Entry DOI: 10.7270/Q24F1R52 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50359389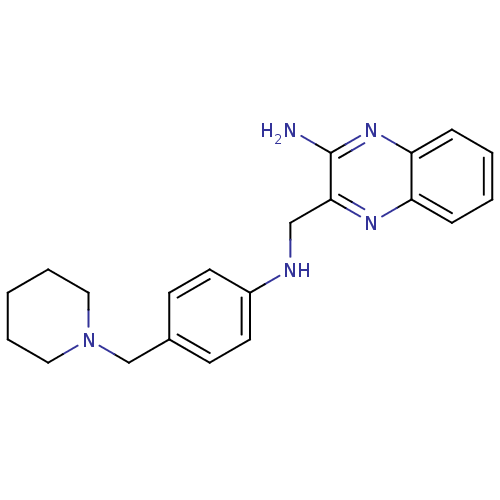 (CHEMBL1929412) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 130 | n/a | n/a | n/a | n/a | n/a | n/a |
Zhejiang University Curated by ChEMBL | Assay Description Antagonist activity at histamine H3 receptor expressed in HEK293 cells co-transfected with pCRE-Luc gene assessed as inhibition of forskolin/histamin... | Bioorg Med Chem 19: 7158-67 (2011) Article DOI: 10.1016/j.bmc.2011.09.061 BindingDB Entry DOI: 10.7270/Q24F1R52 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50359376 (CHEMBL1929400 | CHEMBL1929401) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 171 | n/a | n/a | n/a | n/a | n/a | n/a |
Zhejiang University Curated by ChEMBL | Assay Description Inverse agonist activity at histamine H3 receptor expressed in HEK293 cells co-transfected with pCRE-Luc gene assessed as inhibition of forskolin/his... | Bioorg Med Chem 19: 7158-67 (2011) Article DOI: 10.1016/j.bmc.2011.09.061 BindingDB Entry DOI: 10.7270/Q24F1R52 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50359383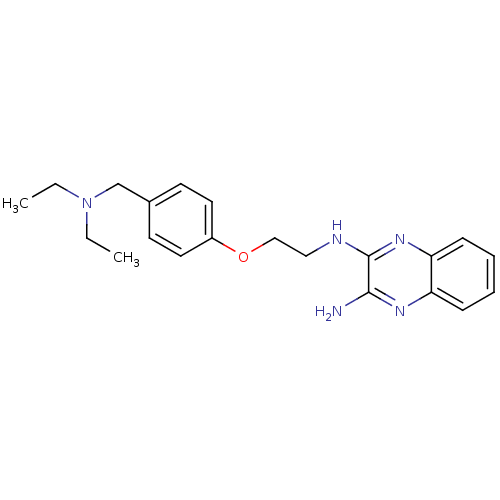 (CHEMBL1929407) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 182 | n/a | n/a | n/a | n/a | n/a | n/a |
Zhejiang University Curated by ChEMBL | Assay Description Antagonist activity at histamine H3 receptor expressed in HEK293 cells co-transfected with pCRE-Luc gene assessed as inhibition of forskolin/histamin... | Bioorg Med Chem 19: 7158-67 (2011) Article DOI: 10.1016/j.bmc.2011.09.061 BindingDB Entry DOI: 10.7270/Q24F1R52 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50359376 (CHEMBL1929400 | CHEMBL1929401) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 189 | n/a | n/a | n/a | n/a | n/a | n/a |
Zhejiang University Curated by ChEMBL | Assay Description Inverse agonist activity at histamine H3 receptor expressed in HEK293 cells co-transfected with pCRE-Luc gene assessed as inhibition of forskolin/his... | Bioorg Med Chem 19: 7158-67 (2011) Article DOI: 10.1016/j.bmc.2011.09.061 BindingDB Entry DOI: 10.7270/Q24F1R52 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50359386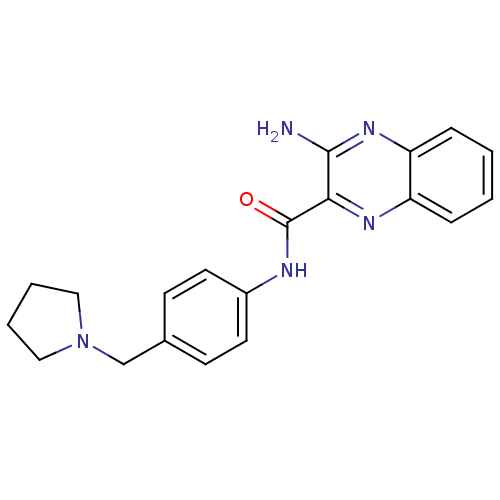 (CHEMBL1929411) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 260 | n/a | n/a | n/a | n/a | n/a | n/a |
Zhejiang University Curated by ChEMBL | Assay Description Antagonist activity at histamine H3 receptor expressed in HEK293 cells co-transfected with pCRE-Luc gene assessed as inhibition of forskolin/histamin... | Bioorg Med Chem 19: 7158-67 (2011) Article DOI: 10.1016/j.bmc.2011.09.061 BindingDB Entry DOI: 10.7270/Q24F1R52 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50359376 (CHEMBL1929400 | CHEMBL1929401) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 280 | n/a | n/a | n/a | n/a | n/a | n/a |
Zhejiang University Curated by ChEMBL | Assay Description Antagonist activity at histamine H3 receptor expressed in HEK293 cells co-transfected with pCRE-Luc gene assessed as inhibition of forskolin/histamin... | Bioorg Med Chem 19: 7158-67 (2011) Article DOI: 10.1016/j.bmc.2011.09.061 BindingDB Entry DOI: 10.7270/Q24F1R52 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Rattus norvegicus (rat)) | BDBM50359376 (CHEMBL1929400 | CHEMBL1929401) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 313 | n/a | n/a | n/a | n/a | n/a | n/a |
Zhejiang University Curated by ChEMBL | Assay Description Inhibition of AChE in rat cortex homogenates using acetylthiocholine iodide as substrate after 15 mins by Ellman's method | Bioorg Med Chem 19: 7158-67 (2011) Article DOI: 10.1016/j.bmc.2011.09.061 BindingDB Entry DOI: 10.7270/Q24F1R52 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50359386 (CHEMBL1929411) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 379 | n/a | n/a | n/a | n/a | n/a | n/a |
Zhejiang University Curated by ChEMBL | Assay Description Antagonist activity at histamine H3 receptor expressed in HEK293 cells co-transfected with pCRE-Luc gene assessed as inhibition of forskolin/histamin... | Bioorg Med Chem 19: 7158-67 (2011) Article DOI: 10.1016/j.bmc.2011.09.061 BindingDB Entry DOI: 10.7270/Q24F1R52 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Rattus norvegicus (rat)) | BDBM50359379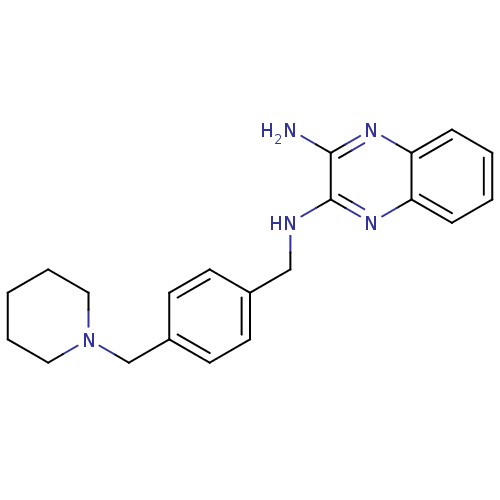 (CHEMBL1929403) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 476 | n/a | n/a | n/a | n/a | n/a | n/a |
Zhejiang University Curated by ChEMBL | Assay Description Inhibition of AChE in rat cortex homogenates using acetylthiocholine iodide as substrate after 15 mins by Ellman's method | Bioorg Med Chem 19: 7158-67 (2011) Article DOI: 10.1016/j.bmc.2011.09.061 BindingDB Entry DOI: 10.7270/Q24F1R52 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Rattus norvegicus (rat)) | BDBM50359376 (CHEMBL1929400 | CHEMBL1929401) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 483 | n/a | n/a | n/a | n/a | n/a | n/a |
Zhejiang University Curated by ChEMBL | Assay Description Inhibition of AChE in rat cortex homogenates using acetylthiocholine iodide as substrate after 15 mins by Ellman's method | Bioorg Med Chem 19: 7158-67 (2011) Article DOI: 10.1016/j.bmc.2011.09.061 BindingDB Entry DOI: 10.7270/Q24F1R52 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50359376 (CHEMBL1929400 | CHEMBL1929401) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 511 | n/a | n/a | n/a | n/a | n/a | n/a |
Zhejiang University Curated by ChEMBL | Assay Description Antagonist activity at histamine H3 receptor expressed in HEK293 cells co-transfected with pCRE-Luc gene assessed as inhibition of forskolin/histamin... | Bioorg Med Chem 19: 7158-67 (2011) Article DOI: 10.1016/j.bmc.2011.09.061 BindingDB Entry DOI: 10.7270/Q24F1R52 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM22914 (CHEMBL260374 | N-cyclohexyl-4-(1H-imidazol-5-yl)pi...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 518 | n/a | n/a | n/a | n/a | n/a | n/a |
Zhejiang University Curated by ChEMBL | Assay Description Antagonist activity at histamine H3 receptor expressed in HEK293 cells co-transfected with pCRE-Luc gene assessed as inhibition of forskolin/histamin... | Bioorg Med Chem 19: 7158-67 (2011) Article DOI: 10.1016/j.bmc.2011.09.061 BindingDB Entry DOI: 10.7270/Q24F1R52 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H4 receptor (Homo sapiens (Human)) | BDBM22914 (CHEMBL260374 | N-cyclohexyl-4-(1H-imidazol-5-yl)pi...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 667 | n/a | n/a | n/a | n/a | n/a | n/a |
Zhejiang University Curated by ChEMBL | Assay Description Antagonist activity at histamine H4 receptor expressed in HEK293 cells co-transfected with pCRE-Luc gene assessed as inhibition of forskolin/histamin... | Bioorg Med Chem 19: 7158-67 (2011) Article DOI: 10.1016/j.bmc.2011.09.061 BindingDB Entry DOI: 10.7270/Q24F1R52 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Rattus norvegicus (rat)) | BDBM50359387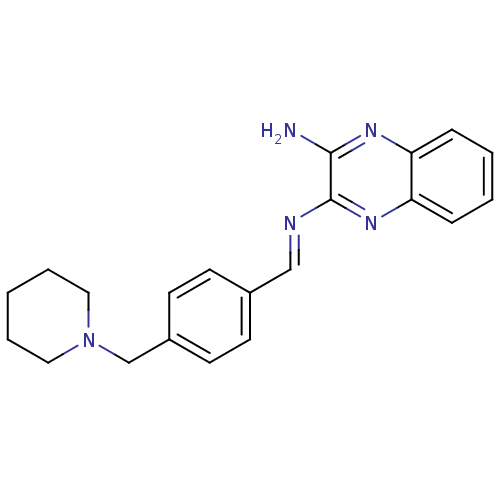 (CHEMBL1929413) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 748 | n/a | n/a | n/a | n/a | n/a | n/a |
Zhejiang University Curated by ChEMBL | Assay Description Inhibition of AChE in rat cortex homogenates using acetylthiocholine iodide as substrate after 15 mins by Ellman's method | Bioorg Med Chem 19: 7158-67 (2011) Article DOI: 10.1016/j.bmc.2011.09.061 BindingDB Entry DOI: 10.7270/Q24F1R52 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Rattus norvegicus (rat)) | BDBM50359385 (CHEMBL1929410) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 892 | n/a | n/a | n/a | n/a | n/a | n/a |
Zhejiang University Curated by ChEMBL | Assay Description Inhibition of AChE in rat cortex homogenates using acetylthiocholine iodide as substrate after 15 mins by Ellman's method | Bioorg Med Chem 19: 7158-67 (2011) Article DOI: 10.1016/j.bmc.2011.09.061 BindingDB Entry DOI: 10.7270/Q24F1R52 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Rattus norvegicus (rat)) | BDBM50359380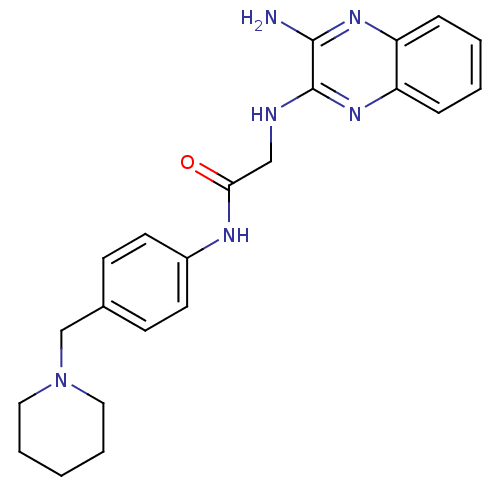 (CHEMBL1929404) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.16E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Zhejiang University Curated by ChEMBL | Assay Description Inhibition of AChE in rat cortex homogenates using acetylthiocholine iodide as substrate after 15 mins by Ellman's method | Bioorg Med Chem 19: 7158-67 (2011) Article DOI: 10.1016/j.bmc.2011.09.061 BindingDB Entry DOI: 10.7270/Q24F1R52 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Rattus norvegicus (rat)) | BDBM50359381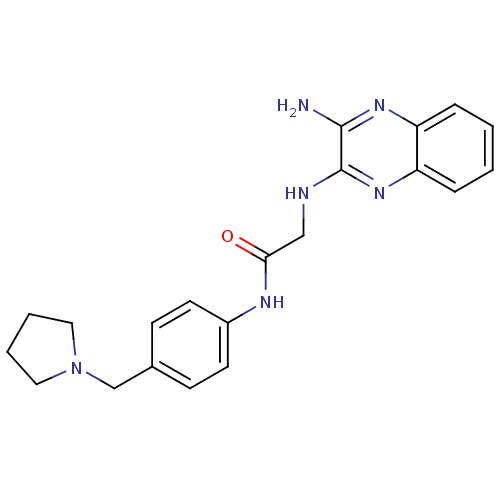 (CHEMBL1929405) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.17E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Zhejiang University Curated by ChEMBL | Assay Description Inhibition of AChE in rat cortex homogenates using acetylthiocholine iodide as substrate after 15 mins by Ellman's method | Bioorg Med Chem 19: 7158-67 (2011) Article DOI: 10.1016/j.bmc.2011.09.061 BindingDB Entry DOI: 10.7270/Q24F1R52 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Rattus norvegicus (rat)) | BDBM50359388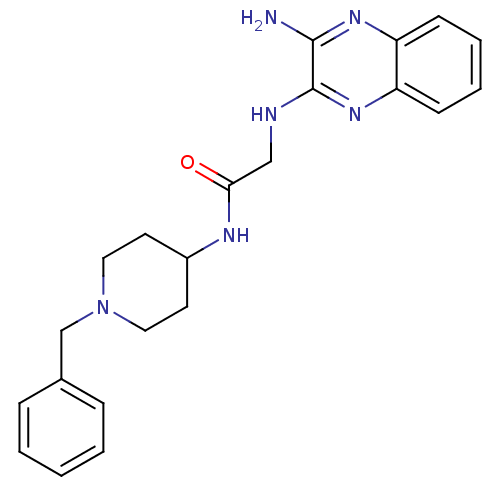 (CHEMBL1929416) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.33E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Zhejiang University Curated by ChEMBL | Assay Description Inhibition of AChE in rat cortex homogenates using acetylthiocholine iodide as substrate after 15 mins by Ellman's method | Bioorg Med Chem 19: 7158-67 (2011) Article DOI: 10.1016/j.bmc.2011.09.061 BindingDB Entry DOI: 10.7270/Q24F1R52 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Rattus norvegicus (rat)) | BDBM50359386 (CHEMBL1929411) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Zhejiang University Curated by ChEMBL | Assay Description Inhibition of AChE in rat cortex homogenates using acetylthiocholine iodide as substrate after 15 mins by Ellman's method | Bioorg Med Chem 19: 7158-67 (2011) Article DOI: 10.1016/j.bmc.2011.09.061 BindingDB Entry DOI: 10.7270/Q24F1R52 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Rattus norvegicus (rat)) | BDBM50359382 (CHEMBL1929406) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Zhejiang University Curated by ChEMBL | Assay Description Inhibition of AChE in rat cortex homogenates using acetylthiocholine iodide as substrate after 15 mins by Ellman's method | Bioorg Med Chem 19: 7158-67 (2011) Article DOI: 10.1016/j.bmc.2011.09.061 BindingDB Entry DOI: 10.7270/Q24F1R52 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Rattus norvegicus (rat)) | BDBM50359384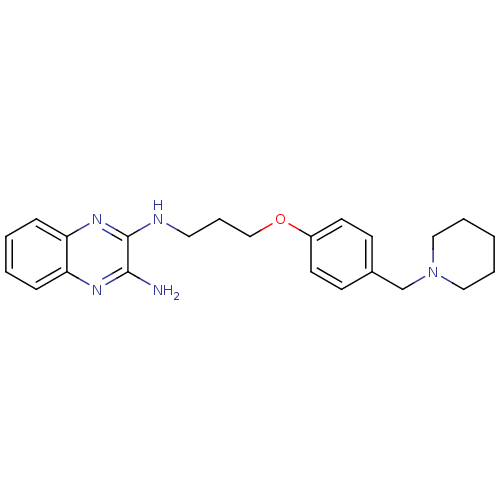 (CHEMBL1929409) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.93E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Zhejiang University Curated by ChEMBL | Assay Description Inhibition of AChE in rat cortex homogenates using acetylthiocholine iodide as substrate after 15 mins by Ellman's method | Bioorg Med Chem 19: 7158-67 (2011) Article DOI: 10.1016/j.bmc.2011.09.061 BindingDB Entry DOI: 10.7270/Q24F1R52 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Rattus norvegicus (rat)) | BDBM50359383 (CHEMBL1929407) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.87E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Zhejiang University Curated by ChEMBL | Assay Description Inhibition of AChE in rat cortex homogenates using acetylthiocholine iodide as substrate after 15 mins by Ellman's method | Bioorg Med Chem 19: 7158-67 (2011) Article DOI: 10.1016/j.bmc.2011.09.061 BindingDB Entry DOI: 10.7270/Q24F1R52 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H4 receptor (Homo sapiens (Human)) | BDBM50359376 (CHEMBL1929400 | CHEMBL1929401) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Zhejiang University Curated by ChEMBL | Assay Description Antagonist activity at histamine H4 receptor expressed in HEK293 cells co-transfected with pCRE-Luc gene assessed as inhibition of forskolin/histamin... | Bioorg Med Chem 19: 7158-67 (2011) Article DOI: 10.1016/j.bmc.2011.09.061 BindingDB Entry DOI: 10.7270/Q24F1R52 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H4 receptor (Homo sapiens (Human)) | BDBM50359376 (CHEMBL1929400 | CHEMBL1929401) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Zhejiang University Curated by ChEMBL | Assay Description Antagonist activity at histamine H4 receptor expressed in HEK293 cells co-transfected with pCRE-Luc gene assessed as inhibition of forskolin/histamin... | Bioorg Med Chem 19: 7158-67 (2011) Article DOI: 10.1016/j.bmc.2011.09.061 BindingDB Entry DOI: 10.7270/Q24F1R52 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||