

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM240835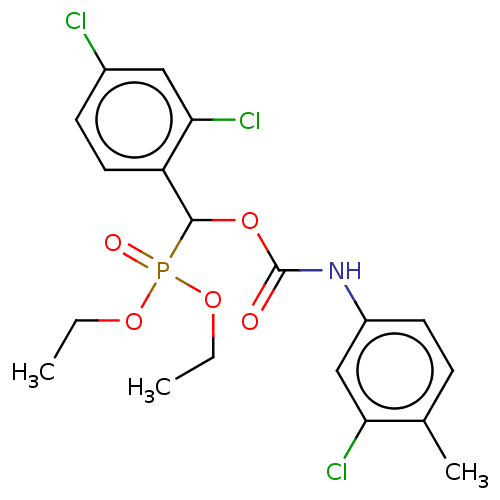 (Diethyl[(3-chloro-4-methylanilinocarbonyl)oxy](2,4...) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 6.36E+3 | n/a | n/a | n/a | n/a | 8.0 | 25 |
Institute for Advanced Studies in Basic Sciences (IASBS) | Assay Description Inhibitory activities of synthesized α-oxycarbanilinophosphonates were determined at 25°C by the colorimetric method of Ellman et al. [Ellman et... | J Enzyme Inhib Med Chem 28: 576-82 (2013) Article DOI: 10.3109/14756366.2012.663362 BindingDB Entry DOI: 10.7270/Q20R9N9H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM240836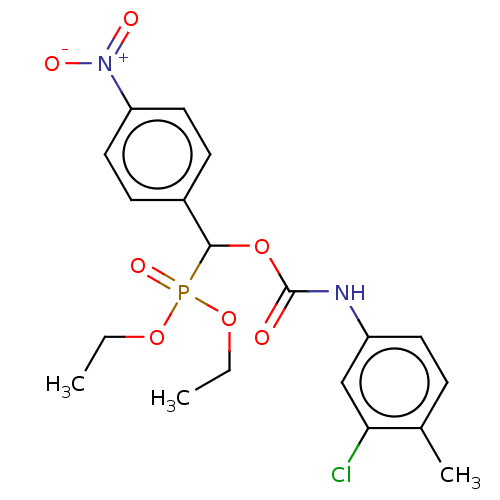 (α-Oxycarbanilinophosphonate, 4i) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 7.47E+3 | n/a | n/a | n/a | n/a | 8.0 | 25 |
Institute for Advanced Studies in Basic Sciences (IASBS) | Assay Description Inhibitory activities of synthesized α-oxycarbanilinophosphonates were determined at 25°C by the colorimetric method of Ellman et al. [Ellman et... | J Enzyme Inhib Med Chem 28: 576-82 (2013) Article DOI: 10.3109/14756366.2012.663362 BindingDB Entry DOI: 10.7270/Q20R9N9H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM240834 (α-Oxycarbanilinophosphonate, 4f) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.04E+4 | n/a | n/a | n/a | n/a | 8.0 | 25 |
Institute for Advanced Studies in Basic Sciences (IASBS) | Assay Description Inhibitory activities of synthesized α-oxycarbanilinophosphonates were determined at 25°C by the colorimetric method of Ellman et al. [Ellman et... | J Enzyme Inhib Med Chem 28: 576-82 (2013) Article DOI: 10.3109/14756366.2012.663362 BindingDB Entry DOI: 10.7270/Q20R9N9H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM240833 (α-Oxycarbanilinophosphonate, 4d) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.61E+5 | n/a | n/a | n/a | n/a | 8.0 | 25 |
Institute for Advanced Studies in Basic Sciences (IASBS) | Assay Description Inhibitory activities of synthesized α-oxycarbanilinophosphonates were determined at 25°C by the colorimetric method of Ellman et al. [Ellman et... | J Enzyme Inhib Med Chem 28: 576-82 (2013) Article DOI: 10.3109/14756366.2012.663362 BindingDB Entry DOI: 10.7270/Q20R9N9H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM240837 (α-Oxycarbanilinophosphonate, 4l) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 4.18E+5 | n/a | n/a | n/a | n/a | 8.0 | 25 |
Institute for Advanced Studies in Basic Sciences (IASBS) | Assay Description Inhibitory activities of synthesized α-oxycarbanilinophosphonates were determined at 25°C by the colorimetric method of Ellman et al. [Ellman et... | J Enzyme Inhib Med Chem 28: 576-82 (2013) Article DOI: 10.3109/14756366.2012.663362 BindingDB Entry DOI: 10.7270/Q20R9N9H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||