

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cholinesterase (Equus caballus (Horse)) | BDBM50396122 (CHEMBL2171326) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of equine serum BuChE using butyrylthiocholine iodide as substrate after 5 mins by Ellman's method | J Med Chem 55: 4309-21 (2012) Article DOI: 10.1021/jm300106z BindingDB Entry DOI: 10.7270/Q21837N7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50396125 (CHEMBL2171323) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of equine serum BuChE using butyrylthiocholine iodide as substrate after 5 mins by Ellman's method | J Med Chem 55: 4309-21 (2012) Article DOI: 10.1021/jm300106z BindingDB Entry DOI: 10.7270/Q21837N7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50396123 (CHEMBL2171325) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of equine serum BuChE using butyrylthiocholine iodide as substrate after 5 mins by Ellman's method | J Med Chem 55: 4309-21 (2012) Article DOI: 10.1021/jm300106z BindingDB Entry DOI: 10.7270/Q21837N7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50396121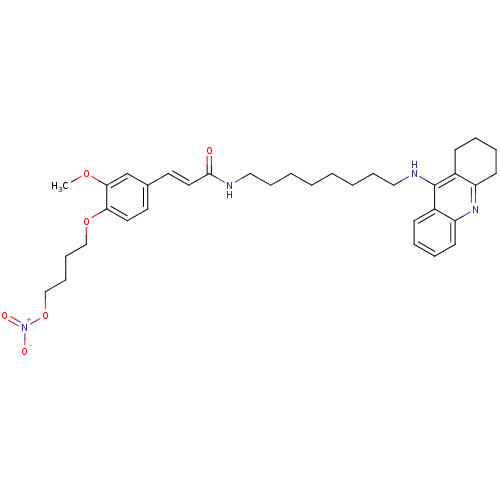 (CHEMBL2171327) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of equine serum BuChE using butyrylthiocholine iodide as substrate after 5 mins by Ellman's method | J Med Chem 55: 4309-21 (2012) Article DOI: 10.1021/jm300106z BindingDB Entry DOI: 10.7270/Q21837N7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50396120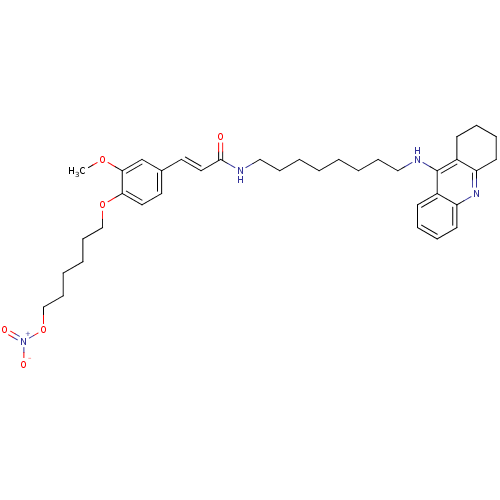 (CHEMBL2169901) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of equine serum BuChE using butyrylthiocholine iodide as substrate after 5 mins by Ellman's method | J Med Chem 55: 4309-21 (2012) Article DOI: 10.1021/jm300106z BindingDB Entry DOI: 10.7270/Q21837N7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50396124 (CHEMBL2171324) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of equine serum BuChE using butyrylthiocholine iodide as substrate after 5 mins by Ellman's method | J Med Chem 55: 4309-21 (2012) Article DOI: 10.1021/jm300106z BindingDB Entry DOI: 10.7270/Q21837N7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM9426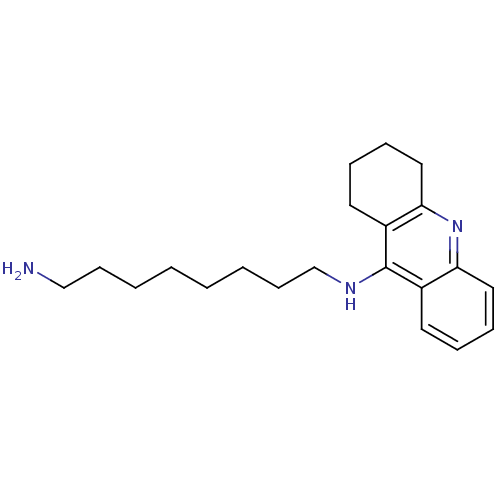 (CHEMBL258928 | Heterodimeric Tacrine-Based Inhibit...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | 2.80 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of equine serum BuChE using butyrylthiocholine iodide as substrate after 5 mins by Ellman's method | J Med Chem 55: 4309-21 (2012) Article DOI: 10.1021/jm300106z BindingDB Entry DOI: 10.7270/Q21837N7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM9426 (CHEMBL258928 | Heterodimeric Tacrine-Based Inhibit...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | 2.90 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of eel AChE using acetylthiocholine iodide as substrate after 5 mins by Ellman's method | J Med Chem 55: 4309-21 (2012) Article DOI: 10.1021/jm300106z BindingDB Entry DOI: 10.7270/Q21837N7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50396133 (CHEMBL2171315) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.60 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of eel AChE using acetylthiocholine iodide as substrate after 5 mins by Ellman's method | J Med Chem 55: 4309-21 (2012) Article DOI: 10.1021/jm300106z BindingDB Entry DOI: 10.7270/Q21837N7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50396125 (CHEMBL2171323) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.70 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of eel AChE using acetylthiocholine iodide as substrate after 5 mins by Ellman's method | J Med Chem 55: 4309-21 (2012) Article DOI: 10.1021/jm300106z BindingDB Entry DOI: 10.7270/Q21837N7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50396126 (CHEMBL2171322) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.40 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of eel AChE using acetylthiocholine iodide as substrate after 5 mins by Ellman's method | J Med Chem 55: 4309-21 (2012) Article DOI: 10.1021/jm300106z BindingDB Entry DOI: 10.7270/Q21837N7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50396129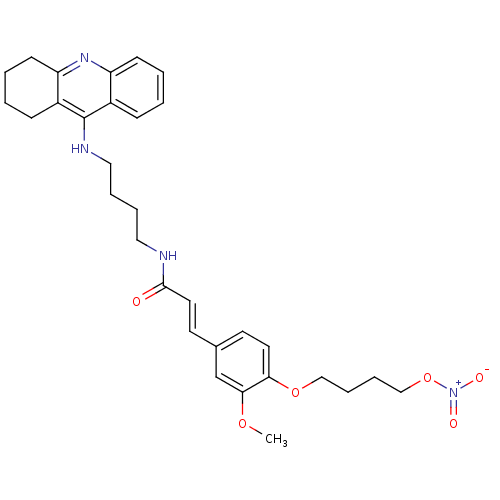 (CHEMBL2171319) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.70 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of equine serum BuChE using butyrylthiocholine iodide as substrate after 5 mins by Ellman's method | J Med Chem 55: 4309-21 (2012) Article DOI: 10.1021/jm300106z BindingDB Entry DOI: 10.7270/Q21837N7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50396124 (CHEMBL2171324) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.80 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of eel AChE using acetylthiocholine iodide as substrate after 5 mins by Ellman's method | J Med Chem 55: 4309-21 (2012) Article DOI: 10.1021/jm300106z BindingDB Entry DOI: 10.7270/Q21837N7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50396127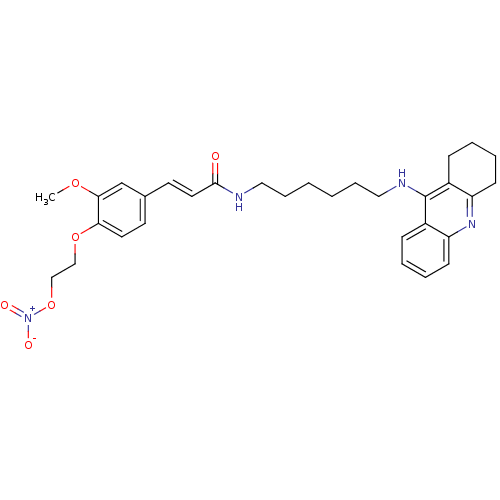 (CHEMBL2171321) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.20 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of equine serum BuChE using butyrylthiocholine iodide as substrate after 5 mins by Ellman's method | J Med Chem 55: 4309-21 (2012) Article DOI: 10.1021/jm300106z BindingDB Entry DOI: 10.7270/Q21837N7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50396123 (CHEMBL2171325) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.5 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of eel AChE using acetylthiocholine iodide as substrate after 5 mins by Ellman's method | J Med Chem 55: 4309-21 (2012) Article DOI: 10.1021/jm300106z BindingDB Entry DOI: 10.7270/Q21837N7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50396126 (CHEMBL2171322) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.60 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of equine serum BuChE using butyrylthiocholine iodide as substrate after 5 mins by Ellman's method | J Med Chem 55: 4309-21 (2012) Article DOI: 10.1021/jm300106z BindingDB Entry DOI: 10.7270/Q21837N7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50396133 (CHEMBL2171315) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6.20 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of equine serum BuChE using butyrylthiocholine iodide as substrate after 5 mins by Ellman's method | J Med Chem 55: 4309-21 (2012) Article DOI: 10.1021/jm300106z BindingDB Entry DOI: 10.7270/Q21837N7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50396135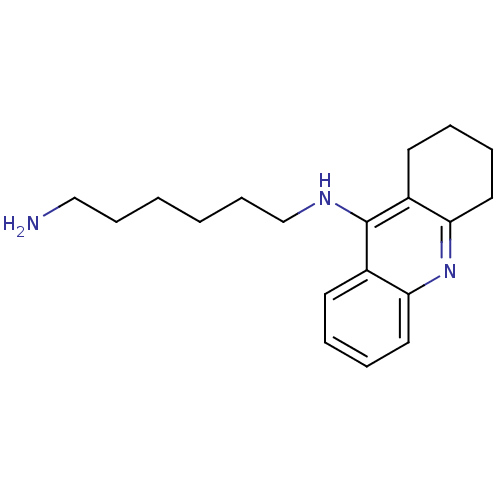 (CHEMBL2171328) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6.60 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of equine serum BuChE using butyrylthiocholine iodide as substrate after 5 mins by Ellman's method | J Med Chem 55: 4309-21 (2012) Article DOI: 10.1021/jm300106z BindingDB Entry DOI: 10.7270/Q21837N7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50396111 (CHEMBL2171311) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 7.60 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of equine serum BuChE using butyrylthiocholine iodide as substrate after 5 mins by Ellman's method | J Med Chem 55: 4309-21 (2012) Article DOI: 10.1021/jm300106z BindingDB Entry DOI: 10.7270/Q21837N7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50396127 (CHEMBL2171321) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 8.30 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of eel AChE using acetylthiocholine iodide as substrate after 5 mins by Ellman's method | J Med Chem 55: 4309-21 (2012) Article DOI: 10.1021/jm300106z BindingDB Entry DOI: 10.7270/Q21837N7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50396112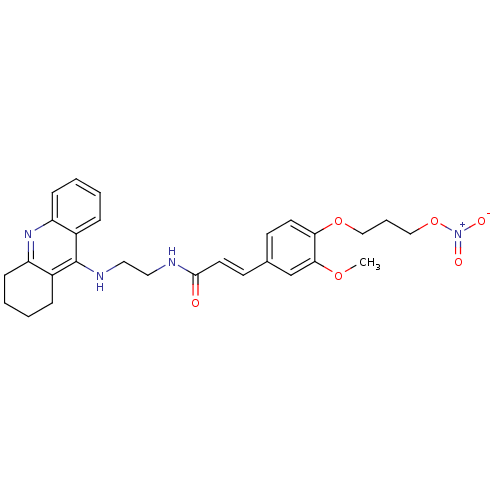 (CHEMBL2171334) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 8.40 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of equine serum BuChE using butyrylthiocholine iodide as substrate after 5 mins by Ellman's method | J Med Chem 55: 4309-21 (2012) Article DOI: 10.1021/jm300106z BindingDB Entry DOI: 10.7270/Q21837N7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50396121 (CHEMBL2171327) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 9.40 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of eel AChE using acetylthiocholine iodide as substrate after 5 mins by Ellman's method | J Med Chem 55: 4309-21 (2012) Article DOI: 10.1021/jm300106z BindingDB Entry DOI: 10.7270/Q21837N7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50377436 (CHEMBL255304) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 9.80 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of equine serum BuChE using butyrylthiocholine iodide as substrate after 5 mins by Ellman's method | J Med Chem 55: 4309-21 (2012) Article DOI: 10.1021/jm300106z BindingDB Entry DOI: 10.7270/Q21837N7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50396122 (CHEMBL2171326) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 10.1 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of eel AChE using acetylthiocholine iodide as substrate after 5 mins by Ellman's method | J Med Chem 55: 4309-21 (2012) Article DOI: 10.1021/jm300106z BindingDB Entry DOI: 10.7270/Q21837N7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50396113 (CHEMBL2171333) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 10.5 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of equine serum BuChE using butyrylthiocholine iodide as substrate after 5 mins by Ellman's method | J Med Chem 55: 4309-21 (2012) Article DOI: 10.1021/jm300106z BindingDB Entry DOI: 10.7270/Q21837N7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM8961 (1,2,3,4-tetrahydro-9-acridinamine | 1,2,3,4-tetrah...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 10.6 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of equine serum BuChE using butyrylthiocholine iodide as substrate after 5 mins by Ellman's method | J Med Chem 55: 4309-21 (2012) Article DOI: 10.1021/jm300106z BindingDB Entry DOI: 10.7270/Q21837N7 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50396120 (CHEMBL2169901) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 10.8 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of eel AChE using acetylthiocholine iodide as substrate after 5 mins by Ellman's method | J Med Chem 55: 4309-21 (2012) Article DOI: 10.1021/jm300106z BindingDB Entry DOI: 10.7270/Q21837N7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50396134 (CHEMBL2171314) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 10.9 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of eel AChE using acetylthiocholine iodide as substrate after 5 mins by Ellman's method | J Med Chem 55: 4309-21 (2012) Article DOI: 10.1021/jm300106z BindingDB Entry DOI: 10.7270/Q21837N7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50377436 (CHEMBL255304) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 11.1 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of eel AChE using acetylthiocholine iodide as substrate after 5 mins by Ellman's method | J Med Chem 55: 4309-21 (2012) Article DOI: 10.1021/jm300106z BindingDB Entry DOI: 10.7270/Q21837N7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50396128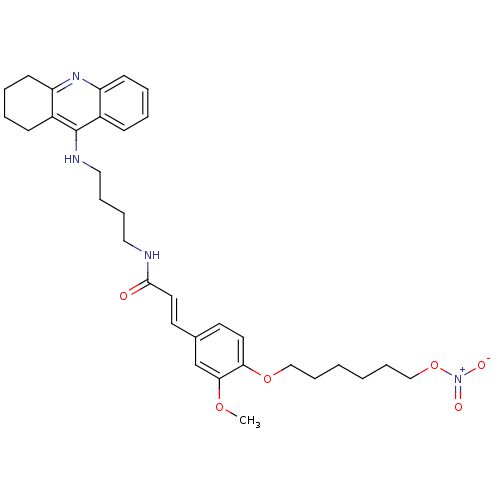 (CHEMBL2171320) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 11.7 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of equine serum BuChE using butyrylthiocholine iodide as substrate after 5 mins by Ellman's method | J Med Chem 55: 4309-21 (2012) Article DOI: 10.1021/jm300106z BindingDB Entry DOI: 10.7270/Q21837N7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50396118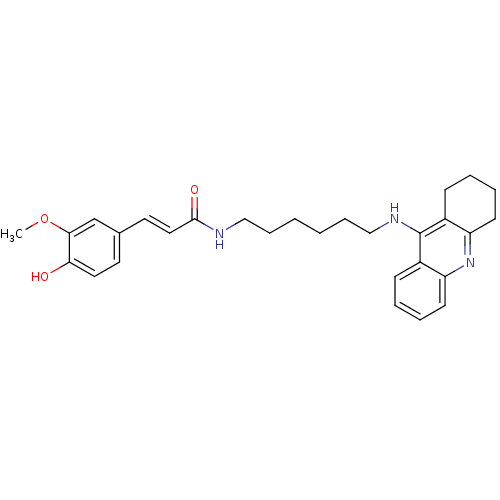 (CHEMBL2171309) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 12.7 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of eel AChE using acetylthiocholine iodide as substrate after 5 mins by Ellman's method | J Med Chem 55: 4309-21 (2012) Article DOI: 10.1021/jm300106z BindingDB Entry DOI: 10.7270/Q21837N7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50396109 (CHEMBL2171313) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 14.4 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of eel AChE using acetylthiocholine iodide as substrate after 5 mins by Ellman's method | J Med Chem 55: 4309-21 (2012) Article DOI: 10.1021/jm300106z BindingDB Entry DOI: 10.7270/Q21837N7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50377435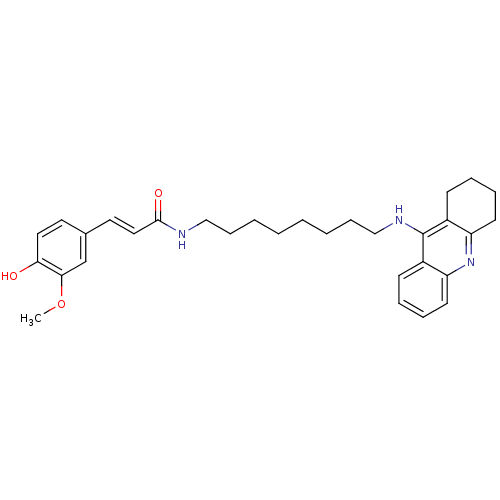 (CHEMBL256919) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 16.9 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of eel AChE using acetylthiocholine iodide as substrate after 5 mins by Ellman's method | J Med Chem 55: 4309-21 (2012) Article DOI: 10.1021/jm300106z BindingDB Entry DOI: 10.7270/Q21837N7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50396113 (CHEMBL2171333) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 17.2 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of eel AChE using acetylthiocholine iodide as substrate after 5 mins by Ellman's method | J Med Chem 55: 4309-21 (2012) Article DOI: 10.1021/jm300106z BindingDB Entry DOI: 10.7270/Q21837N7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50396134 (CHEMBL2171314) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 17.7 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of equine serum BuChE using butyrylthiocholine iodide as substrate after 5 mins by Ellman's method | J Med Chem 55: 4309-21 (2012) Article DOI: 10.1021/jm300106z BindingDB Entry DOI: 10.7270/Q21837N7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50396132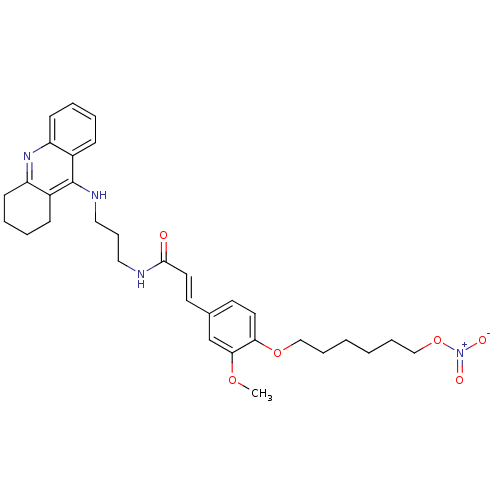 (CHEMBL2171316) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 19.3 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of equine serum BuChE using butyrylthiocholine iodide as substrate after 5 mins by Ellman's method | J Med Chem 55: 4309-21 (2012) Article DOI: 10.1021/jm300106z BindingDB Entry DOI: 10.7270/Q21837N7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50396129 (CHEMBL2171319) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 19.5 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of eel AChE using acetylthiocholine iodide as substrate after 5 mins by Ellman's method | J Med Chem 55: 4309-21 (2012) Article DOI: 10.1021/jm300106z BindingDB Entry DOI: 10.7270/Q21837N7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50377431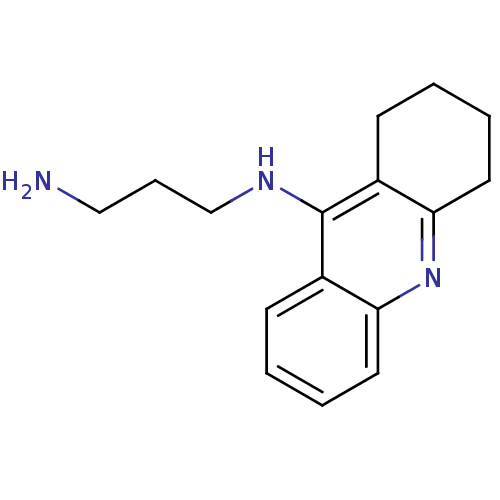 (CHEMBL258744) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 19.7 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of equine serum BuChE using butyrylthiocholine iodide as substrate after 5 mins by Ellman's method | J Med Chem 55: 4309-21 (2012) Article DOI: 10.1021/jm300106z BindingDB Entry DOI: 10.7270/Q21837N7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50396112 (CHEMBL2171334) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 19.9 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of eel AChE using acetylthiocholine iodide as substrate after 5 mins by Ellman's method | J Med Chem 55: 4309-21 (2012) Article DOI: 10.1021/jm300106z BindingDB Entry DOI: 10.7270/Q21837N7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50396131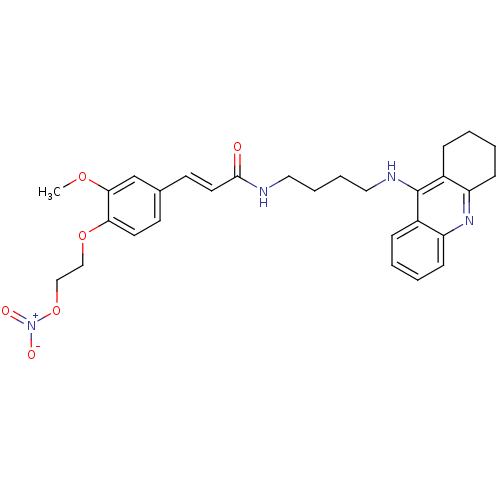 (CHEMBL2171317) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 20.2 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of equine serum BuChE using butyrylthiocholine iodide as substrate after 5 mins by Ellman's method | J Med Chem 55: 4309-21 (2012) Article DOI: 10.1021/jm300106z BindingDB Entry DOI: 10.7270/Q21837N7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50396110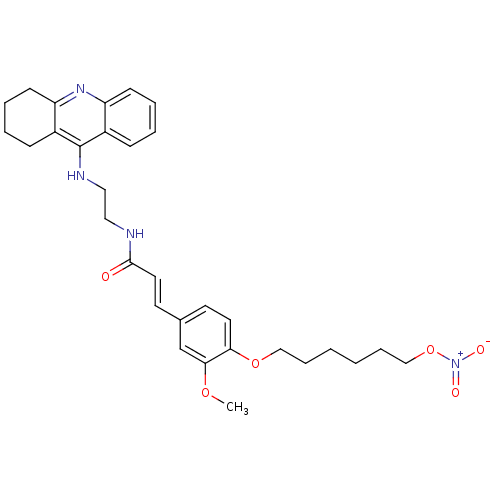 (CHEMBL2171312) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 21.8 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of equine serum BuChE using butyrylthiocholine iodide as substrate after 5 mins by Ellman's method | J Med Chem 55: 4309-21 (2012) Article DOI: 10.1021/jm300106z BindingDB Entry DOI: 10.7270/Q21837N7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50396132 (CHEMBL2171316) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 21.8 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of eel AChE using acetylthiocholine iodide as substrate after 5 mins by Ellman's method | J Med Chem 55: 4309-21 (2012) Article DOI: 10.1021/jm300106z BindingDB Entry DOI: 10.7270/Q21837N7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50396131 (CHEMBL2171317) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 22.1 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of eel AChE using acetylthiocholine iodide as substrate after 5 mins by Ellman's method | J Med Chem 55: 4309-21 (2012) Article DOI: 10.1021/jm300106z BindingDB Entry DOI: 10.7270/Q21837N7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50377433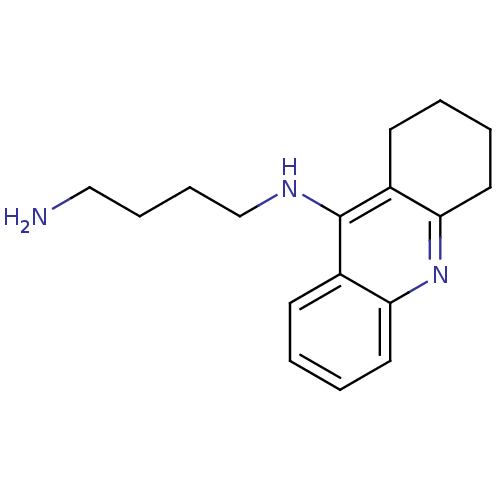 (CHEMBL258926) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 22.9 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of equine serum BuChE using butyrylthiocholine iodide as substrate after 5 mins by Ellman's method | J Med Chem 55: 4309-21 (2012) Article DOI: 10.1021/jm300106z BindingDB Entry DOI: 10.7270/Q21837N7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50396130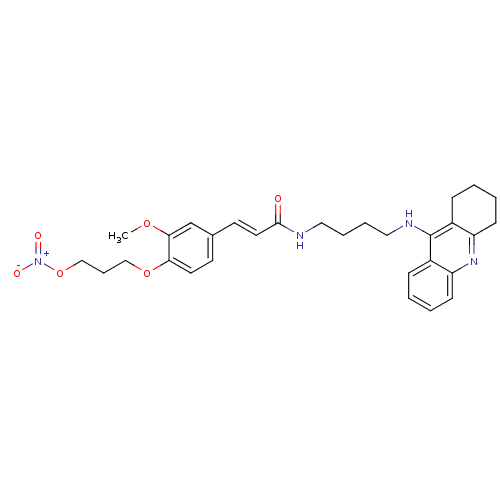 (CHEMBL2171318) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 23.6 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of equine serum BuChE using butyrylthiocholine iodide as substrate after 5 mins by Ellman's method | J Med Chem 55: 4309-21 (2012) Article DOI: 10.1021/jm300106z BindingDB Entry DOI: 10.7270/Q21837N7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50396135 (CHEMBL2171328) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 23.7 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of eel AChE using acetylthiocholine iodide as substrate after 5 mins by Ellman's method | J Med Chem 55: 4309-21 (2012) Article DOI: 10.1021/jm300106z BindingDB Entry DOI: 10.7270/Q21837N7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50396109 (CHEMBL2171313) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 24.9 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of equine serum BuChE using butyrylthiocholine iodide as substrate after 5 mins by Ellman's method | J Med Chem 55: 4309-21 (2012) Article DOI: 10.1021/jm300106z BindingDB Entry DOI: 10.7270/Q21837N7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50396118 (CHEMBL2171309) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 26.3 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of equine serum BuChE using butyrylthiocholine iodide as substrate after 5 mins by Ellman's method | J Med Chem 55: 4309-21 (2012) Article DOI: 10.1021/jm300106z BindingDB Entry DOI: 10.7270/Q21837N7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50396111 (CHEMBL2171311) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 27.1 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of eel AChE using acetylthiocholine iodide as substrate after 5 mins by Ellman's method | J Med Chem 55: 4309-21 (2012) Article DOI: 10.1021/jm300106z BindingDB Entry DOI: 10.7270/Q21837N7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50377435 (CHEMBL256919) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 27.4 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of equine serum BuChE using butyrylthiocholine iodide as substrate after 5 mins by Ellman's method | J Med Chem 55: 4309-21 (2012) Article DOI: 10.1021/jm300106z BindingDB Entry DOI: 10.7270/Q21837N7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50396128 (CHEMBL2171320) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 27.9 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of eel AChE using acetylthiocholine iodide as substrate after 5 mins by Ellman's method | J Med Chem 55: 4309-21 (2012) Article DOI: 10.1021/jm300106z BindingDB Entry DOI: 10.7270/Q21837N7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50377434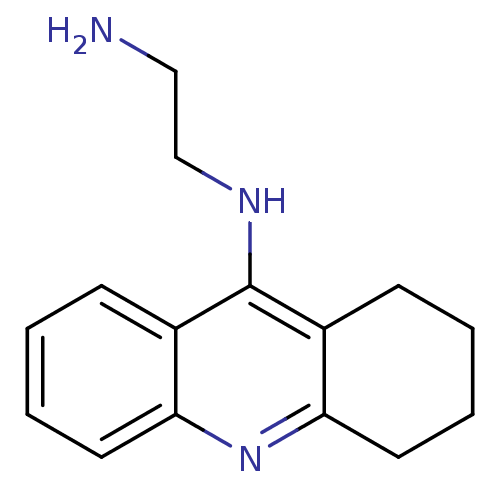 (CHEMBL407765) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 30.9 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of equine serum BuChE using butyrylthiocholine iodide as substrate after 5 mins by Ellman's method | J Med Chem 55: 4309-21 (2012) Article DOI: 10.1021/jm300106z BindingDB Entry DOI: 10.7270/Q21837N7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50377437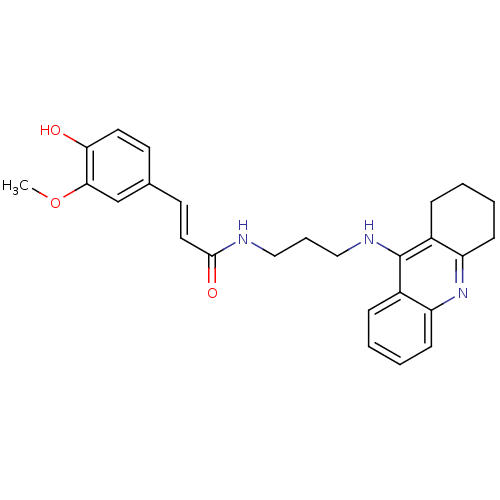 (CHEMBL255502) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 31.1 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of equine serum BuChE using butyrylthiocholine iodide as substrate after 5 mins by Ellman's method | J Med Chem 55: 4309-21 (2012) Article DOI: 10.1021/jm300106z BindingDB Entry DOI: 10.7270/Q21837N7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50377438 (CHEMBL403083) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 37.5 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of equine serum BuChE using butyrylthiocholine iodide as substrate after 5 mins by Ellman's method | J Med Chem 55: 4309-21 (2012) Article DOI: 10.1021/jm300106z BindingDB Entry DOI: 10.7270/Q21837N7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50377433 (CHEMBL258926) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 39.3 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of eel AChE using acetylthiocholine iodide as substrate after 5 mins by Ellman's method | J Med Chem 55: 4309-21 (2012) Article DOI: 10.1021/jm300106z BindingDB Entry DOI: 10.7270/Q21837N7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50396119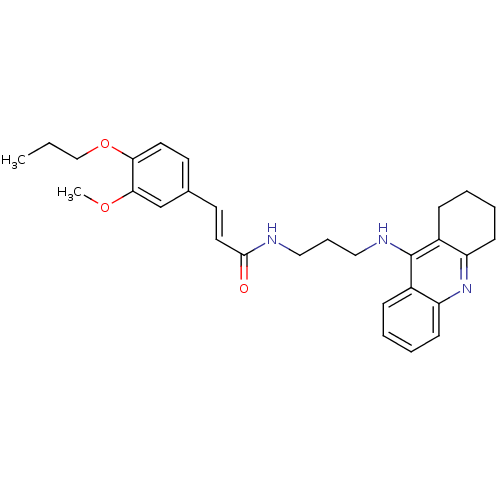 (CHEMBL2171308) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 40.3 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of equine serum BuChE using butyrylthiocholine iodide as substrate after 5 mins by Ellman's method | J Med Chem 55: 4309-21 (2012) Article DOI: 10.1021/jm300106z BindingDB Entry DOI: 10.7270/Q21837N7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50396110 (CHEMBL2171312) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 40.6 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of eel AChE using acetylthiocholine iodide as substrate after 5 mins by Ellman's method | J Med Chem 55: 4309-21 (2012) Article DOI: 10.1021/jm300106z BindingDB Entry DOI: 10.7270/Q21837N7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50396130 (CHEMBL2171318) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 44.3 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of eel AChE using acetylthiocholine iodide as substrate after 5 mins by Ellman's method | J Med Chem 55: 4309-21 (2012) Article DOI: 10.1021/jm300106z BindingDB Entry DOI: 10.7270/Q21837N7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50377437 (CHEMBL255502) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 56.7 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of eel AChE using acetylthiocholine iodide as substrate after 5 mins by Ellman's method | J Med Chem 55: 4309-21 (2012) Article DOI: 10.1021/jm300106z BindingDB Entry DOI: 10.7270/Q21837N7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50377438 (CHEMBL403083) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 62 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of eel AChE using acetylthiocholine iodide as substrate after 5 mins by Ellman's method | J Med Chem 55: 4309-21 (2012) Article DOI: 10.1021/jm300106z BindingDB Entry DOI: 10.7270/Q21837N7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM8961 (1,2,3,4-tetrahydro-9-acridinamine | 1,2,3,4-tetrah...) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 69.8 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of eel AChE using acetylthiocholine iodide as substrate after 5 mins by Ellman's method | J Med Chem 55: 4309-21 (2012) Article DOI: 10.1021/jm300106z BindingDB Entry DOI: 10.7270/Q21837N7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50396119 (CHEMBL2171308) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 69.8 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of eel AChE using acetylthiocholine iodide as substrate after 5 mins by Ellman's method | J Med Chem 55: 4309-21 (2012) Article DOI: 10.1021/jm300106z BindingDB Entry DOI: 10.7270/Q21837N7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50377434 (CHEMBL407765) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 71.4 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of eel AChE using acetylthiocholine iodide as substrate after 5 mins by Ellman's method | J Med Chem 55: 4309-21 (2012) Article DOI: 10.1021/jm300106z BindingDB Entry DOI: 10.7270/Q21837N7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50377431 (CHEMBL258744) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 79.5 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of eel AChE using acetylthiocholine iodide as substrate after 5 mins by Ellman's method | J Med Chem 55: 4309-21 (2012) Article DOI: 10.1021/jm300106z BindingDB Entry DOI: 10.7270/Q21837N7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50396114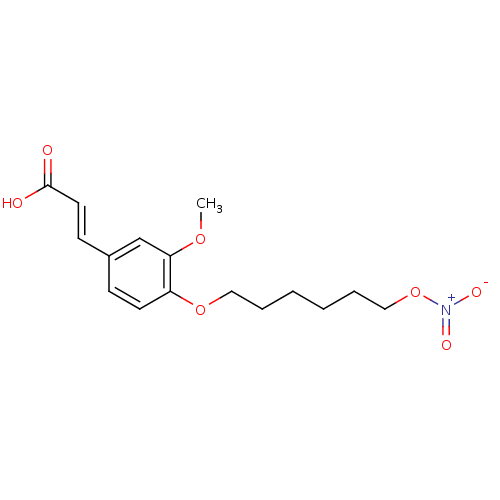 (CHEMBL2171332) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of equine serum BuChE using butyrylthiocholine iodide as substrate after 5 mins by Ellman's method | J Med Chem 55: 4309-21 (2012) Article DOI: 10.1021/jm300106z BindingDB Entry DOI: 10.7270/Q21837N7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50396116 (CHEMBL2171330) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of eel AChE using acetylthiocholine iodide as substrate after 5 mins by Ellman's method | J Med Chem 55: 4309-21 (2012) Article DOI: 10.1021/jm300106z BindingDB Entry DOI: 10.7270/Q21837N7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50396117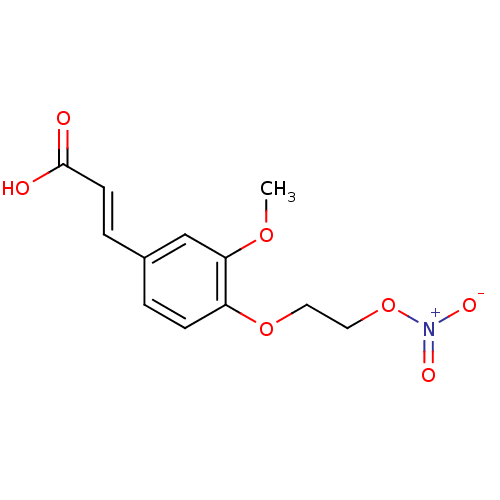 (CHEMBL2171329) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of equine serum BuChE using butyrylthiocholine iodide as substrate after 5 mins by Ellman's method | J Med Chem 55: 4309-21 (2012) Article DOI: 10.1021/jm300106z BindingDB Entry DOI: 10.7270/Q21837N7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50396117 (CHEMBL2171329) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of eel AChE using acetylthiocholine iodide as substrate after 5 mins by Ellman's method | J Med Chem 55: 4309-21 (2012) Article DOI: 10.1021/jm300106z BindingDB Entry DOI: 10.7270/Q21837N7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50396115 (CHEMBL2171331) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of eel AChE using acetylthiocholine iodide as substrate after 5 mins by Ellman's method | J Med Chem 55: 4309-21 (2012) Article DOI: 10.1021/jm300106z BindingDB Entry DOI: 10.7270/Q21837N7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50396115 (CHEMBL2171331) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of equine serum BuChE using butyrylthiocholine iodide as substrate after 5 mins by Ellman's method | J Med Chem 55: 4309-21 (2012) Article DOI: 10.1021/jm300106z BindingDB Entry DOI: 10.7270/Q21837N7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50396116 (CHEMBL2171330) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of equine serum BuChE using butyrylthiocholine iodide as substrate after 5 mins by Ellman's method | J Med Chem 55: 4309-21 (2012) Article DOI: 10.1021/jm300106z BindingDB Entry DOI: 10.7270/Q21837N7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50396114 (CHEMBL2171332) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of eel AChE using acetylthiocholine iodide as substrate after 5 mins by Ellman's method | J Med Chem 55: 4309-21 (2012) Article DOI: 10.1021/jm300106z BindingDB Entry DOI: 10.7270/Q21837N7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||