 Found 60 hits of Enzyme Inhibition Constant Data
Found 60 hits of Enzyme Inhibition Constant Data Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Nicotinamide phosphoribosyltransferase
(Homo sapiens (Human)) | BDBM50439306
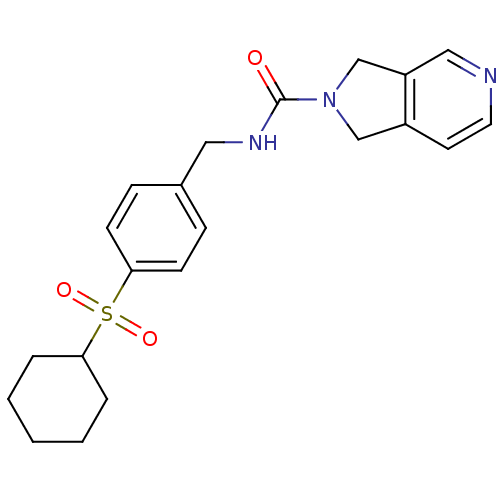
(CHEMBL2417572)Show SMILES O=C(NCc1ccc(cc1)S(=O)(=O)C1CCCCC1)N1Cc2ccncc2C1 Show InChI InChI=1S/C21H25N3O3S/c25-21(24-14-17-10-11-22-13-18(17)15-24)23-12-16-6-8-20(9-7-16)28(26,27)19-4-2-1-3-5-19/h6-11,13,19H,1-5,12,14-15H2,(H,23,25) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech Inc
Curated by ChEMBL
| Assay Description
Inhibition of human NAMPT using NAM/PRPP as substrate incubated for 15 mins prior to substrate addition measured after 30 mins by mass spectrometric ... |
Bioorg Med Chem Lett 23: 4875-85 (2013)
Article DOI: 10.1016/j.bmcl.2013.06.090
BindingDB Entry DOI: 10.7270/Q25T3MWP |
More data for this
Ligand-Target Pair | |
Nicotinamide phosphoribosyltransferase
(Homo sapiens (Human)) | BDBM50439308
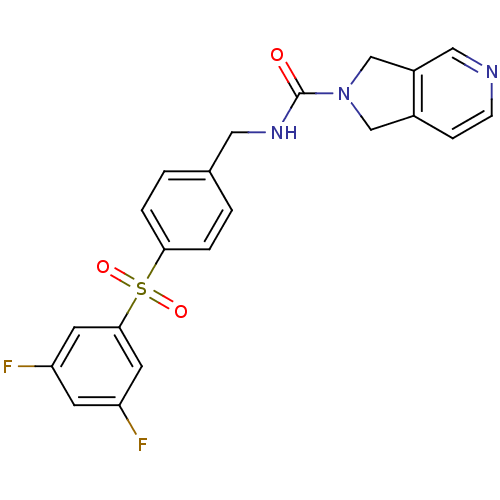
(CHEMBL2419514)Show SMILES Fc1cc(F)cc(c1)S(=O)(=O)c1ccc(CNC(=O)N2Cc3ccncc3C2)cc1 Show InChI InChI=1S/C21H17F2N3O3S/c22-17-7-18(23)9-20(8-17)30(28,29)19-3-1-14(2-4-19)10-25-21(27)26-12-15-5-6-24-11-16(15)13-26/h1-9,11H,10,12-13H2,(H,25,27) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 7.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech Inc
Curated by ChEMBL
| Assay Description
Inhibition of human NAMPT using NAM/PRPP as substrate incubated for 15 mins prior to substrate addition measured after 30 mins by mass spectrometric ... |
Bioorg Med Chem Lett 23: 4875-85 (2013)
Article DOI: 10.1016/j.bmcl.2013.06.090
BindingDB Entry DOI: 10.7270/Q25T3MWP |
More data for this
Ligand-Target Pair | |
Nicotinamide phosphoribosyltransferase
(Homo sapiens (Human)) | BDBM50439318

(CHEMBL2419536 | US10696692, Example 292)Show SMILES O=C(NCc1ccc(cc1)S(=O)(=O)N1CCC(CC1)N1CCCC1)N1Cc2ccncc2C1 Show InChI InChI=1S/C24H31N5O3S/c30-24(28-17-20-7-10-25-16-21(20)18-28)26-15-19-3-5-23(6-4-19)33(31,32)29-13-8-22(9-14-29)27-11-1-2-12-27/h3-7,10,16,22H,1-2,8-9,11-15,17-18H2,(H,26,30) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 7.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech Inc
Curated by ChEMBL
| Assay Description
Inhibition of human NAMPT using NAM/PRPP as substrate incubated for 15 mins prior to substrate addition measured after 30 mins by mass spectrometric ... |
Bioorg Med Chem Lett 23: 4875-85 (2013)
Article DOI: 10.1016/j.bmcl.2013.06.090
BindingDB Entry DOI: 10.7270/Q25T3MWP |
More data for this
Ligand-Target Pair | |
Nicotinamide phosphoribosyltransferase
(Homo sapiens (Human)) | BDBM50439310

(CHEMBL2419512)Show SMILES FC(F)(F)c1cccc(c1)S(=O)(=O)c1ccc(CNC(=O)N2Cc3ccncc3C2)cc1 Show InChI InChI=1S/C22H18F3N3O3S/c23-22(24,25)18-2-1-3-20(10-18)32(30,31)19-6-4-15(5-7-19)11-27-21(29)28-13-16-8-9-26-12-17(16)14-28/h1-10,12H,11,13-14H2,(H,27,29) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech Inc
Curated by ChEMBL
| Assay Description
Inhibition of human NAMPT using NAM/PRPP as substrate incubated for 15 mins prior to substrate addition measured after 30 mins by mass spectrometric ... |
Bioorg Med Chem Lett 23: 4875-85 (2013)
Article DOI: 10.1016/j.bmcl.2013.06.090
BindingDB Entry DOI: 10.7270/Q25T3MWP |
More data for this
Ligand-Target Pair | |
Nicotinamide phosphoribosyltransferase
(Homo sapiens (Human)) | BDBM50438976

(CHEMBL2419504)Show SMILES O=C(NCc1ccc(cc1)S(=O)(=O)c1ccccc1)c1cc2ccncc2o1 Show InChI InChI=1S/C21H16N2O4S/c24-21(19-12-16-10-11-22-14-20(16)27-19)23-13-15-6-8-18(9-7-15)28(25,26)17-4-2-1-3-5-17/h1-12,14H,13H2,(H,23,24) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech Inc
Curated by ChEMBL
| Assay Description
Inhibition of human NAMPT using NAM/PRPP as substrate incubated for 15 mins prior to substrate addition measured after 30 mins by mass spectrometric ... |
Bioorg Med Chem Lett 23: 4875-85 (2013)
Article DOI: 10.1016/j.bmcl.2013.06.090
BindingDB Entry DOI: 10.7270/Q25T3MWP |
More data for this
Ligand-Target Pair | |
Nicotinamide phosphoribosyltransferase
(Homo sapiens (Human)) | BDBM50439295

(CHEMBL2419525 | US10696692, Example 289)Show SMILES O=C(NCc1ccc(cc1)S(=O)(=O)C1CCN(CC1)C1CCOCC1)N1Cc2ccncc2C1 Show InChI InChI=1S/C25H32N4O4S/c30-25(29-17-20-5-10-26-16-21(20)18-29)27-15-19-1-3-23(4-2-19)34(31,32)24-6-11-28(12-7-24)22-8-13-33-14-9-22/h1-5,10,16,22,24H,6-9,11-15,17-18H2,(H,27,30) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech Inc
Curated by ChEMBL
| Assay Description
Inhibition of human NAMPT using NAM/PRPP as substrate incubated for 15 mins prior to substrate addition measured after 30 mins by mass spectrometric ... |
Bioorg Med Chem Lett 23: 4875-85 (2013)
Article DOI: 10.1016/j.bmcl.2013.06.090
BindingDB Entry DOI: 10.7270/Q25T3MWP |
More data for this
Ligand-Target Pair | |
Nicotinamide phosphoribosyltransferase
(Homo sapiens (Human)) | BDBM50439303

(CHEMBL2419515)Show SMILES Cc1ccc(cn1)S(=O)(=O)c1ccc(CNC(=O)N2Cc3ccncc3C2)cc1 Show InChI InChI=1S/C21H20N4O3S/c1-15-2-5-20(12-23-15)29(27,28)19-6-3-16(4-7-19)10-24-21(26)25-13-17-8-9-22-11-18(17)14-25/h2-9,11-12H,10,13-14H2,1H3,(H,24,26) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech Inc
Curated by ChEMBL
| Assay Description
Inhibition of human NAMPT using NAM/PRPP as substrate incubated for 15 mins prior to substrate addition measured after 30 mins by mass spectrometric ... |
Bioorg Med Chem Lett 23: 4875-85 (2013)
Article DOI: 10.1016/j.bmcl.2013.06.090
BindingDB Entry DOI: 10.7270/Q25T3MWP |
More data for this
Ligand-Target Pair | |
Nicotinamide phosphoribosyltransferase
(Homo sapiens (Human)) | BDBM50439314

(CHEMBL2419508)Show SMILES O=C(NCc1ccc(cc1)S(=O)(=O)c1ccccc1)N1Cc2ccccc2C1 Show InChI InChI=1S/C22H20N2O3S/c25-22(24-15-18-6-4-5-7-19(18)16-24)23-14-17-10-12-21(13-11-17)28(26,27)20-8-2-1-3-9-20/h1-13H,14-16H2,(H,23,25) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech Inc
Curated by ChEMBL
| Assay Description
Inhibition of human NAMPT using NAM/PRPP as substrate incubated for 15 mins prior to substrate addition measured after 30 mins by mass spectrometric ... |
Bioorg Med Chem Lett 23: 4875-85 (2013)
Article DOI: 10.1016/j.bmcl.2013.06.090
BindingDB Entry DOI: 10.7270/Q25T3MWP |
More data for this
Ligand-Target Pair | |
Nicotinamide phosphoribosyltransferase
(Homo sapiens (Human)) | BDBM50439304
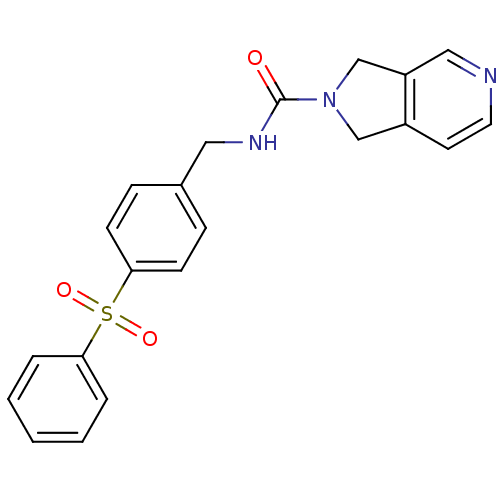
(CHEMBL2419505)Show SMILES O=C(NCc1ccc(cc1)S(=O)(=O)c1ccccc1)N1Cc2ccncc2C1 Show InChI InChI=1S/C21H19N3O3S/c25-21(24-14-17-10-11-22-13-18(17)15-24)23-12-16-6-8-20(9-7-16)28(26,27)19-4-2-1-3-5-19/h1-11,13H,12,14-15H2,(H,23,25) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech Inc
Curated by ChEMBL
| Assay Description
Inhibition of human NAMPT using NAM/PRPP as substrate incubated for 15 mins prior to substrate addition measured after 30 mins by mass spectrometric ... |
Bioorg Med Chem Lett 23: 4875-85 (2013)
Article DOI: 10.1016/j.bmcl.2013.06.090
BindingDB Entry DOI: 10.7270/Q25T3MWP |
More data for this
Ligand-Target Pair | |
Nicotinamide phosphoribosyltransferase
(Homo sapiens (Human)) | BDBM50439309

(CHEMBL2419513)Show SMILES FC(F)(F)c1cncc(c1)S(=O)(=O)c1ccc(CNC(=O)N2Cc3ccncc3C2)cc1 Show InChI InChI=1S/C21H17F3N4O3S/c22-21(23,24)17-7-19(11-26-10-17)32(30,31)18-3-1-14(2-4-18)8-27-20(29)28-12-15-5-6-25-9-16(15)13-28/h1-7,9-11H,8,12-13H2,(H,27,29) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech Inc
Curated by ChEMBL
| Assay Description
Inhibition of human NAMPT using NAM/PRPP as substrate incubated for 15 mins prior to substrate addition measured after 30 mins by mass spectrometric ... |
Bioorg Med Chem Lett 23: 4875-85 (2013)
Article DOI: 10.1016/j.bmcl.2013.06.090
BindingDB Entry DOI: 10.7270/Q25T3MWP |
More data for this
Ligand-Target Pair | |
Nicotinamide phosphoribosyltransferase
(Homo sapiens (Human)) | BDBM50439302

(CHEMBL2419516)Show SMILES Nc1ccc(cn1)S(=O)(=O)c1ccc(CNC(=O)N2Cc3ccncc3C2)cc1 Show InChI InChI=1S/C20H19N5O3S/c21-19-6-5-18(11-23-19)29(27,28)17-3-1-14(2-4-17)9-24-20(26)25-12-15-7-8-22-10-16(15)13-25/h1-8,10-11H,9,12-13H2,(H2,21,23)(H,24,26) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 17 | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech Inc
Curated by ChEMBL
| Assay Description
Inhibition of human NAMPT using NAM/PRPP as substrate incubated for 15 mins prior to substrate addition measured after 30 mins by mass spectrometric ... |
Bioorg Med Chem Lett 23: 4875-85 (2013)
Article DOI: 10.1016/j.bmcl.2013.06.090
BindingDB Entry DOI: 10.7270/Q25T3MWP |
More data for this
Ligand-Target Pair | |
Nicotinamide phosphoribosyltransferase
(Homo sapiens (Human)) | BDBM50439307
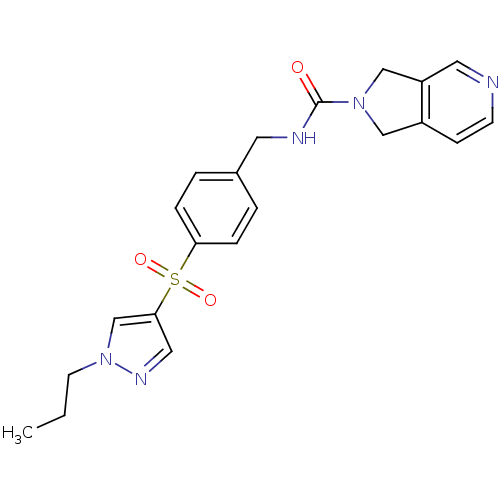
(CHEMBL2419517)Show SMILES CCCn1cc(cn1)S(=O)(=O)c1ccc(CNC(=O)N2Cc3ccncc3C2)cc1 Show InChI InChI=1S/C21H23N5O3S/c1-2-9-26-15-20(12-24-26)30(28,29)19-5-3-16(4-6-19)10-23-21(27)25-13-17-7-8-22-11-18(17)14-25/h3-8,11-12,15H,2,9-10,13-14H2,1H3,(H,23,27) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 17 | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech Inc
Curated by ChEMBL
| Assay Description
Inhibition of human NAMPT using NAM/PRPP as substrate incubated for 15 mins prior to substrate addition measured after 30 mins by mass spectrometric ... |
Bioorg Med Chem Lett 23: 4875-85 (2013)
Article DOI: 10.1016/j.bmcl.2013.06.090
BindingDB Entry DOI: 10.7270/Q25T3MWP |
More data for this
Ligand-Target Pair | |
Nicotinamide phosphoribosyltransferase
(Homo sapiens (Human)) | BDBM50439317

(CHEMBL2419502)Show SMILES O=C(NCc1ccc(cc1)S(=O)(=O)c1ccccc1)NCc1cccnc1 Show InChI InChI=1S/C20H19N3O3S/c24-20(23-15-17-5-4-12-21-13-17)22-14-16-8-10-19(11-9-16)27(25,26)18-6-2-1-3-7-18/h1-13H,14-15H2,(H2,22,23,24) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 19 | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech Inc
Curated by ChEMBL
| Assay Description
Inhibition of human NAMPT using NAM/PRPP as substrate incubated for 15 mins prior to substrate addition measured after 30 mins by mass spectrometric ... |
Bioorg Med Chem Lett 23: 4875-85 (2013)
Article DOI: 10.1016/j.bmcl.2013.06.090
BindingDB Entry DOI: 10.7270/Q25T3MWP |
More data for this
Ligand-Target Pair | |
Nicotinamide phosphoribosyltransferase
(Homo sapiens (Human)) | BDBM50439319

(CHEMBL2419535 | US10696692, Example 291)Show SMILES O=C(NCc1ccc(cc1)S(=O)(=O)N1CC2(C1)CCOCC2)N1Cc2ccncc2C1 Show InChI InChI=1S/C22H26N4O4S/c27-21(25-13-18-5-8-23-12-19(18)14-25)24-11-17-1-3-20(4-2-17)31(28,29)26-15-22(16-26)6-9-30-10-7-22/h1-5,8,12H,6-7,9-11,13-16H2,(H,24,27) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech Inc
Curated by ChEMBL
| Assay Description
Inhibition of human NAMPT using NAM/PRPP as substrate incubated for 15 mins prior to substrate addition measured after 30 mins by mass spectrometric ... |
Bioorg Med Chem Lett 23: 4875-85 (2013)
Article DOI: 10.1016/j.bmcl.2013.06.090
BindingDB Entry DOI: 10.7270/Q25T3MWP |
More data for this
Ligand-Target Pair | |
Nicotinamide phosphoribosyltransferase
(Homo sapiens (Human)) | BDBM50438986
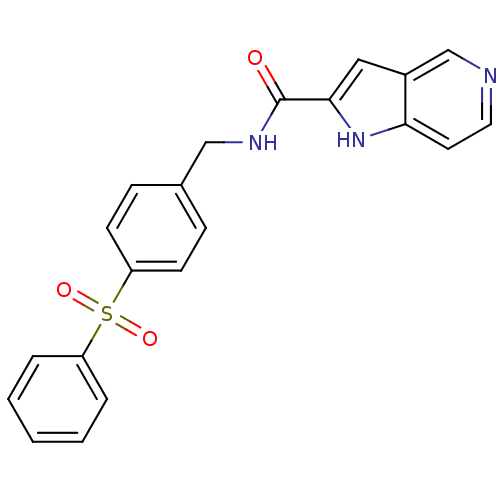
(CHEMBL2419503)Show SMILES O=C(NCc1ccc(cc1)S(=O)(=O)c1ccccc1)c1cc2cnccc2[nH]1 Show InChI InChI=1S/C21H17N3O3S/c25-21(20-12-16-14-22-11-10-19(16)24-20)23-13-15-6-8-18(9-7-15)28(26,27)17-4-2-1-3-5-17/h1-12,14,24H,13H2,(H,23,25) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech Inc
Curated by ChEMBL
| Assay Description
Inhibition of human NAMPT using NAM/PRPP as substrate incubated for 15 mins prior to substrate addition measured after 30 mins by mass spectrometric ... |
Bioorg Med Chem Lett 23: 4875-85 (2013)
Article DOI: 10.1016/j.bmcl.2013.06.090
BindingDB Entry DOI: 10.7270/Q25T3MWP |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Nicotinamide phosphoribosyltransferase
(Homo sapiens (Human)) | BDBM50439301
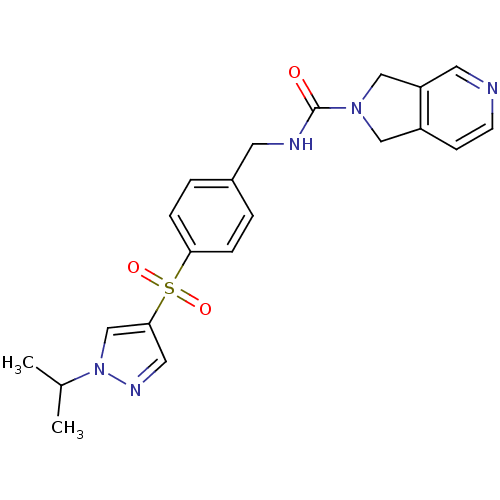
(CHEMBL2419518)Show SMILES CC(C)n1cc(cn1)S(=O)(=O)c1ccc(CNC(=O)N2Cc3ccncc3C2)cc1 Show InChI InChI=1S/C21H23N5O3S/c1-15(2)26-14-20(11-24-26)30(28,29)19-5-3-16(4-6-19)9-23-21(27)25-12-17-7-8-22-10-18(17)13-25/h3-8,10-11,14-15H,9,12-13H2,1-2H3,(H,23,27) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech Inc
Curated by ChEMBL
| Assay Description
Inhibition of human NAMPT using NAM/PRPP as substrate incubated for 15 mins prior to substrate addition measured after 30 mins by mass spectrometric ... |
Bioorg Med Chem Lett 23: 4875-85 (2013)
Article DOI: 10.1016/j.bmcl.2013.06.090
BindingDB Entry DOI: 10.7270/Q25T3MWP |
More data for this
Ligand-Target Pair | |
Nicotinamide phosphoribosyltransferase
(Homo sapiens (Human)) | BDBM50439320

(CHEMBL2419534 | US10696692, Example 278)Show SMILES O=C(NCc1ccc(cc1)S(=O)(=O)N1CCN(CC1)C1CCOCC1)N1Cc2ccncc2C1 Show InChI InChI=1S/C24H31N5O4S/c30-24(28-17-20-5-8-25-16-21(20)18-28)26-15-19-1-3-23(4-2-19)34(31,32)29-11-9-27(10-12-29)22-6-13-33-14-7-22/h1-5,8,16,22H,6-7,9-15,17-18H2,(H,26,30) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 22 | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech Inc
Curated by ChEMBL
| Assay Description
Inhibition of human NAMPT using NAM/PRPP as substrate incubated for 15 mins prior to substrate addition measured after 30 mins by mass spectrometric ... |
Bioorg Med Chem Lett 23: 4875-85 (2013)
Article DOI: 10.1016/j.bmcl.2013.06.090
BindingDB Entry DOI: 10.7270/Q25T3MWP |
More data for this
Ligand-Target Pair | |
Nicotinamide phosphoribosyltransferase
(Homo sapiens (Human)) | BDBM50439300

(CHEMBL2419520 | US10696692, Example 294)Show SMILES O=C(NCc1ccc(cc1)S(=O)(=O)C1CCOCC1)N1Cc2ccncc2C1 Show InChI InChI=1S/C20H23N3O4S/c24-20(23-13-16-5-8-21-12-17(16)14-23)22-11-15-1-3-18(4-2-15)28(25,26)19-6-9-27-10-7-19/h1-5,8,12,19H,6-7,9-11,13-14H2,(H,22,24) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 26 | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech Inc
Curated by ChEMBL
| Assay Description
Inhibition of human NAMPT using NAM/PRPP as substrate incubated for 15 mins prior to substrate addition measured after 30 mins by mass spectrometric ... |
Bioorg Med Chem Lett 23: 4875-85 (2013)
Article DOI: 10.1016/j.bmcl.2013.06.090
BindingDB Entry DOI: 10.7270/Q25T3MWP |
More data for this
Ligand-Target Pair | |
Nicotinamide phosphoribosyltransferase
(Homo sapiens (Human)) | BDBM50439297

(CHEMBL2419523 | US10696692, Example 311)Show SMILES CCC(=O)N1CCC(CC1)S(=O)(=O)c1ccc(CNC(=O)N2Cc3ccncc3C2)cc1 Show InChI InChI=1S/C23H28N4O4S/c1-2-22(28)26-11-8-21(9-12-26)32(30,31)20-5-3-17(4-6-20)13-25-23(29)27-15-18-7-10-24-14-19(18)16-27/h3-7,10,14,21H,2,8-9,11-13,15-16H2,1H3,(H,25,29) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 27 | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech Inc
Curated by ChEMBL
| Assay Description
Inhibition of human NAMPT using NAM/PRPP as substrate incubated for 15 mins prior to substrate addition measured after 30 mins by mass spectrometric ... |
Bioorg Med Chem Lett 23: 4875-85 (2013)
Article DOI: 10.1016/j.bmcl.2013.06.090
BindingDB Entry DOI: 10.7270/Q25T3MWP |
More data for this
Ligand-Target Pair | |
Nicotinamide phosphoribosyltransferase
(Homo sapiens (Human)) | BDBM50439290
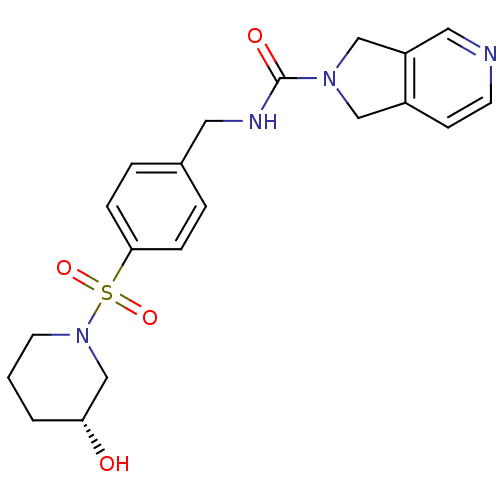
(CHEMBL2419530 | US10696692, Example 306)Show SMILES O[C@@H]1CCCN(C1)S(=O)(=O)c1ccc(CNC(=O)N2Cc3ccncc3C2)cc1 |r| Show InChI InChI=1S/C20H24N4O4S/c25-18-2-1-9-24(14-18)29(27,28)19-5-3-15(4-6-19)10-22-20(26)23-12-16-7-8-21-11-17(16)13-23/h3-8,11,18,25H,1-2,9-10,12-14H2,(H,22,26)/t18-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 28 | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech Inc
Curated by ChEMBL
| Assay Description
Inhibition of human NAMPT using NAM/PRPP as substrate incubated for 15 mins prior to substrate addition measured after 30 mins by mass spectrometric ... |
Bioorg Med Chem Lett 23: 4875-85 (2013)
Article DOI: 10.1016/j.bmcl.2013.06.090
BindingDB Entry DOI: 10.7270/Q25T3MWP |
More data for this
Ligand-Target Pair | |
Nicotinamide phosphoribosyltransferase
(Homo sapiens (Human)) | BDBM50439296
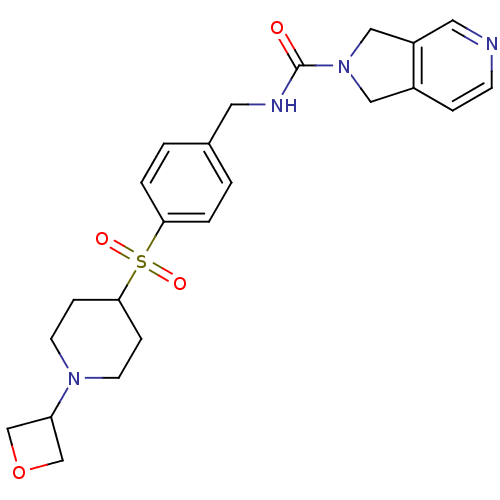
(CHEMBL2419524 | US10696692, Example 287)Show SMILES O=C(NCc1ccc(cc1)S(=O)(=O)C1CCN(CC1)C1COC1)N1Cc2ccncc2C1 Show InChI InChI=1S/C23H28N4O4S/c28-23(27-13-18-5-8-24-12-19(18)14-27)25-11-17-1-3-21(4-2-17)32(29,30)22-6-9-26(10-7-22)20-15-31-16-20/h1-5,8,12,20,22H,6-7,9-11,13-16H2,(H,25,28) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 31 | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech Inc
Curated by ChEMBL
| Assay Description
Inhibition of human NAMPT using NAM/PRPP as substrate incubated for 15 mins prior to substrate addition measured after 30 mins by mass spectrometric ... |
Bioorg Med Chem Lett 23: 4875-85 (2013)
Article DOI: 10.1016/j.bmcl.2013.06.090
BindingDB Entry DOI: 10.7270/Q25T3MWP |
More data for this
Ligand-Target Pair | |
Nicotinamide phosphoribosyltransferase
(Homo sapiens (Human)) | BDBM50439322

(CHEMBL2419532 | US10696692, Example 288)Show SMILES CN1CCN(CC1)S(=O)(=O)c1ccc(CNC(=O)N2Cc3ccncc3C2)cc1 Show InChI InChI=1S/C20H25N5O3S/c1-23-8-10-25(11-9-23)29(27,28)19-4-2-16(3-5-19)12-22-20(26)24-14-17-6-7-21-13-18(17)15-24/h2-7,13H,8-12,14-15H2,1H3,(H,22,26) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 38 | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech Inc
Curated by ChEMBL
| Assay Description
Inhibition of human NAMPT using NAM/PRPP as substrate incubated for 15 mins prior to substrate addition measured after 30 mins by mass spectrometric ... |
Bioorg Med Chem Lett 23: 4875-85 (2013)
Article DOI: 10.1016/j.bmcl.2013.06.090
BindingDB Entry DOI: 10.7270/Q25T3MWP |
More data for this
Ligand-Target Pair | |
Nicotinamide phosphoribosyltransferase
(Homo sapiens (Human)) | BDBM50439299

(CHEMBL2419521 | US10696692, Example 268)Show SMILES O=C(NCc1ccc(cc1)S(=O)(=O)C1CCCOC1)N1Cc2ccncc2C1 Show InChI InChI=1S/C20H23N3O4S/c24-20(23-12-16-7-8-21-11-17(16)13-23)22-10-15-3-5-18(6-4-15)28(25,26)19-2-1-9-27-14-19/h3-8,11,19H,1-2,9-10,12-14H2,(H,22,24) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 44 | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech Inc
Curated by ChEMBL
| Assay Description
Inhibition of human NAMPT using NAM/PRPP as substrate incubated for 15 mins prior to substrate addition measured after 30 mins by mass spectrometric ... |
Bioorg Med Chem Lett 23: 4875-85 (2013)
Article DOI: 10.1016/j.bmcl.2013.06.090
BindingDB Entry DOI: 10.7270/Q25T3MWP |
More data for this
Ligand-Target Pair | |
Nicotinamide phosphoribosyltransferase
(Homo sapiens (Human)) | BDBM50439291

(CHEMBL2419529 | US10696692, Example 318)Show SMILES OC1CCN(CC1)S(=O)(=O)c1ccc(CNC(=O)N2Cc3ccncc3C2)cc1 Show InChI InChI=1S/C20H24N4O4S/c25-18-6-9-24(10-7-18)29(27,28)19-3-1-15(2-4-19)11-22-20(26)23-13-16-5-8-21-12-17(16)14-23/h1-5,8,12,18,25H,6-7,9-11,13-14H2,(H,22,26) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 44 | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech Inc
Curated by ChEMBL
| Assay Description
Inhibition of human NAMPT using NAM/PRPP as substrate incubated for 15 mins prior to substrate addition measured after 30 mins by mass spectrometric ... |
Bioorg Med Chem Lett 23: 4875-85 (2013)
Article DOI: 10.1016/j.bmcl.2013.06.090
BindingDB Entry DOI: 10.7270/Q25T3MWP |
More data for this
Ligand-Target Pair | |
Nicotinamide phosphoribosyltransferase
(Homo sapiens (Human)) | BDBM50439294

(CHEMBL2419526 | US10696692, Example 309)Show SMILES CN1CCC(CC1)S(=O)(=O)c1ccc(CNC(=O)N2Cc3ccncc3C2)cc1 Show InChI InChI=1S/C21H26N4O3S/c1-24-10-7-20(8-11-24)29(27,28)19-4-2-16(3-5-19)12-23-21(26)25-14-17-6-9-22-13-18(17)15-25/h2-6,9,13,20H,7-8,10-12,14-15H2,1H3,(H,23,26) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 48 | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech Inc
Curated by ChEMBL
| Assay Description
Inhibition of human NAMPT using NAM/PRPP as substrate incubated for 15 mins prior to substrate addition measured after 30 mins by mass spectrometric ... |
Bioorg Med Chem Lett 23: 4875-85 (2013)
Article DOI: 10.1016/j.bmcl.2013.06.090
BindingDB Entry DOI: 10.7270/Q25T3MWP |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C9
(Homo sapiens (Human)) | BDBM50439304

(CHEMBL2419505)Show SMILES O=C(NCc1ccc(cc1)S(=O)(=O)c1ccccc1)N1Cc2ccncc2C1 Show InChI InChI=1S/C21H19N3O3S/c25-21(24-14-17-10-11-22-13-18(17)15-24)23-12-16-6-8-20(9-7-16)28(26,27)19-4-2-1-3-5-19/h1-11,13H,12,14-15H2,(H,23,25) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 54 | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech Inc
Curated by ChEMBL
| Assay Description
Inhibition of CYP2C9 in human liver microsomes using (S)- warfarin as substrate by LC-MS/MS analysis |
Bioorg Med Chem Lett 23: 4875-85 (2013)
Article DOI: 10.1016/j.bmcl.2013.06.090
BindingDB Entry DOI: 10.7270/Q25T3MWP |
More data for this
Ligand-Target Pair | |
Nicotinamide phosphoribosyltransferase
(Homo sapiens (Human)) | BDBM50439293

(CHEMBL2419527 | US10696692, Example 316)Show SMILES CC(C)N1CCC(CC1)S(=O)(=O)c1ccc(CNC(=O)N2Cc3ccncc3C2)cc1 Show InChI InChI=1S/C23H30N4O3S/c1-17(2)26-11-8-22(9-12-26)31(29,30)21-5-3-18(4-6-21)13-25-23(28)27-15-19-7-10-24-14-20(19)16-27/h3-7,10,14,17,22H,8-9,11-13,15-16H2,1-2H3,(H,25,28) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 58 | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech Inc
Curated by ChEMBL
| Assay Description
Inhibition of human NAMPT using NAM/PRPP as substrate incubated for 15 mins prior to substrate addition measured after 30 mins by mass spectrometric ... |
Bioorg Med Chem Lett 23: 4875-85 (2013)
Article DOI: 10.1016/j.bmcl.2013.06.090
BindingDB Entry DOI: 10.7270/Q25T3MWP |
More data for this
Ligand-Target Pair | |
Nicotinamide phosphoribosyltransferase
(Homo sapiens (Human)) | BDBM50439321

(CHEMBL2419533 | US10696692, Example 280)Show SMILES O=C(NCc1ccc(cc1)S(=O)(=O)N1CCN(CC1)C1COC1)N1Cc2ccncc2C1 Show InChI InChI=1S/C22H27N5O4S/c28-22(26-13-18-5-6-23-12-19(18)14-26)24-11-17-1-3-21(4-2-17)32(29,30)27-9-7-25(8-10-27)20-15-31-16-20/h1-6,12,20H,7-11,13-16H2,(H,24,28) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 59 | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech Inc
Curated by ChEMBL
| Assay Description
Inhibition of human NAMPT using NAM/PRPP as substrate incubated for 15 mins prior to substrate addition measured after 30 mins by mass spectrometric ... |
Bioorg Med Chem Lett 23: 4875-85 (2013)
Article DOI: 10.1016/j.bmcl.2013.06.090
BindingDB Entry DOI: 10.7270/Q25T3MWP |
More data for this
Ligand-Target Pair | |
Nicotinamide phosphoribosyltransferase
(Homo sapiens (Human)) | BDBM50439315

(CHEMBL2419507)Show SMILES O=C(NCc1ccc(cc1)S(=O)(=O)c1ccccc1)N1Cc2cccnc2C1 Show InChI InChI=1S/C21H19N3O3S/c25-21(24-14-17-5-4-12-22-20(17)15-24)23-13-16-8-10-19(11-9-16)28(26,27)18-6-2-1-3-7-18/h1-12H,13-15H2,(H,23,25) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 60 | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech Inc
Curated by ChEMBL
| Assay Description
Inhibition of human NAMPT using NAM/PRPP as substrate incubated for 15 mins prior to substrate addition measured after 30 mins by mass spectrometric ... |
Bioorg Med Chem Lett 23: 4875-85 (2013)
Article DOI: 10.1016/j.bmcl.2013.06.090
BindingDB Entry DOI: 10.7270/Q25T3MWP |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C9
(Homo sapiens (Human)) | BDBM50439302

(CHEMBL2419516)Show SMILES Nc1ccc(cn1)S(=O)(=O)c1ccc(CNC(=O)N2Cc3ccncc3C2)cc1 Show InChI InChI=1S/C20H19N5O3S/c21-19-6-5-18(11-23-19)29(27,28)17-3-1-14(2-4-17)9-24-20(26)25-12-15-7-8-22-10-16(15)13-25/h1-8,10-11H,9,12-13H2,(H2,21,23)(H,24,26) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 65 | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech Inc
Curated by ChEMBL
| Assay Description
Inhibition of CYP2C9 in human liver microsomes using (S)- warfarin as substrate by LC-MS/MS analysis |
Bioorg Med Chem Lett 23: 4875-85 (2013)
Article DOI: 10.1016/j.bmcl.2013.06.090
BindingDB Entry DOI: 10.7270/Q25T3MWP |
More data for this
Ligand-Target Pair | |
Nicotinamide phosphoribosyltransferase
(Homo sapiens (Human)) | BDBM50439292
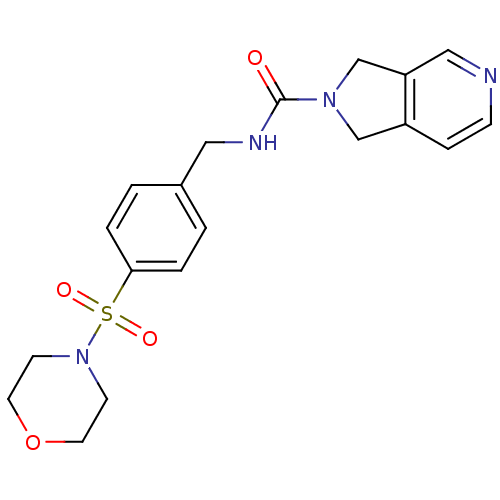
(CHEMBL2419528)Show SMILES O=C(NCc1ccc(cc1)S(=O)(=O)N1CCOCC1)N1Cc2ccncc2C1 Show InChI InChI=1S/C19H22N4O4S/c24-19(22-13-16-5-6-20-12-17(16)14-22)21-11-15-1-3-18(4-2-15)28(25,26)23-7-9-27-10-8-23/h1-6,12H,7-11,13-14H2,(H,21,24) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 71 | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech Inc
Curated by ChEMBL
| Assay Description
Inhibition of human NAMPT using NAM/PRPP as substrate incubated for 15 mins prior to substrate addition measured after 30 mins by mass spectrometric ... |
Bioorg Med Chem Lett 23: 4875-85 (2013)
Article DOI: 10.1016/j.bmcl.2013.06.090
BindingDB Entry DOI: 10.7270/Q25T3MWP |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C9
(Homo sapiens (Human)) | BDBM50439301

(CHEMBL2419518)Show SMILES CC(C)n1cc(cn1)S(=O)(=O)c1ccc(CNC(=O)N2Cc3ccncc3C2)cc1 Show InChI InChI=1S/C21H23N5O3S/c1-15(2)26-14-20(11-24-26)30(28,29)19-5-3-16(4-6-19)9-23-21(27)25-12-17-7-8-22-10-18(17)13-25/h3-8,10-11,14-15H,9,12-13H2,1-2H3,(H,23,27) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 73 | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech Inc
Curated by ChEMBL
| Assay Description
Inhibition of CYP2C9 in human liver microsomes using (S)- warfarin as substrate by LC-MS/MS analysis |
Bioorg Med Chem Lett 23: 4875-85 (2013)
Article DOI: 10.1016/j.bmcl.2013.06.090
BindingDB Entry DOI: 10.7270/Q25T3MWP |
More data for this
Ligand-Target Pair | |
Nicotinamide phosphoribosyltransferase
(Homo sapiens (Human)) | BDBM50439316
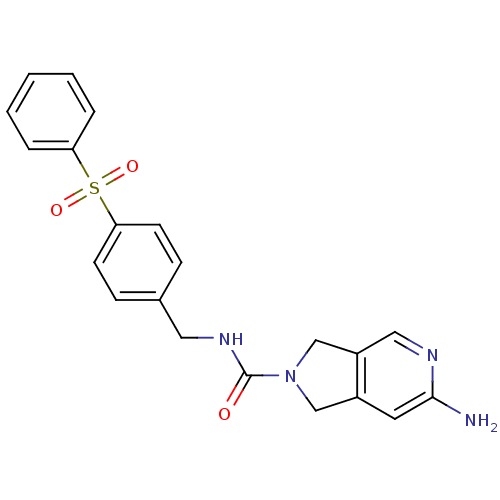
(CHEMBL2419506)Show SMILES Nc1cc2CN(Cc2cn1)C(=O)NCc1ccc(cc1)S(=O)(=O)c1ccccc1 Show InChI InChI=1S/C21H20N4O3S/c22-20-10-16-13-25(14-17(16)12-23-20)21(26)24-11-15-6-8-19(9-7-15)29(27,28)18-4-2-1-3-5-18/h1-10,12H,11,13-14H2,(H2,22,23)(H,24,26) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 75 | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech Inc
Curated by ChEMBL
| Assay Description
Inhibition of human NAMPT using NAM/PRPP as substrate incubated for 15 mins prior to substrate addition measured after 30 mins by mass spectrometric ... |
Bioorg Med Chem Lett 23: 4875-85 (2013)
Article DOI: 10.1016/j.bmcl.2013.06.090
BindingDB Entry DOI: 10.7270/Q25T3MWP |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C9
(Homo sapiens (Human)) | BDBM50439303

(CHEMBL2419515)Show SMILES Cc1ccc(cn1)S(=O)(=O)c1ccc(CNC(=O)N2Cc3ccncc3C2)cc1 Show InChI InChI=1S/C21H20N4O3S/c1-15-2-5-20(12-23-15)29(27,28)19-6-3-16(4-7-19)10-24-21(26)25-13-17-8-9-22-11-18(17)14-25/h2-9,11-12H,10,13-14H2,1H3,(H,24,26) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 77 | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech Inc
Curated by ChEMBL
| Assay Description
Inhibition of CYP2C9 in human liver microsomes using (S)- warfarin as substrate by LC-MS/MS analysis |
Bioorg Med Chem Lett 23: 4875-85 (2013)
Article DOI: 10.1016/j.bmcl.2013.06.090
BindingDB Entry DOI: 10.7270/Q25T3MWP |
More data for this
Ligand-Target Pair | |
Nicotinamide phosphoribosyltransferase
(Homo sapiens (Human)) | BDBM50439305

(CHEMBL2419519)Show SMILES FC1(F)CCC(CC1)S(=O)(=O)c1ccc(CNC(=O)N2Cc3ccncc3C2)cc1 Show InChI InChI=1S/C21H23F2N3O3S/c22-21(23)8-5-19(6-9-21)30(28,29)18-3-1-15(2-4-18)11-25-20(27)26-13-16-7-10-24-12-17(16)14-26/h1-4,7,10,12,19H,5-6,8-9,11,13-14H2,(H,25,27) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 78 | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech Inc
Curated by ChEMBL
| Assay Description
Inhibition of human NAMPT using NAM/PRPP as substrate incubated for 15 mins prior to substrate addition measured after 30 mins by mass spectrometric ... |
Bioorg Med Chem Lett 23: 4875-85 (2013)
Article DOI: 10.1016/j.bmcl.2013.06.090
BindingDB Entry DOI: 10.7270/Q25T3MWP |
More data for this
Ligand-Target Pair | |
Nicotinamide phosphoribosyltransferase
(Homo sapiens (Human)) | BDBM50439298

(CHEMBL2419522 | US10696692, Example 265)Show SMILES CC(=O)N1CCC(CC1)S(=O)(=O)c1ccc(CNC(=O)N2Cc3ccncc3C2)cc1 Show InChI InChI=1S/C22H26N4O4S/c1-16(27)25-10-7-21(8-11-25)31(29,30)20-4-2-17(3-5-20)12-24-22(28)26-14-18-6-9-23-13-19(18)15-26/h2-6,9,13,21H,7-8,10-12,14-15H2,1H3,(H,24,28) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 83 | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech Inc
Curated by ChEMBL
| Assay Description
Inhibition of human NAMPT using NAM/PRPP as substrate incubated for 15 mins prior to substrate addition measured after 30 mins by mass spectrometric ... |
Bioorg Med Chem Lett 23: 4875-85 (2013)
Article DOI: 10.1016/j.bmcl.2013.06.090
BindingDB Entry DOI: 10.7270/Q25T3MWP |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C9
(Homo sapiens (Human)) | BDBM50439295

(CHEMBL2419525 | US10696692, Example 289)Show SMILES O=C(NCc1ccc(cc1)S(=O)(=O)C1CCN(CC1)C1CCOCC1)N1Cc2ccncc2C1 Show InChI InChI=1S/C25H32N4O4S/c30-25(29-17-20-5-10-26-16-21(20)18-29)27-15-19-1-3-23(4-2-19)34(31,32)24-6-11-28(12-7-24)22-8-13-33-14-9-22/h1-5,10,16,22,24H,6-9,11-15,17-18H2,(H,27,30) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 270 | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech Inc
Curated by ChEMBL
| Assay Description
Inhibition of CYP2C9 in human liver microsomes using (S)- warfarin as substrate by LC-MS/MS analysis |
Bioorg Med Chem Lett 23: 4875-85 (2013)
Article DOI: 10.1016/j.bmcl.2013.06.090
BindingDB Entry DOI: 10.7270/Q25T3MWP |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C9
(Homo sapiens (Human)) | BDBM50439296

(CHEMBL2419524 | US10696692, Example 287)Show SMILES O=C(NCc1ccc(cc1)S(=O)(=O)C1CCN(CC1)C1COC1)N1Cc2ccncc2C1 Show InChI InChI=1S/C23H28N4O4S/c28-23(27-13-18-5-8-24-12-19(18)14-27)25-11-17-1-3-21(4-2-17)32(29,30)22-6-9-26(10-7-22)20-15-31-16-20/h1-5,8,12,20,22H,6-7,9-11,13-16H2,(H,25,28) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 300 | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech Inc
Curated by ChEMBL
| Assay Description
Inhibition of CYP2C9 in human liver microsomes using (S)- warfarin as substrate by LC-MS/MS analysis |
Bioorg Med Chem Lett 23: 4875-85 (2013)
Article DOI: 10.1016/j.bmcl.2013.06.090
BindingDB Entry DOI: 10.7270/Q25T3MWP |
More data for this
Ligand-Target Pair | |
Nicotinamide phosphoribosyltransferase
(Homo sapiens (Human)) | BDBM50439313

(CHEMBL2419509)Show SMILES O=C(NCc1ccc(cc1)S(=O)(=O)c1ccccc1)N1CCc2ccncc2C1 Show InChI InChI=1S/C22H21N3O3S/c26-22(25-13-11-18-10-12-23-15-19(18)16-25)24-14-17-6-8-21(9-7-17)29(27,28)20-4-2-1-3-5-20/h1-10,12,15H,11,13-14,16H2,(H,24,26) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 310 | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech Inc
Curated by ChEMBL
| Assay Description
Inhibition of human NAMPT using NAM/PRPP as substrate incubated for 15 mins prior to substrate addition measured after 30 mins by mass spectrometric ... |
Bioorg Med Chem Lett 23: 4875-85 (2013)
Article DOI: 10.1016/j.bmcl.2013.06.090
BindingDB Entry DOI: 10.7270/Q25T3MWP |
More data for this
Ligand-Target Pair | |
Nicotinamide phosphoribosyltransferase
(Homo sapiens (Human)) | BDBM50439323

(CHEMBL2419531 | US10696692, Example 307)Show SMILES O[C@H]1CCCN(C1)S(=O)(=O)c1ccc(CNC(=O)N2Cc3ccncc3C2)cc1 |r| Show InChI InChI=1S/C20H24N4O4S/c25-18-2-1-9-24(14-18)29(27,28)19-5-3-15(4-6-19)10-22-20(26)23-12-16-7-8-21-11-17(16)13-23/h3-8,11,18,25H,1-2,9-10,12-14H2,(H,22,26)/t18-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 770 | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech Inc
Curated by ChEMBL
| Assay Description
Inhibition of human NAMPT using NAM/PRPP as substrate incubated for 15 mins prior to substrate addition measured after 30 mins by mass spectrometric ... |
Bioorg Med Chem Lett 23: 4875-85 (2013)
Article DOI: 10.1016/j.bmcl.2013.06.090
BindingDB Entry DOI: 10.7270/Q25T3MWP |
More data for this
Ligand-Target Pair | |
Nicotinamide phosphoribosyltransferase
(Homo sapiens (Human)) | BDBM50439311
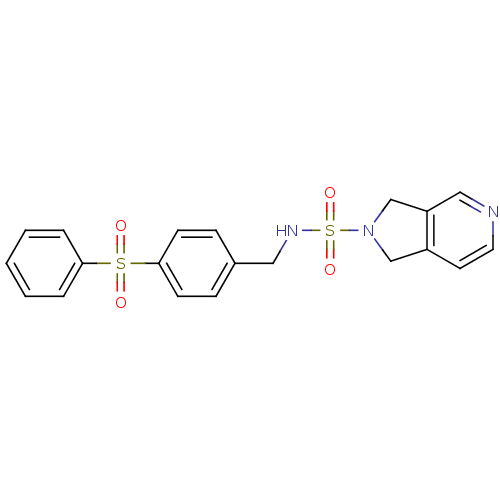
(CHEMBL2419511)Show SMILES O=S(=O)(NCc1ccc(cc1)S(=O)(=O)c1ccccc1)N1Cc2ccncc2C1 Show InChI InChI=1S/C20H19N3O4S2/c24-28(25,19-4-2-1-3-5-19)20-8-6-16(7-9-20)12-22-29(26,27)23-14-17-10-11-21-13-18(17)15-23/h1-11,13,22H,12,14-15H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech Inc
Curated by ChEMBL
| Assay Description
Inhibition of human NAMPT using NAM/PRPP as substrate incubated for 15 mins prior to substrate addition measured after 30 mins by mass spectrometric ... |
Bioorg Med Chem Lett 23: 4875-85 (2013)
Article DOI: 10.1016/j.bmcl.2013.06.090
BindingDB Entry DOI: 10.7270/Q25T3MWP |
More data for this
Ligand-Target Pair | |
Nicotinamide phosphoribosyltransferase
(Homo sapiens (Human)) | BDBM50439312
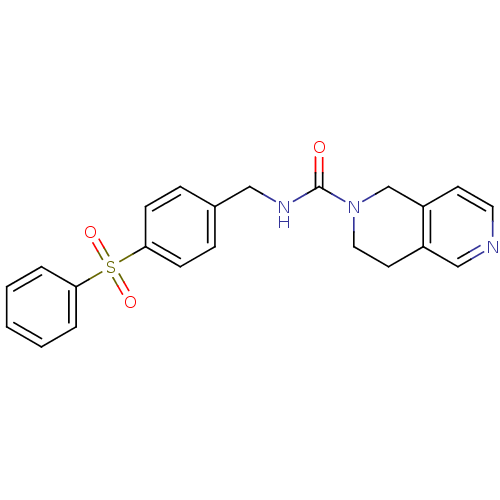
(CHEMBL2419510)Show SMILES O=C(NCc1ccc(cc1)S(=O)(=O)c1ccccc1)N1CCc2cnccc2C1 Show InChI InChI=1S/C22H21N3O3S/c26-22(25-13-11-18-15-23-12-10-19(18)16-25)24-14-17-6-8-21(9-7-17)29(27,28)20-4-2-1-3-5-20/h1-10,12,15H,11,13-14,16H2,(H,24,26) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech Inc
Curated by ChEMBL
| Assay Description
Inhibition of human NAMPT using NAM/PRPP as substrate incubated for 15 mins prior to substrate addition measured after 30 mins by mass spectrometric ... |
Bioorg Med Chem Lett 23: 4875-85 (2013)
Article DOI: 10.1016/j.bmcl.2013.06.090
BindingDB Entry DOI: 10.7270/Q25T3MWP |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C19
(Homo sapiens (Human)) | BDBM50439304

(CHEMBL2419505)Show SMILES O=C(NCc1ccc(cc1)S(=O)(=O)c1ccccc1)N1Cc2ccncc2C1 Show InChI InChI=1S/C21H19N3O3S/c25-21(24-14-17-10-11-22-13-18(17)15-24)23-12-16-6-8-20(9-7-16)28(26,27)19-4-2-1-3-5-19/h1-11,13H,12,14-15H2,(H,23,25) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech Inc
Curated by ChEMBL
| Assay Description
Inhibition of CYP2C19 in human liver microsomes using (S)-mephenytoin as substrate by LC-MS/MS analysis |
Bioorg Med Chem Lett 23: 4875-85 (2013)
Article DOI: 10.1016/j.bmcl.2013.06.090
BindingDB Entry DOI: 10.7270/Q25T3MWP |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2D6
(Homo sapiens (Human)) | BDBM50439304

(CHEMBL2419505)Show SMILES O=C(NCc1ccc(cc1)S(=O)(=O)c1ccccc1)N1Cc2ccncc2C1 Show InChI InChI=1S/C21H19N3O3S/c25-21(24-14-17-10-11-22-13-18(17)15-24)23-12-16-6-8-20(9-7-16)28(26,27)19-4-2-1-3-5-19/h1-11,13H,12,14-15H2,(H,23,25) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech Inc
Curated by ChEMBL
| Assay Description
Inhibition of CYP2D6 in human liver microsomes using dextromethorphan as substrate by LC-MS/MS analysis |
Bioorg Med Chem Lett 23: 4875-85 (2013)
Article DOI: 10.1016/j.bmcl.2013.06.090
BindingDB Entry DOI: 10.7270/Q25T3MWP |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C19
(Homo sapiens (Human)) | BDBM50439302

(CHEMBL2419516)Show SMILES Nc1ccc(cn1)S(=O)(=O)c1ccc(CNC(=O)N2Cc3ccncc3C2)cc1 Show InChI InChI=1S/C20H19N5O3S/c21-19-6-5-18(11-23-19)29(27,28)17-3-1-14(2-4-17)9-24-20(26)25-12-15-7-8-22-10-16(15)13-25/h1-8,10-11H,9,12-13H2,(H2,21,23)(H,24,26) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech Inc
Curated by ChEMBL
| Assay Description
Inhibition of CYP2C19 in human liver microsomes using (S)-mephenytoin as substrate by LC-MS/MS analysis |
Bioorg Med Chem Lett 23: 4875-85 (2013)
Article DOI: 10.1016/j.bmcl.2013.06.090
BindingDB Entry DOI: 10.7270/Q25T3MWP |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C19
(Homo sapiens (Human)) | BDBM50439301

(CHEMBL2419518)Show SMILES CC(C)n1cc(cn1)S(=O)(=O)c1ccc(CNC(=O)N2Cc3ccncc3C2)cc1 Show InChI InChI=1S/C21H23N5O3S/c1-15(2)26-14-20(11-24-26)30(28,29)19-5-3-16(4-6-19)9-23-21(27)25-12-17-7-8-22-10-18(17)13-25/h3-8,10-11,14-15H,9,12-13H2,1-2H3,(H,23,27) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech Inc
Curated by ChEMBL
| Assay Description
Inhibition of CYP2C19 in human liver microsomes using (S)-mephenytoin as substrate by LC-MS/MS analysis |
Bioorg Med Chem Lett 23: 4875-85 (2013)
Article DOI: 10.1016/j.bmcl.2013.06.090
BindingDB Entry DOI: 10.7270/Q25T3MWP |
More data for this
Ligand-Target Pair | |
Cytochrome P450 1A2
(Homo sapiens (Human)) | BDBM50439295

(CHEMBL2419525 | US10696692, Example 289)Show SMILES O=C(NCc1ccc(cc1)S(=O)(=O)C1CCN(CC1)C1CCOCC1)N1Cc2ccncc2C1 Show InChI InChI=1S/C25H32N4O4S/c30-25(29-17-20-5-10-26-16-21(20)18-29)27-15-19-1-3-23(4-2-19)34(31,32)24-6-11-28(12-7-24)22-8-13-33-14-9-22/h1-5,10,16,22,24H,6-9,11-15,17-18H2,(H,27,30) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech Inc
Curated by ChEMBL
| Assay Description
Inhibition of CYP1A2 in human liver microsomes using phenacetin as substrate by LC-MS/MS analysis |
Bioorg Med Chem Lett 23: 4875-85 (2013)
Article DOI: 10.1016/j.bmcl.2013.06.090
BindingDB Entry DOI: 10.7270/Q25T3MWP |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2D6
(Homo sapiens (Human)) | BDBM50439295

(CHEMBL2419525 | US10696692, Example 289)Show SMILES O=C(NCc1ccc(cc1)S(=O)(=O)C1CCN(CC1)C1CCOCC1)N1Cc2ccncc2C1 Show InChI InChI=1S/C25H32N4O4S/c30-25(29-17-20-5-10-26-16-21(20)18-29)27-15-19-1-3-23(4-2-19)34(31,32)24-6-11-28(12-7-24)22-8-13-33-14-9-22/h1-5,10,16,22,24H,6-9,11-15,17-18H2,(H,27,30) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech Inc
Curated by ChEMBL
| Assay Description
Inhibition of CYP2D6 in human liver microsomes using dextromethorphan as substrate by LC-MS/MS analysis |
Bioorg Med Chem Lett 23: 4875-85 (2013)
Article DOI: 10.1016/j.bmcl.2013.06.090
BindingDB Entry DOI: 10.7270/Q25T3MWP |
More data for this
Ligand-Target Pair | |
Cytochrome P450 1A2
(Homo sapiens (Human)) | BDBM50439296

(CHEMBL2419524 | US10696692, Example 287)Show SMILES O=C(NCc1ccc(cc1)S(=O)(=O)C1CCN(CC1)C1COC1)N1Cc2ccncc2C1 Show InChI InChI=1S/C23H28N4O4S/c28-23(27-13-18-5-8-24-12-19(18)14-27)25-11-17-1-3-21(4-2-17)32(29,30)22-6-9-26(10-7-22)20-15-31-16-20/h1-5,8,12,20,22H,6-7,9-11,13-16H2,(H,25,28) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech Inc
Curated by ChEMBL
| Assay Description
Inhibition of CYP1A2 in human liver microsomes using phenacetin as substrate by LC-MS/MS analysis |
Bioorg Med Chem Lett 23: 4875-85 (2013)
Article DOI: 10.1016/j.bmcl.2013.06.090
BindingDB Entry DOI: 10.7270/Q25T3MWP |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2D6
(Homo sapiens (Human)) | BDBM50439303

(CHEMBL2419515)Show SMILES Cc1ccc(cn1)S(=O)(=O)c1ccc(CNC(=O)N2Cc3ccncc3C2)cc1 Show InChI InChI=1S/C21H20N4O3S/c1-15-2-5-20(12-23-15)29(27,28)19-6-3-16(4-7-19)10-24-21(26)25-13-17-8-9-22-11-18(17)14-25/h2-9,11-12H,10,13-14H2,1H3,(H,24,26) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech Inc
Curated by ChEMBL
| Assay Description
Inhibition of CYP2D6 in human liver microsomes using dextromethorphan as substrate by LC-MS/MS analysis |
Bioorg Med Chem Lett 23: 4875-85 (2013)
Article DOI: 10.1016/j.bmcl.2013.06.090
BindingDB Entry DOI: 10.7270/Q25T3MWP |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2D6
(Homo sapiens (Human)) | BDBM50439296

(CHEMBL2419524 | US10696692, Example 287)Show SMILES O=C(NCc1ccc(cc1)S(=O)(=O)C1CCN(CC1)C1COC1)N1Cc2ccncc2C1 Show InChI InChI=1S/C23H28N4O4S/c28-23(27-13-18-5-8-24-12-19(18)14-27)25-11-17-1-3-21(4-2-17)32(29,30)22-6-9-26(10-7-22)20-15-31-16-20/h1-5,8,12,20,22H,6-7,9-11,13-16H2,(H,25,28) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech Inc
Curated by ChEMBL
| Assay Description
Inhibition of CYP2D6 in human liver microsomes using dextromethorphan as substrate by LC-MS/MS analysis |
Bioorg Med Chem Lett 23: 4875-85 (2013)
Article DOI: 10.1016/j.bmcl.2013.06.090
BindingDB Entry DOI: 10.7270/Q25T3MWP |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2D6
(Homo sapiens (Human)) | BDBM50439302

(CHEMBL2419516)Show SMILES Nc1ccc(cn1)S(=O)(=O)c1ccc(CNC(=O)N2Cc3ccncc3C2)cc1 Show InChI InChI=1S/C20H19N5O3S/c21-19-6-5-18(11-23-19)29(27,28)17-3-1-14(2-4-17)9-24-20(26)25-12-15-7-8-22-10-16(15)13-25/h1-8,10-11H,9,12-13H2,(H2,21,23)(H,24,26) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech Inc
Curated by ChEMBL
| Assay Description
Inhibition of CYP2D6 in human liver microsomes using dextromethorphan as substrate by LC-MS/MS analysis |
Bioorg Med Chem Lett 23: 4875-85 (2013)
Article DOI: 10.1016/j.bmcl.2013.06.090
BindingDB Entry DOI: 10.7270/Q25T3MWP |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2D6
(Homo sapiens (Human)) | BDBM50439301

(CHEMBL2419518)Show SMILES CC(C)n1cc(cn1)S(=O)(=O)c1ccc(CNC(=O)N2Cc3ccncc3C2)cc1 Show InChI InChI=1S/C21H23N5O3S/c1-15(2)26-14-20(11-24-26)30(28,29)19-5-3-16(4-6-19)9-23-21(27)25-12-17-7-8-22-10-18(17)13-25/h3-8,10-11,14-15H,9,12-13H2,1-2H3,(H,23,27) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech Inc
Curated by ChEMBL
| Assay Description
Inhibition of CYP2D6 in human liver microsomes using dextromethorphan as substrate by LC-MS/MS analysis |
Bioorg Med Chem Lett 23: 4875-85 (2013)
Article DOI: 10.1016/j.bmcl.2013.06.090
BindingDB Entry DOI: 10.7270/Q25T3MWP |
More data for this
Ligand-Target Pair | |
Cytochrome P450 1A2
(Homo sapiens (Human)) | BDBM50439303

(CHEMBL2419515)Show SMILES Cc1ccc(cn1)S(=O)(=O)c1ccc(CNC(=O)N2Cc3ccncc3C2)cc1 Show InChI InChI=1S/C21H20N4O3S/c1-15-2-5-20(12-23-15)29(27,28)19-6-3-16(4-7-19)10-24-21(26)25-13-17-8-9-22-11-18(17)14-25/h2-9,11-12H,10,13-14H2,1H3,(H,24,26) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech Inc
Curated by ChEMBL
| Assay Description
Inhibition of CYP1A2 in human liver microsomes using phenacetin as substrate by LC-MS/MS analysis |
Bioorg Med Chem Lett 23: 4875-85 (2013)
Article DOI: 10.1016/j.bmcl.2013.06.090
BindingDB Entry DOI: 10.7270/Q25T3MWP |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C19
(Homo sapiens (Human)) | BDBM50439296

(CHEMBL2419524 | US10696692, Example 287)Show SMILES O=C(NCc1ccc(cc1)S(=O)(=O)C1CCN(CC1)C1COC1)N1Cc2ccncc2C1 Show InChI InChI=1S/C23H28N4O4S/c28-23(27-13-18-5-8-24-12-19(18)14-27)25-11-17-1-3-21(4-2-17)32(29,30)22-6-9-26(10-7-22)20-15-31-16-20/h1-5,8,12,20,22H,6-7,9-11,13-16H2,(H,25,28) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech Inc
Curated by ChEMBL
| Assay Description
Inhibition of CYP2C19 in human liver microsomes using (S)-mephenytoin as substrate by LC-MS/MS analysis |
Bioorg Med Chem Lett 23: 4875-85 (2013)
Article DOI: 10.1016/j.bmcl.2013.06.090
BindingDB Entry DOI: 10.7270/Q25T3MWP |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C19
(Homo sapiens (Human)) | BDBM50439295

(CHEMBL2419525 | US10696692, Example 289)Show SMILES O=C(NCc1ccc(cc1)S(=O)(=O)C1CCN(CC1)C1CCOCC1)N1Cc2ccncc2C1 Show InChI InChI=1S/C25H32N4O4S/c30-25(29-17-20-5-10-26-16-21(20)18-29)27-15-19-1-3-23(4-2-19)34(31,32)24-6-11-28(12-7-24)22-8-13-33-14-9-22/h1-5,10,16,22,24H,6-9,11-15,17-18H2,(H,27,30) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech Inc
Curated by ChEMBL
| Assay Description
Inhibition of CYP2C19 in human liver microsomes using (S)-mephenytoin as substrate by LC-MS/MS analysis |
Bioorg Med Chem Lett 23: 4875-85 (2013)
Article DOI: 10.1016/j.bmcl.2013.06.090
BindingDB Entry DOI: 10.7270/Q25T3MWP |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C19
(Homo sapiens (Human)) | BDBM50439303

(CHEMBL2419515)Show SMILES Cc1ccc(cn1)S(=O)(=O)c1ccc(CNC(=O)N2Cc3ccncc3C2)cc1 Show InChI InChI=1S/C21H20N4O3S/c1-15-2-5-20(12-23-15)29(27,28)19-6-3-16(4-7-19)10-24-21(26)25-13-17-8-9-22-11-18(17)14-25/h2-9,11-12H,10,13-14H2,1H3,(H,24,26) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech Inc
Curated by ChEMBL
| Assay Description
Inhibition of CYP2C19 in human liver microsomes using (S)-mephenytoin as substrate by LC-MS/MS analysis |
Bioorg Med Chem Lett 23: 4875-85 (2013)
Article DOI: 10.1016/j.bmcl.2013.06.090
BindingDB Entry DOI: 10.7270/Q25T3MWP |
More data for this
Ligand-Target Pair | |
Cytochrome P450 1A2
(Homo sapiens (Human)) | BDBM50439304

(CHEMBL2419505)Show SMILES O=C(NCc1ccc(cc1)S(=O)(=O)c1ccccc1)N1Cc2ccncc2C1 Show InChI InChI=1S/C21H19N3O3S/c25-21(24-14-17-10-11-22-13-18(17)15-24)23-12-16-6-8-20(9-7-16)28(26,27)19-4-2-1-3-5-19/h1-11,13H,12,14-15H2,(H,23,25) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech Inc
Curated by ChEMBL
| Assay Description
Inhibition of CYP1A2 in human liver microsomes using phenacetin as substrate by LC-MS/MS analysis |
Bioorg Med Chem Lett 23: 4875-85 (2013)
Article DOI: 10.1016/j.bmcl.2013.06.090
BindingDB Entry DOI: 10.7270/Q25T3MWP |
More data for this
Ligand-Target Pair | |
Cytochrome P450 1A2
(Homo sapiens (Human)) | BDBM50439302

(CHEMBL2419516)Show SMILES Nc1ccc(cn1)S(=O)(=O)c1ccc(CNC(=O)N2Cc3ccncc3C2)cc1 Show InChI InChI=1S/C20H19N5O3S/c21-19-6-5-18(11-23-19)29(27,28)17-3-1-14(2-4-17)9-24-20(26)25-12-15-7-8-22-10-16(15)13-25/h1-8,10-11H,9,12-13H2,(H2,21,23)(H,24,26) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech Inc
Curated by ChEMBL
| Assay Description
Inhibition of CYP1A2 in human liver microsomes using phenacetin as substrate by LC-MS/MS analysis |
Bioorg Med Chem Lett 23: 4875-85 (2013)
Article DOI: 10.1016/j.bmcl.2013.06.090
BindingDB Entry DOI: 10.7270/Q25T3MWP |
More data for this
Ligand-Target Pair | |
Cytochrome P450 1A2
(Homo sapiens (Human)) | BDBM50439301

(CHEMBL2419518)Show SMILES CC(C)n1cc(cn1)S(=O)(=O)c1ccc(CNC(=O)N2Cc3ccncc3C2)cc1 Show InChI InChI=1S/C21H23N5O3S/c1-15(2)26-14-20(11-24-26)30(28,29)19-5-3-16(4-6-19)9-23-21(27)25-12-17-7-8-22-10-18(17)13-25/h3-8,10-11,14-15H,9,12-13H2,1-2H3,(H,23,27) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech Inc
Curated by ChEMBL
| Assay Description
Inhibition of CYP1A2 in human liver microsomes using phenacetin as substrate by LC-MS/MS analysis |
Bioorg Med Chem Lett 23: 4875-85 (2013)
Article DOI: 10.1016/j.bmcl.2013.06.090
BindingDB Entry DOI: 10.7270/Q25T3MWP |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data