

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cholinesterase (Equus caballus (Horse)) | BDBM8961 (1,2,3,4-tetrahydro-9-acridinamine | 1,2,3,4-tetrah...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 3.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ de Franche Comt£ Curated by ChEMBL | Assay Description Inhibition of BuChE in equine serum using butyrylthiocholine iodide as substrate preincubated with enzyme for 10 mins prior to substrate challenge me... | Bioorg Med Chem 22: 2496-507 (2014) Article DOI: 10.1016/j.bmc.2014.02.046 BindingDB Entry DOI: 10.7270/Q28K7BMT | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM8960 ((+/-)-2-[(1-benzylpiperidin-4-yl)methyl]-5,6-dimet...) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 33 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ de Franche Comt£ Curated by ChEMBL | Assay Description Inhibition of electric eel AChE using acetylthiocholine iodide as substrate preincubated with enzyme for 10 mins prior to substrate challenge measure... | Bioorg Med Chem 22: 2496-507 (2014) Article DOI: 10.1016/j.bmc.2014.02.046 BindingDB Entry DOI: 10.7270/Q28K7BMT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM8961 (1,2,3,4-tetrahydro-9-acridinamine | 1,2,3,4-tetrah...) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 65 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ de Franche Comt£ Curated by ChEMBL | Assay Description Inhibition of electric eel AChE using acetylthiocholine iodide as substrate preincubated with enzyme for 10 mins prior to substrate challenge measure... | Bioorg Med Chem 22: 2496-507 (2014) Article DOI: 10.1016/j.bmc.2014.02.046 BindingDB Entry DOI: 10.7270/Q28K7BMT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50002842 (CHEMBL3233934) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 110 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ de Franche Comt£ Curated by ChEMBL | Assay Description Inhibition of electric eel AChE using acetylthiocholine iodide as substrate preincubated with enzyme for 10 mins prior to substrate challenge measure... | Bioorg Med Chem 22: 2496-507 (2014) Article DOI: 10.1016/j.bmc.2014.02.046 BindingDB Entry DOI: 10.7270/Q28K7BMT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50002842 (CHEMBL3233934) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 230 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ de Franche Comt£ Curated by ChEMBL | Assay Description Inhibition of AChE in human erythrocytes using acetylthiocholine iodide as substrate preincubated with enzyme for 10 mins prior to substrate challeng... | Bioorg Med Chem 22: 2496-507 (2014) Article DOI: 10.1016/j.bmc.2014.02.046 BindingDB Entry DOI: 10.7270/Q28K7BMT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50002846 (CHEMBL3233938) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 270 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ de Franche Comt£ Curated by ChEMBL | Assay Description Inhibition of electric eel AChE using acetylthiocholine iodide as substrate preincubated with enzyme for 10 mins prior to substrate challenge measure... | Bioorg Med Chem 22: 2496-507 (2014) Article DOI: 10.1016/j.bmc.2014.02.046 BindingDB Entry DOI: 10.7270/Q28K7BMT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50002848 (CHEMBL3233940) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 330 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ de Franche Comt£ Curated by ChEMBL | Assay Description Inhibition of BuChE in equine serum using butyrylthiocholine iodide as substrate preincubated with enzyme for 10 mins prior to substrate challenge me... | Bioorg Med Chem 22: 2496-507 (2014) Article DOI: 10.1016/j.bmc.2014.02.046 BindingDB Entry DOI: 10.7270/Q28K7BMT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50002853 (CHEMBL3233927) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 370 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ de Franche Comt£ Curated by ChEMBL | Assay Description Inhibition of electric eel AChE using acetylthiocholine iodide as substrate preincubated with enzyme for 10 mins prior to substrate challenge measure... | Bioorg Med Chem 22: 2496-507 (2014) Article DOI: 10.1016/j.bmc.2014.02.046 BindingDB Entry DOI: 10.7270/Q28K7BMT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50002841 (CHEMBL3233933) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 380 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ de Franche Comt£ Curated by ChEMBL | Assay Description Inhibition of electric eel AChE using acetylthiocholine iodide as substrate preincubated with enzyme for 10 mins prior to substrate challenge measure... | Bioorg Med Chem 22: 2496-507 (2014) Article DOI: 10.1016/j.bmc.2014.02.046 BindingDB Entry DOI: 10.7270/Q28K7BMT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50002854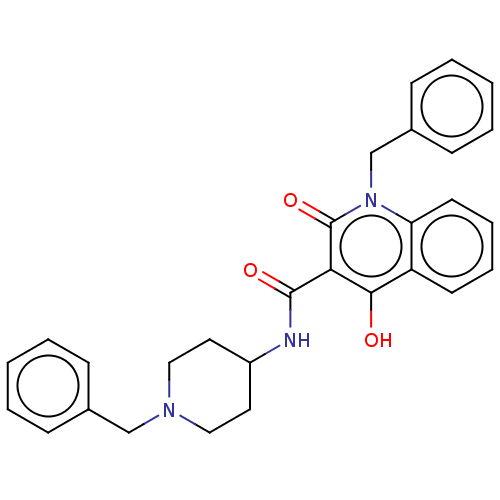 (CHEMBL3233928) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 390 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ de Franche Comt£ Curated by ChEMBL | Assay Description Inhibition of electric eel AChE using acetylthiocholine iodide as substrate preincubated with enzyme for 10 mins prior to substrate challenge measure... | Bioorg Med Chem 22: 2496-507 (2014) Article DOI: 10.1016/j.bmc.2014.02.046 BindingDB Entry DOI: 10.7270/Q28K7BMT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50002839 (CHEMBL3233931) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 470 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ de Franche Comt£ Curated by ChEMBL | Assay Description Inhibition of electric eel AChE using acetylthiocholine iodide as substrate preincubated with enzyme for 10 mins prior to substrate challenge measure... | Bioorg Med Chem 22: 2496-507 (2014) Article DOI: 10.1016/j.bmc.2014.02.046 BindingDB Entry DOI: 10.7270/Q28K7BMT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50002843 (CHEMBL3233935) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 480 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ de Franche Comt£ Curated by ChEMBL | Assay Description Inhibition of electric eel AChE using acetylthiocholine iodide as substrate preincubated with enzyme for 10 mins prior to substrate challenge measure... | Bioorg Med Chem 22: 2496-507 (2014) Article DOI: 10.1016/j.bmc.2014.02.046 BindingDB Entry DOI: 10.7270/Q28K7BMT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50002855 (CHEMBL3233929) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 490 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ de Franche Comt£ Curated by ChEMBL | Assay Description Inhibition of electric eel AChE using acetylthiocholine iodide as substrate preincubated with enzyme for 10 mins prior to substrate challenge measure... | Bioorg Med Chem 22: 2496-507 (2014) Article DOI: 10.1016/j.bmc.2014.02.046 BindingDB Entry DOI: 10.7270/Q28K7BMT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50002848 (CHEMBL3233940) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 560 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ de Franche Comt£ Curated by ChEMBL | Assay Description Inhibition of electric eel AChE using acetylthiocholine iodide as substrate preincubated with enzyme for 10 mins prior to substrate challenge measure... | Bioorg Med Chem 22: 2496-507 (2014) Article DOI: 10.1016/j.bmc.2014.02.046 BindingDB Entry DOI: 10.7270/Q28K7BMT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50002843 (CHEMBL3233935) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 980 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ de Franche Comt£ Curated by ChEMBL | Assay Description Inhibition of AChE in human erythrocytes using acetylthiocholine iodide as substrate preincubated with enzyme for 10 mins prior to substrate challeng... | Bioorg Med Chem 22: 2496-507 (2014) Article DOI: 10.1016/j.bmc.2014.02.046 BindingDB Entry DOI: 10.7270/Q28K7BMT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50002841 (CHEMBL3233933) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.14E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ de Franche Comt£ Curated by ChEMBL | Assay Description Inhibition of BuChE in equine serum using butyrylthiocholine iodide as substrate preincubated with enzyme for 10 mins prior to substrate challenge me... | Bioorg Med Chem 22: 2496-507 (2014) Article DOI: 10.1016/j.bmc.2014.02.046 BindingDB Entry DOI: 10.7270/Q28K7BMT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50002851 (CHEMBL3233664) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.41E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ de Franche Comt£ Curated by ChEMBL | Assay Description Inhibition of electric eel AChE using acetylthiocholine iodide as substrate preincubated with enzyme for 10 mins prior to substrate challenge measure... | Bioorg Med Chem 22: 2496-507 (2014) Article DOI: 10.1016/j.bmc.2014.02.046 BindingDB Entry DOI: 10.7270/Q28K7BMT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50002852 (CHEMBL3233926) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.52E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ de Franche Comt£ Curated by ChEMBL | Assay Description Inhibition of electric eel AChE using acetylthiocholine iodide as substrate preincubated with enzyme for 10 mins prior to substrate challenge measure... | Bioorg Med Chem 22: 2496-507 (2014) Article DOI: 10.1016/j.bmc.2014.02.046 BindingDB Entry DOI: 10.7270/Q28K7BMT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM8960 ((+/-)-2-[(1-benzylpiperidin-4-yl)methyl]-5,6-dimet...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 1.82E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ de Franche Comt£ Curated by ChEMBL | Assay Description Inhibition of BuChE in equine serum using butyrylthiocholine iodide as substrate preincubated with enzyme for 10 mins prior to substrate challenge me... | Bioorg Med Chem 22: 2496-507 (2014) Article DOI: 10.1016/j.bmc.2014.02.046 BindingDB Entry DOI: 10.7270/Q28K7BMT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50002845 (CHEMBL3233937) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.02E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ de Franche Comt£ Curated by ChEMBL | Assay Description Inhibition of electric eel AChE using acetylthiocholine iodide as substrate preincubated with enzyme for 10 mins prior to substrate challenge measure... | Bioorg Med Chem 22: 2496-507 (2014) Article DOI: 10.1016/j.bmc.2014.02.046 BindingDB Entry DOI: 10.7270/Q28K7BMT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50002850 (CHEMBL3233663) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.27E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ de Franche Comt£ Curated by ChEMBL | Assay Description Inhibition of electric eel AChE using acetylthiocholine iodide as substrate preincubated with enzyme for 10 mins prior to substrate challenge measure... | Bioorg Med Chem 22: 2496-507 (2014) Article DOI: 10.1016/j.bmc.2014.02.046 BindingDB Entry DOI: 10.7270/Q28K7BMT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50002837 (CHEMBL3233930) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.46E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ de Franche Comt£ Curated by ChEMBL | Assay Description Inhibition of electric eel AChE using acetylthiocholine iodide as substrate preincubated with enzyme for 10 mins prior to substrate challenge measure... | Bioorg Med Chem 22: 2496-507 (2014) Article DOI: 10.1016/j.bmc.2014.02.046 BindingDB Entry DOI: 10.7270/Q28K7BMT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50002844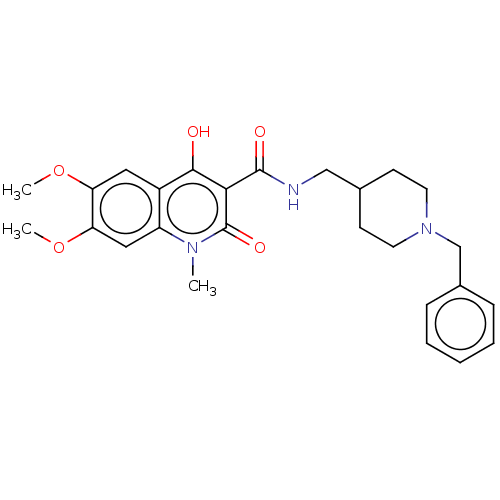 (CHEMBL3233936) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 2.93E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ de Franche Comt£ Curated by ChEMBL | Assay Description Inhibition of electric eel AChE using acetylthiocholine iodide as substrate preincubated with enzyme for 10 mins prior to substrate challenge measure... | Bioorg Med Chem 22: 2496-507 (2014) Article DOI: 10.1016/j.bmc.2014.02.046 BindingDB Entry DOI: 10.7270/Q28K7BMT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50002849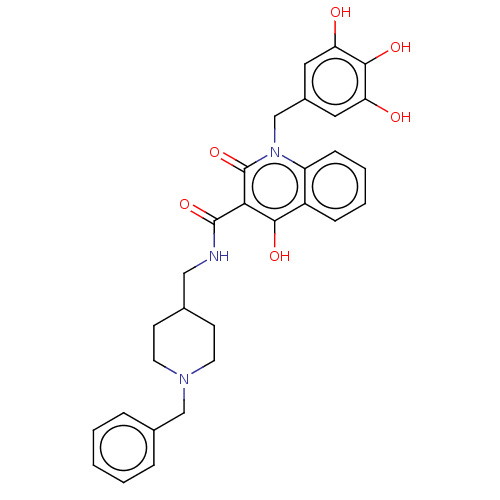 (CHEMBL3233941) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.89E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ de Franche Comt£ Curated by ChEMBL | Assay Description Inhibition of electric eel AChE using acetylthiocholine iodide as substrate preincubated with enzyme for 10 mins prior to substrate challenge measure... | Bioorg Med Chem 22: 2496-507 (2014) Article DOI: 10.1016/j.bmc.2014.02.046 BindingDB Entry DOI: 10.7270/Q28K7BMT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50002853 (CHEMBL3233927) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 4.26E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ de Franche Comt£ Curated by ChEMBL | Assay Description Inhibition of BuChE in equine serum using butyrylthiocholine iodide as substrate preincubated with enzyme for 10 mins prior to substrate challenge me... | Bioorg Med Chem 22: 2496-507 (2014) Article DOI: 10.1016/j.bmc.2014.02.046 BindingDB Entry DOI: 10.7270/Q28K7BMT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50002851 (CHEMBL3233664) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 6.02E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ de Franche Comt£ Curated by ChEMBL | Assay Description Inhibition of BuChE in equine serum using butyrylthiocholine iodide as substrate preincubated with enzyme for 10 mins prior to substrate challenge me... | Bioorg Med Chem 22: 2496-507 (2014) Article DOI: 10.1016/j.bmc.2014.02.046 BindingDB Entry DOI: 10.7270/Q28K7BMT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50002854 (CHEMBL3233928) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ de Franche Comt£ Curated by ChEMBL | Assay Description Inhibition of BuChE in equine serum using butyrylthiocholine iodide as substrate preincubated with enzyme for 10 mins prior to substrate challenge me... | Bioorg Med Chem 22: 2496-507 (2014) Article DOI: 10.1016/j.bmc.2014.02.046 BindingDB Entry DOI: 10.7270/Q28K7BMT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50002840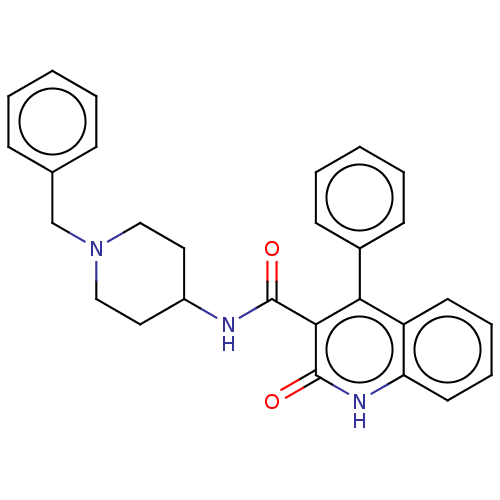 (CHEMBL3233932) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ de Franche Comt£ Curated by ChEMBL | Assay Description Inhibition of electric eel AChE using acetylthiocholine iodide as substrate preincubated with enzyme for 10 mins prior to substrate challenge measure... | Bioorg Med Chem 22: 2496-507 (2014) Article DOI: 10.1016/j.bmc.2014.02.046 BindingDB Entry DOI: 10.7270/Q28K7BMT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50002844 (CHEMBL3233936) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ de Franche Comt£ Curated by ChEMBL | Assay Description Inhibition of BuChE in equine serum using butyrylthiocholine iodide as substrate preincubated with enzyme for 10 mins prior to substrate challenge me... | Bioorg Med Chem 22: 2496-507 (2014) Article DOI: 10.1016/j.bmc.2014.02.046 BindingDB Entry DOI: 10.7270/Q28K7BMT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50002847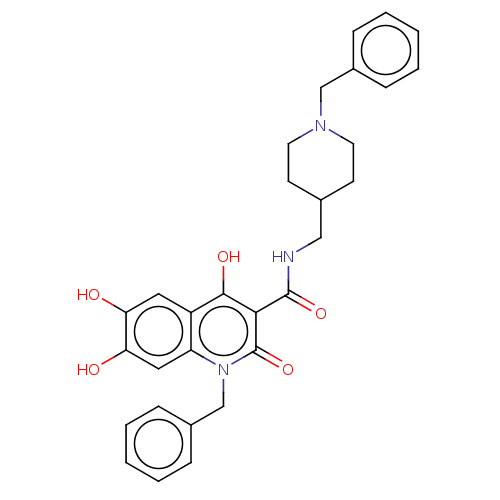 (CHEMBL3233939) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ de Franche Comt£ Curated by ChEMBL | Assay Description Inhibition of electric eel AChE using acetylthiocholine iodide as substrate preincubated with enzyme for 10 mins prior to substrate challenge measure... | Bioorg Med Chem 22: 2496-507 (2014) Article DOI: 10.1016/j.bmc.2014.02.046 BindingDB Entry DOI: 10.7270/Q28K7BMT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50002850 (CHEMBL3233663) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ de Franche Comt£ Curated by ChEMBL | Assay Description Inhibition of BuChE in equine serum using butyrylthiocholine iodide as substrate preincubated with enzyme for 10 mins prior to substrate challenge me... | Bioorg Med Chem 22: 2496-507 (2014) Article DOI: 10.1016/j.bmc.2014.02.046 BindingDB Entry DOI: 10.7270/Q28K7BMT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50002840 (CHEMBL3233932) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ de Franche Comt£ Curated by ChEMBL | Assay Description Inhibition of BuChE in equine serum using butyrylthiocholine iodide as substrate preincubated with enzyme for 10 mins prior to substrate challenge me... | Bioorg Med Chem 22: 2496-507 (2014) Article DOI: 10.1016/j.bmc.2014.02.046 BindingDB Entry DOI: 10.7270/Q28K7BMT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50002839 (CHEMBL3233931) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ de Franche Comt£ Curated by ChEMBL | Assay Description Inhibition of BuChE in equine serum using butyrylthiocholine iodide as substrate preincubated with enzyme for 10 mins prior to substrate challenge me... | Bioorg Med Chem 22: 2496-507 (2014) Article DOI: 10.1016/j.bmc.2014.02.046 BindingDB Entry DOI: 10.7270/Q28K7BMT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50002837 (CHEMBL3233930) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ de Franche Comt£ Curated by ChEMBL | Assay Description Inhibition of BuChE in equine serum using butyrylthiocholine iodide as substrate preincubated with enzyme for 10 mins prior to substrate challenge me... | Bioorg Med Chem 22: 2496-507 (2014) Article DOI: 10.1016/j.bmc.2014.02.046 BindingDB Entry DOI: 10.7270/Q28K7BMT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50002847 (CHEMBL3233939) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ de Franche Comt£ Curated by ChEMBL | Assay Description Inhibition of BuChE in equine serum using butyrylthiocholine iodide as substrate preincubated with enzyme for 10 mins prior to substrate challenge me... | Bioorg Med Chem 22: 2496-507 (2014) Article DOI: 10.1016/j.bmc.2014.02.046 BindingDB Entry DOI: 10.7270/Q28K7BMT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50002855 (CHEMBL3233929) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ de Franche Comt£ Curated by ChEMBL | Assay Description Inhibition of BuChE in equine serum using butyrylthiocholine iodide as substrate preincubated with enzyme for 10 mins prior to substrate challenge me... | Bioorg Med Chem 22: 2496-507 (2014) Article DOI: 10.1016/j.bmc.2014.02.046 BindingDB Entry DOI: 10.7270/Q28K7BMT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50002852 (CHEMBL3233926) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ de Franche Comt£ Curated by ChEMBL | Assay Description Inhibition of BuChE in equine serum using butyrylthiocholine iodide as substrate preincubated with enzyme for 10 mins prior to substrate challenge me... | Bioorg Med Chem 22: 2496-507 (2014) Article DOI: 10.1016/j.bmc.2014.02.046 BindingDB Entry DOI: 10.7270/Q28K7BMT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50002849 (CHEMBL3233941) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ de Franche Comt£ Curated by ChEMBL | Assay Description Inhibition of BuChE in equine serum using butyrylthiocholine iodide as substrate preincubated with enzyme for 10 mins prior to substrate challenge me... | Bioorg Med Chem 22: 2496-507 (2014) Article DOI: 10.1016/j.bmc.2014.02.046 BindingDB Entry DOI: 10.7270/Q28K7BMT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50002843 (CHEMBL3233935) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ de Franche Comt£ Curated by ChEMBL | Assay Description Inhibition of BuChE in equine serum using butyrylthiocholine iodide as substrate preincubated with enzyme for 10 mins prior to substrate challenge me... | Bioorg Med Chem 22: 2496-507 (2014) Article DOI: 10.1016/j.bmc.2014.02.046 BindingDB Entry DOI: 10.7270/Q28K7BMT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50002845 (CHEMBL3233937) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ de Franche Comt£ Curated by ChEMBL | Assay Description Inhibition of BuChE in equine serum using butyrylthiocholine iodide as substrate preincubated with enzyme for 10 mins prior to substrate challenge me... | Bioorg Med Chem 22: 2496-507 (2014) Article DOI: 10.1016/j.bmc.2014.02.046 BindingDB Entry DOI: 10.7270/Q28K7BMT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50002846 (CHEMBL3233938) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >6.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ de Franche Comt£ Curated by ChEMBL | Assay Description Inhibition of BuChE in equine serum using butyrylthiocholine iodide as substrate preincubated with enzyme for 10 mins prior to substrate challenge me... | Bioorg Med Chem 22: 2496-507 (2014) Article DOI: 10.1016/j.bmc.2014.02.046 BindingDB Entry DOI: 10.7270/Q28K7BMT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50002842 (CHEMBL3233934) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | >6.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ de Franche Comt£ Curated by ChEMBL | Assay Description Inhibition of BuChE in equine serum using butyrylthiocholine iodide as substrate preincubated with enzyme for 10 mins prior to substrate challenge me... | Bioorg Med Chem 22: 2496-507 (2014) Article DOI: 10.1016/j.bmc.2014.02.046 BindingDB Entry DOI: 10.7270/Q28K7BMT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||