

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| RAC-alpha serine/threonine-protein kinase (Homo sapiens (Human)) | BDBM15148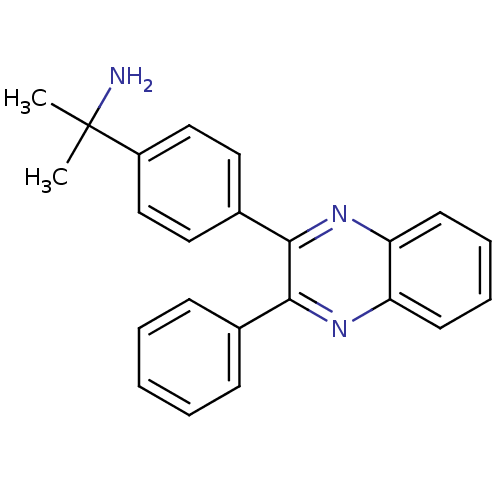 (2,3-diphenylquinoxaline 1 | 2-[4-(3-phenylquinoxal...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | 3.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Vanderbilt University Medical Center Curated by ChEMBL | Assay Description Inhibition of Akt1 (unknown origin) | J Med Chem 57: 7485-98 (2014) Article DOI: 10.1021/jm5011786 BindingDB Entry DOI: 10.7270/Q2XG9SQ5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| RAC-beta serine/threonine-protein kinase (Homo sapiens (Human)) | BDBM15148 (2,3-diphenylquinoxaline 1 | 2-[4-(3-phenylquinoxal...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | 2.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Vanderbilt University Medical Center Curated by ChEMBL | Assay Description Inhibition of Akt2 (unknown origin) | J Med Chem 57: 7485-98 (2014) Article DOI: 10.1021/jm5011786 BindingDB Entry DOI: 10.7270/Q2XG9SQ5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| RAC-alpha serine/threonine-protein kinase (Homo sapiens (Human)) | BDBM15148 (2,3-diphenylquinoxaline 1 | 2-[4-(3-phenylquinoxal...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | >2.50E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Vanderbilt University Medical Center Curated by ChEMBL | Assay Description Inhibition of Akt1 PH domain (unknown origin) | J Med Chem 57: 7485-98 (2014) Article DOI: 10.1021/jm5011786 BindingDB Entry DOI: 10.7270/Q2XG9SQ5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| RAC-beta serine/threonine-protein kinase (Homo sapiens (Human)) | BDBM15148 (2,3-diphenylquinoxaline 1 | 2-[4-(3-phenylquinoxal...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | >2.50E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Vanderbilt University Medical Center Curated by ChEMBL | Assay Description Inhibition of Akt2 PH domain (unknown origin) | J Med Chem 57: 7485-98 (2014) Article DOI: 10.1021/jm5011786 BindingDB Entry DOI: 10.7270/Q2XG9SQ5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase Sgk1 (Homo sapiens (Human)) | BDBM15148 (2,3-diphenylquinoxaline 1 | 2-[4-(3-phenylquinoxal...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | >2.50E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Vanderbilt University Medical Center Curated by ChEMBL | Assay Description Inhibition of SGK (unknown origin) | J Med Chem 57: 7485-98 (2014) Article DOI: 10.1021/jm5011786 BindingDB Entry DOI: 10.7270/Q2XG9SQ5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| RAC-gamma serine/threonine-protein kinase (Homo sapiens (Human)) | BDBM15148 (2,3-diphenylquinoxaline 1 | 2-[4-(3-phenylquinoxal...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | >2.50E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Vanderbilt University Medical Center Curated by ChEMBL | Assay Description Inhibition of Akt3 (unknown origin) | J Med Chem 57: 7485-98 (2014) Article DOI: 10.1021/jm5011786 BindingDB Entry DOI: 10.7270/Q2XG9SQ5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M5 (Homo sapiens (Human)) | BDBM50156074 (1,2-bis(3-fluorobenzylidene)hydrazine | CHEMBL3718...) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | 2.60E+3 | n/a | n/a | n/a | n/a |
Vanderbilt University Medical Center Curated by ChEMBL | Assay Description Positive allosteric modulator activity at human muscarinic acetylcholine receptor M5 expressed in CHO cells by fluorometric imaging plate reader | J Med Chem 57: 7485-98 (2014) Article DOI: 10.1021/jm5011786 BindingDB Entry DOI: 10.7270/Q2XG9SQ5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M5 (Homo sapiens (Human)) | BDBM50024560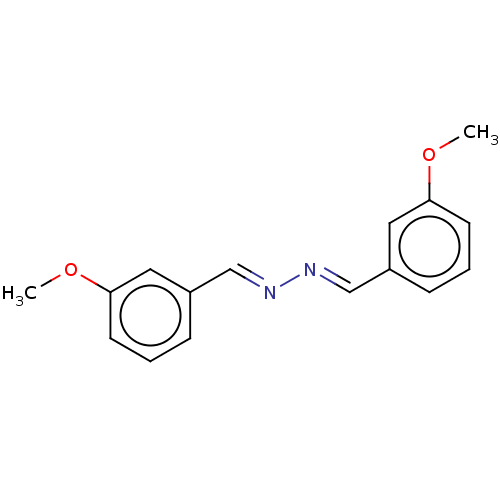 (CHEMBL3334989) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | 3.00E+3 | n/a | n/a | n/a | n/a |
Vanderbilt University Medical Center Curated by ChEMBL | Assay Description Negative allosteric modulator activity at human muscarinic acetylcholine receptor M5 expressed in CHO cells by fluorometric imaging plate reader | J Med Chem 57: 7485-98 (2014) Article DOI: 10.1021/jm5011786 BindingDB Entry DOI: 10.7270/Q2XG9SQ5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M5 (Homo sapiens (Human)) | BDBM50024561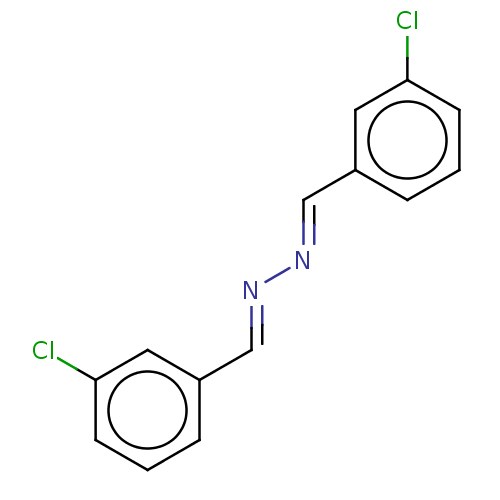 (CHEMBL3334990) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 7.60E+3 | n/a | n/a | n/a | n/a |
Vanderbilt University Medical Center Curated by ChEMBL | Assay Description Silent allosteric modulator activity at human muscarinic acetylcholine receptor M5 expressed in CHO cells by fluorometric imaging plate reader | J Med Chem 57: 7485-98 (2014) Article DOI: 10.1021/jm5011786 BindingDB Entry DOI: 10.7270/Q2XG9SQ5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M5 (Homo sapiens (Human)) | BDBM50024542 (CHEMBL3334991) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 33 | n/a | n/a | n/a | n/a |
Vanderbilt University Medical Center Curated by ChEMBL | Assay Description Positive allosteric modulator activity at human muscarinic acetylcholine receptor M5 expressed in CHO cells by fluorometric imaging plate reader | J Med Chem 57: 7485-98 (2014) Article DOI: 10.1021/jm5011786 BindingDB Entry DOI: 10.7270/Q2XG9SQ5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M5 (Homo sapiens (Human)) | BDBM50024562 (CHEMBL2431187) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 400 | n/a | n/a | n/a | n/a |
Vanderbilt University Medical Center Curated by ChEMBL | Assay Description Allosteric agonist activity at human muscarinic acetylcholine receptor M5 | J Med Chem 57: 7485-98 (2014) Article DOI: 10.1021/jm5011786 BindingDB Entry DOI: 10.7270/Q2XG9SQ5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||