 Found 18 hits of Enzyme Inhibition Constant Data
Found 18 hits of Enzyme Inhibition Constant Data Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Group 10 secretory phospholipase A2
(Homo sapiens (Human)) | BDBM50262998
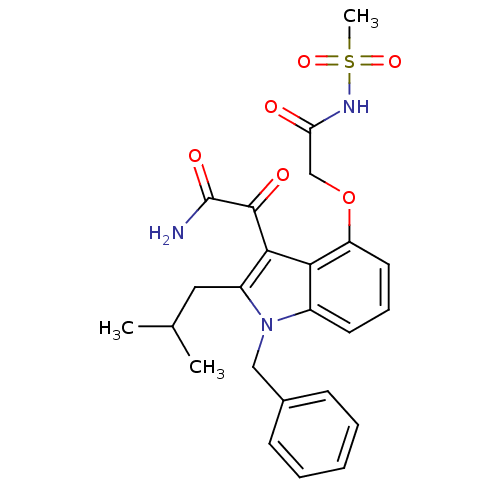
(CHEMBL477548 | mesyl-2-(3-(2-amino-2-oxoacetyl)-1-...)Show SMILES CC(C)Cc1c(C(=O)C(N)=O)c2c(OCC(=O)NS(C)(=O)=O)cccc2n1Cc1ccccc1 Show InChI InChI=1S/C24H27N3O6S/c1-15(2)12-18-22(23(29)24(25)30)21-17(27(18)13-16-8-5-4-6-9-16)10-7-11-19(21)33-14-20(28)26-34(3,31)32/h4-11,15H,12-14H2,1-3H3,(H2,25,30)(H,26,28) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Binding affinity to sPLA2X (unknown origin) |
Bioorg Med Chem Lett 24: 5251-5 (2014)
Article DOI: 10.1016/j.bmcl.2014.09.058
BindingDB Entry DOI: 10.7270/Q2668FS8 |
More data for this
Ligand-Target Pair | |
Group 10 secretory phospholipase A2
(Homo sapiens (Human)) | BDBM50031106
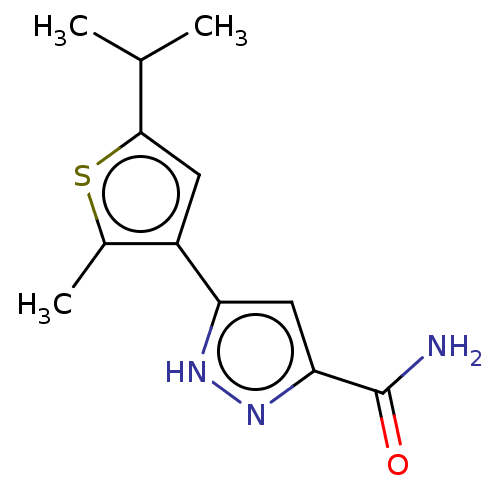
(CHEMBL3337975)Show InChI InChI=1S/C12H15N3OS/c1-6(2)11-4-8(7(3)17-11)9-5-10(12(13)16)15-14-9/h4-6H,1-3H3,(H2,13,16)(H,14,15) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >51 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of human sPLA2X using 1,2-bis(heptanoylthio) glycerophosphocholine substrate incubated for 30 mins |
Bioorg Med Chem Lett 24: 5251-5 (2014)
Article DOI: 10.1016/j.bmcl.2014.09.058
BindingDB Entry DOI: 10.7270/Q2668FS8 |
More data for this
Ligand-Target Pair | |
Group 10 secretory phospholipase A2
(Homo sapiens (Human)) | BDBM50055366

((3-Aminooxalyl-1-benzyl-2-ethyl-1H-indol-4-yloxy)-...)Show SMILES CCc1c(C(=O)C(N)=O)c2c(OCC(O)=O)cccc2n1Cc1ccccc1 Show InChI InChI=1S/C21H20N2O5/c1-2-14-19(20(26)21(22)27)18-15(9-6-10-16(18)28-12-17(24)25)23(14)11-13-7-4-3-5-8-13/h3-10H,2,11-12H2,1H3,(H2,22,27)(H,24,25) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 107 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of human sPLA2X using 1,2-bis(heptanoylthio) glycerophosphocholine substrate incubated for 30 mins |
Bioorg Med Chem Lett 24: 5251-5 (2014)
Article DOI: 10.1016/j.bmcl.2014.09.058
BindingDB Entry DOI: 10.7270/Q2668FS8 |
More data for this
Ligand-Target Pair | |
Group 10 secretory phospholipase A2
(Homo sapiens (Human)) | BDBM50031105
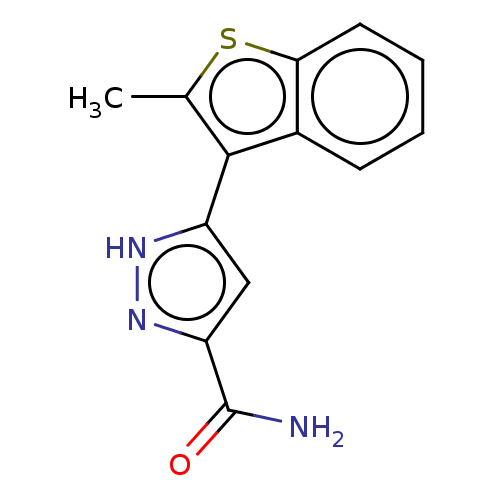
(CHEMBL3337976)Show InChI InChI=1S/C13H11N3OS/c1-7-12(8-4-2-3-5-11(8)18-7)9-6-10(13(14)17)16-15-9/h2-6H,1H3,(H2,14,17)(H,15,16) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 174 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of human sPLA2X using 1,2-bis(heptanoylthio) glycerophosphocholine substrate incubated for 30 mins |
Bioorg Med Chem Lett 24: 5251-5 (2014)
Article DOI: 10.1016/j.bmcl.2014.09.058
BindingDB Entry DOI: 10.7270/Q2668FS8 |
More data for this
Ligand-Target Pair | |
Group 10 secretory phospholipase A2
(Homo sapiens (Human)) | BDBM50031107

(CHEMBL3337972)Show InChI InChI=1S/C10H11N3OS/c1-5-3-7(6(2)15-5)8-4-9(10(11)14)13-12-8/h3-4H,1-2H3,(H2,11,14)(H,12,13) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 316 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of human sPLA2X using 1,2-bis(heptanoylthio) glycerophosphocholine substrate incubated for 30 mins |
Bioorg Med Chem Lett 24: 5251-5 (2014)
Article DOI: 10.1016/j.bmcl.2014.09.058
BindingDB Entry DOI: 10.7270/Q2668FS8 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Group 10 secretory phospholipase A2
(Homo sapiens (Human)) | BDBM50031104
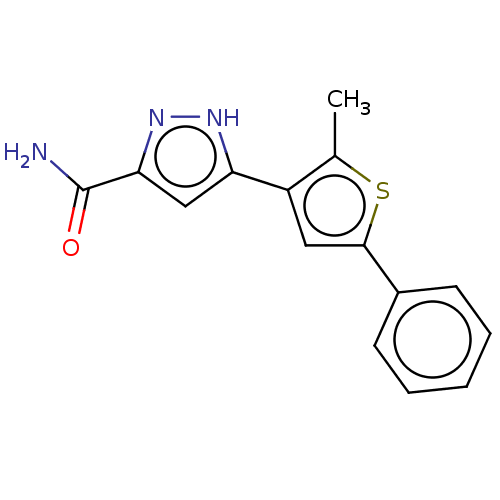
(CHEMBL3337977)Show InChI InChI=1S/C15H13N3OS/c1-9-11(12-8-13(15(16)19)18-17-12)7-14(20-9)10-5-3-2-4-6-10/h2-8H,1H3,(H2,16,19)(H,17,18) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 347 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of human sPLA2X using 1,2-bis(heptanoylthio) glycerophosphocholine substrate incubated for 30 mins |
Bioorg Med Chem Lett 24: 5251-5 (2014)
Article DOI: 10.1016/j.bmcl.2014.09.058
BindingDB Entry DOI: 10.7270/Q2668FS8 |
More data for this
Ligand-Target Pair | |
Group 10 secretory phospholipase A2
(Homo sapiens (Human)) | BDBM50031103

(CHEMBL3337978)Show InChI InChI=1S/C8H5Cl2N3OS/c9-6-1-3(7(10)15-6)4-2-5(8(11)14)13-12-4/h1-2H,(H2,11,14)(H,12,13) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 398 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of human sPLA2X using 1,2-bis(heptanoylthio) glycerophosphocholine substrate incubated for 30 mins |
Bioorg Med Chem Lett 24: 5251-5 (2014)
Article DOI: 10.1016/j.bmcl.2014.09.058
BindingDB Entry DOI: 10.7270/Q2668FS8 |
More data for this
Ligand-Target Pair | |
Group 10 secretory phospholipase A2
(Homo sapiens (Human)) | BDBM50031102
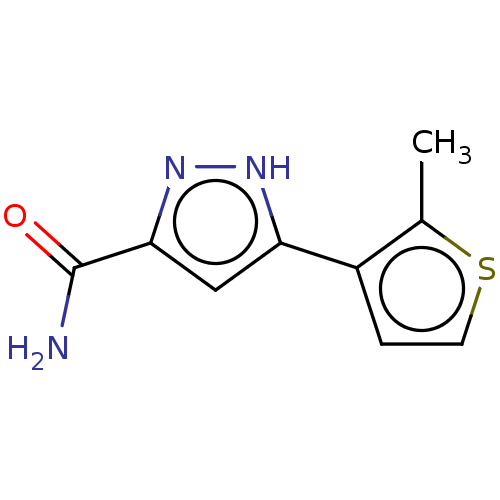
(CHEMBL3337979)Show InChI InChI=1S/C9H9N3OS/c1-5-6(2-3-14-5)7-4-8(9(10)13)12-11-7/h2-4H,1H3,(H2,10,13)(H,11,12) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 631 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of human sPLA2X using 1,2-bis(heptanoylthio) glycerophosphocholine substrate incubated for 30 mins |
Bioorg Med Chem Lett 24: 5251-5 (2014)
Article DOI: 10.1016/j.bmcl.2014.09.058
BindingDB Entry DOI: 10.7270/Q2668FS8 |
More data for this
Ligand-Target Pair | |
Group 10 secretory phospholipase A2
(Homo sapiens (Human)) | BDBM50031112
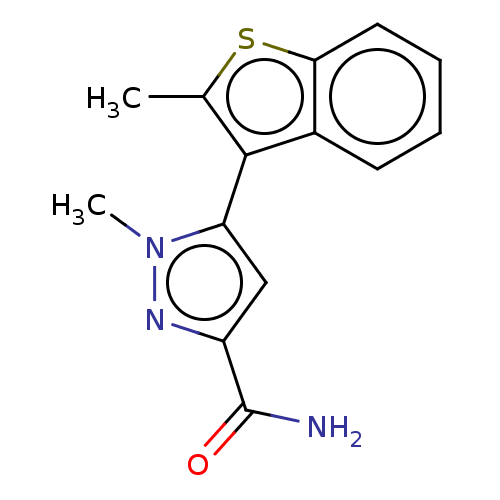
(CHEMBL3337982)Show SMILES Cc1sc2ccccc2c1-c1cc(nn1C)C(N)=O |(18.5,-9.27,;18.5,-7.73,;17.25,-6.82,;17.73,-5.36,;16.96,-4.04,;17.72,-2.7,;19.26,-2.7,;20.03,-4.03,;19.27,-5.36,;19.75,-6.82,;21.21,-7.3,;21.68,-8.77,;23.22,-8.78,;23.7,-7.31,;22.46,-6.4,;22.46,-4.86,;24.12,-10.03,;25.65,-9.88,;23.49,-11.43,)| Show InChI InChI=1S/C14H13N3OS/c1-8-13(9-5-3-4-6-12(9)19-8)11-7-10(14(15)18)16-17(11)2/h3-7H,1-2H3,(H2,15,18) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 851 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of human sPLA2X using 1,2-bis(heptanoylthio) glycerophosphocholine substrate incubated for 30 mins |
Bioorg Med Chem Lett 24: 5251-5 (2014)
Article DOI: 10.1016/j.bmcl.2014.09.058
BindingDB Entry DOI: 10.7270/Q2668FS8 |
More data for this
Ligand-Target Pair | |
Group 10 secretory phospholipase A2
(Homo sapiens (Human)) | BDBM50031113
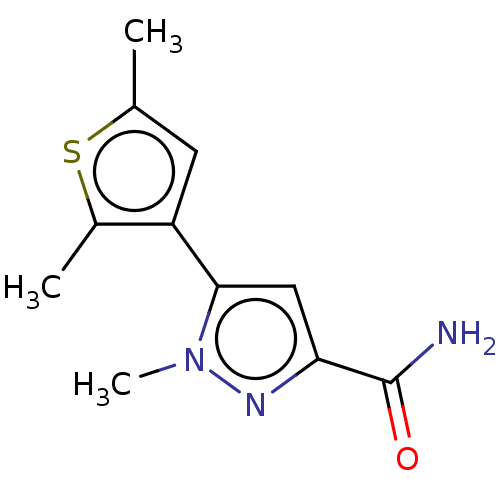
(CHEMBL3337983)Show InChI InChI=1S/C11H13N3OS/c1-6-4-8(7(2)16-6)10-5-9(11(12)15)13-14(10)3/h4-5H,1-3H3,(H2,12,15) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 851 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of human sPLA2X using 1,2-bis(heptanoylthio) glycerophosphocholine substrate incubated for 30 mins |
Bioorg Med Chem Lett 24: 5251-5 (2014)
Article DOI: 10.1016/j.bmcl.2014.09.058
BindingDB Entry DOI: 10.7270/Q2668FS8 |
More data for this
Ligand-Target Pair | |
Group 10 secretory phospholipase A2
(Homo sapiens (Human)) | BDBM50031114
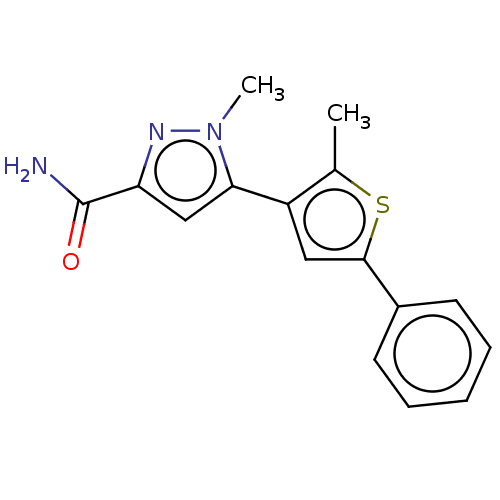
(CHEMBL3337984)Show InChI InChI=1S/C16H15N3OS/c1-10-12(14-9-13(16(17)20)18-19(14)2)8-15(21-10)11-6-4-3-5-7-11/h3-9H,1-2H3,(H2,17,20) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of human sPLA2X using 1,2-bis(heptanoylthio) glycerophosphocholine substrate incubated for 30 mins |
Bioorg Med Chem Lett 24: 5251-5 (2014)
Article DOI: 10.1016/j.bmcl.2014.09.058
BindingDB Entry DOI: 10.7270/Q2668FS8 |
More data for this
Ligand-Target Pair | |
Group 10 secretory phospholipase A2
(Homo sapiens (Human)) | BDBM50031101
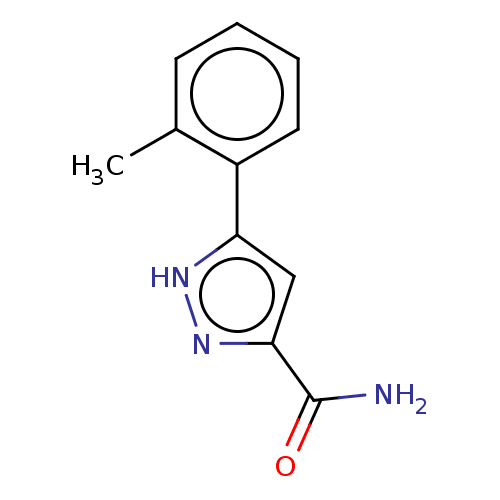
(CHEMBL3337980)Show InChI InChI=1S/C11H11N3O/c1-7-4-2-3-5-8(7)9-6-10(11(12)15)14-13-9/h2-6H,1H3,(H2,12,15)(H,13,14) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.75E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of human sPLA2X using 1,2-bis(heptanoylthio) glycerophosphocholine substrate incubated for 30 mins |
Bioorg Med Chem Lett 24: 5251-5 (2014)
Article DOI: 10.1016/j.bmcl.2014.09.058
BindingDB Entry DOI: 10.7270/Q2668FS8 |
More data for this
Ligand-Target Pair | |
Group 10 secretory phospholipase A2
(Homo sapiens (Human)) | BDBM50031100
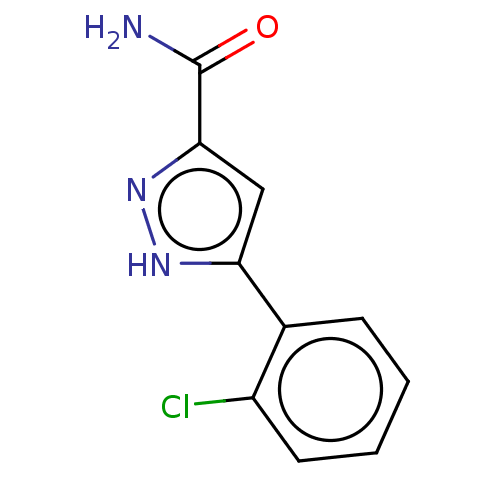
(CHEMBL3337981)Show InChI InChI=1S/C10H8ClN3O/c11-7-4-2-1-3-6(7)8-5-9(10(12)15)14-13-8/h1-5H,(H2,12,15)(H,13,14) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 9.33E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of human sPLA2X using 1,2-bis(heptanoylthio) glycerophosphocholine substrate incubated for 30 mins |
Bioorg Med Chem Lett 24: 5251-5 (2014)
Article DOI: 10.1016/j.bmcl.2014.09.058
BindingDB Entry DOI: 10.7270/Q2668FS8 |
More data for this
Ligand-Target Pair | |
Group 10 secretory phospholipase A2
(Homo sapiens (Human)) | BDBM50031107

(CHEMBL3337972)Show InChI InChI=1S/C10H11N3OS/c1-5-3-7(6(2)15-5)8-4-9(10(11)14)13-12-8/h3-4H,1-2H3,(H2,11,14)(H,12,13) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Binding affinity to sPLA2X (unknown origin) by NMR spectroscopy based displacement assay |
Bioorg Med Chem Lett 24: 5251-5 (2014)
Article DOI: 10.1016/j.bmcl.2014.09.058
BindingDB Entry DOI: 10.7270/Q2668FS8 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Group 10 secretory phospholipase A2
(Homo sapiens (Human)) | BDBM50031111
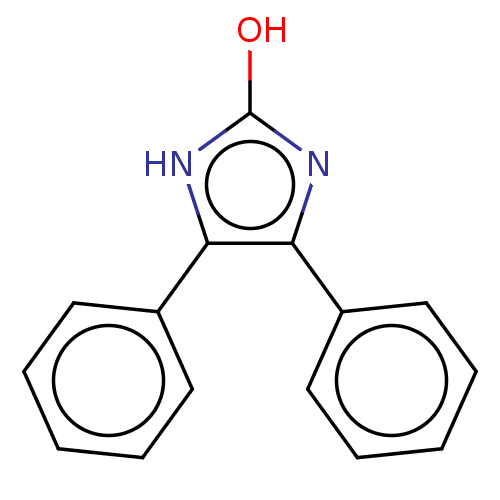
(CHEMBL1527011)Show InChI InChI=1S/C15H12N2O/c18-15-16-13(11-7-3-1-4-8-11)14(17-15)12-9-5-2-6-10-12/h1-10H,(H2,16,17,18) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 4.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Binding affinity to sPLA2X (unknown origin) by NMR spectroscopy based displacement assay |
Bioorg Med Chem Lett 24: 5251-5 (2014)
Article DOI: 10.1016/j.bmcl.2014.09.058
BindingDB Entry DOI: 10.7270/Q2668FS8 |
More data for this
Ligand-Target Pair | |
Group 10 secretory phospholipase A2
(Homo sapiens (Human)) | BDBM50031110
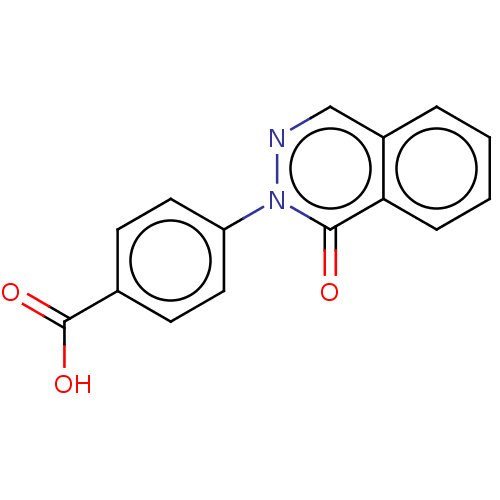
(CHEMBL3337973)Show InChI InChI=1S/C15H10N2O3/c18-14-13-4-2-1-3-11(13)9-16-17(14)12-7-5-10(6-8-12)15(19)20/h1-9H,(H,19,20) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Binding affinity to sPLA2X (unknown origin) by NMR spectroscopy based displacement assay |
Bioorg Med Chem Lett 24: 5251-5 (2014)
Article DOI: 10.1016/j.bmcl.2014.09.058
BindingDB Entry DOI: 10.7270/Q2668FS8 |
More data for this
Ligand-Target Pair | |
Group 10 secretory phospholipase A2
(Homo sapiens (Human)) | BDBM50031109

(CHEMBL3337974)Show InChI InChI=1S/C12H9NO3S/c14-12(15)9-6-17-11(13-9)8-1-2-10-7(5-8)3-4-16-10/h1-2,5-6H,3-4H2,(H,14,15) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 7.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Binding affinity to sPLA2X (unknown origin) by NMR spectroscopy based displacement assay |
Bioorg Med Chem Lett 24: 5251-5 (2014)
Article DOI: 10.1016/j.bmcl.2014.09.058
BindingDB Entry DOI: 10.7270/Q2668FS8 |
More data for this
Ligand-Target Pair | |
Group 10 secretory phospholipase A2
(Homo sapiens (Human)) | BDBM50055804
(2-(1-Benzyl-6-methoxy-2-methyl-1H-indol-3-yl)-2-hy...)Show InChI InChI=1S/C19H20N2O3/c1-12-17(18(22)19(20)23)15-9-8-14(24-2)10-16(15)21(12)11-13-6-4-3-5-7-13/h3-10,18,22H,11H2,1-2H3,(H2,20,23) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2.50E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Binding affinity to sPLA2X (unknown origin) |
Bioorg Med Chem Lett 24: 5251-5 (2014)
Article DOI: 10.1016/j.bmcl.2014.09.058
BindingDB Entry DOI: 10.7270/Q2668FS8 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data