

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cytochrome P450 1A2 (Homo sapiens (Human)) | BDBM50081468 (CHEMBL3422028) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 56 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Inhibition of human recombinant CYP1A2 assessed as metabolism of 3-cyano-7-ethoxycoumarin after 30 mins by fluorescence assay | J Med Chem 58: 2623-48 (2015) Article DOI: 10.1021/jm501218e BindingDB Entry DOI: 10.7270/Q2TX3H32 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 1A2 (Homo sapiens (Human)) | BDBM50435005 (CHEMBL2386285) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 76 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Inhibition of human recombinant CYP1A2 assessed as metabolism of 3-cyano-7-ethoxycoumarin after 30 mins by fluorescence assay | J Med Chem 58: 2623-48 (2015) Article DOI: 10.1021/jm501218e BindingDB Entry DOI: 10.7270/Q2TX3H32 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 3A4 (Homo sapiens (Human)) | BDBM50435005 (CHEMBL2386285) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 375 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Inhibition of human recombinant CYP3A4 assessed as metabolism of 7-benzyloxy-4-trifluoromethylcoumarin to HFC after 30 mins by fluorescence assay | J Med Chem 58: 2623-48 (2015) Article DOI: 10.1021/jm501218e BindingDB Entry DOI: 10.7270/Q2TX3H32 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2D6 (Homo sapiens (Human)) | BDBM50081468 (CHEMBL3422028) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 423 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Inhibition of human recombinant CYP2D6 after 30 mins by fluorescence assay | J Med Chem 58: 2623-48 (2015) Article DOI: 10.1021/jm501218e BindingDB Entry DOI: 10.7270/Q2TX3H32 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2A6 (Homo sapiens (Human)) | BDBM50081468 (CHEMBL3422028) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 710 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Inhibition of human recombinant CYP2A6 assessed as metabolism of coumarin to 7-hydroxycoumarin after 30 mins by fluorescence assay | J Med Chem 58: 2623-48 (2015) Article DOI: 10.1021/jm501218e BindingDB Entry DOI: 10.7270/Q2TX3H32 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 3A5 (Homo sapiens (Human)) | BDBM50435005 (CHEMBL2386285) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 829 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Inhibition of human recombinant CYP3A5 assessed as metabolism of 7-benzyloxy-4-trifluoromethylcoumarin to HFC after 30 mins by fluorescence assay | J Med Chem 58: 2623-48 (2015) Article DOI: 10.1021/jm501218e BindingDB Entry DOI: 10.7270/Q2TX3H32 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 3A5 (Homo sapiens (Human)) | BDBM50081468 (CHEMBL3422028) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 855 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Inhibition of human recombinant CYP3A5 assessed as metabolism of 7-benzyloxy-4-trifluoromethylcoumarin to HFC after 30 mins by fluorescence assay | J Med Chem 58: 2623-48 (2015) Article DOI: 10.1021/jm501218e BindingDB Entry DOI: 10.7270/Q2TX3H32 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 3A4 (Homo sapiens (Human)) | BDBM50081468 (CHEMBL3422028) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.64E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Inhibition of human recombinant CYP3A4 assessed as metabolism of 7-benzyloxy-4-trifluoromethylcoumarin to HFC after 30 mins by fluorescence assay | J Med Chem 58: 2623-48 (2015) Article DOI: 10.1021/jm501218e BindingDB Entry DOI: 10.7270/Q2TX3H32 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2A6 (Homo sapiens (Human)) | BDBM50435005 (CHEMBL2386285) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.18E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Inhibition of human recombinant CYP2A6 assessed as metabolism of coumarin to 7-hydroxycoumarin after 30 mins by fluorescence assay | J Med Chem 58: 2623-48 (2015) Article DOI: 10.1021/jm501218e BindingDB Entry DOI: 10.7270/Q2TX3H32 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM13061 (4,4 -(1H-1,2,4-triazol-1-ylmethanediyl)dibenzonitr...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid UniChem Patents Similars | DrugBank Article PubMed | n/a | n/a | 5.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Inhibition of human recombinant aromatase using 7-methoxy-trifluoromethylcoumarin as substrate assessed as formation of fluorescent metabolite after ... | J Med Chem 58: 2623-48 (2015) Article DOI: 10.1021/jm501218e BindingDB Entry DOI: 10.7270/Q2TX3H32 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM50081410 (CHEMBL3422041) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 17 | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Inhibition of human recombinant aromatase using 7-methoxy-trifluoromethylcoumarin as substrate assessed as formation of fluorescent metabolite after ... | J Med Chem 58: 2623-48 (2015) Article DOI: 10.1021/jm501218e BindingDB Entry DOI: 10.7270/Q2TX3H32 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM50081468 (CHEMBL3422028) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 45 | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Inhibition of human recombinant aromatase using 7-methoxy-trifluoromethylcoumarin as substrate assessed as formation of fluorescent metabolite after ... | J Med Chem 58: 2623-48 (2015) Article DOI: 10.1021/jm501218e BindingDB Entry DOI: 10.7270/Q2TX3H32 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM50081463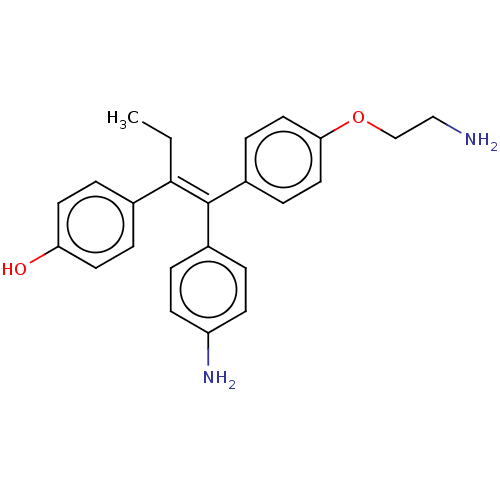 (CHEMBL3422033) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 48 | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Inhibition of human recombinant aromatase using 7-methoxy-trifluoromethylcoumarin as substrate assessed as formation of fluorescent metabolite after ... | J Med Chem 58: 2623-48 (2015) Article DOI: 10.1021/jm501218e BindingDB Entry DOI: 10.7270/Q2TX3H32 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM50081416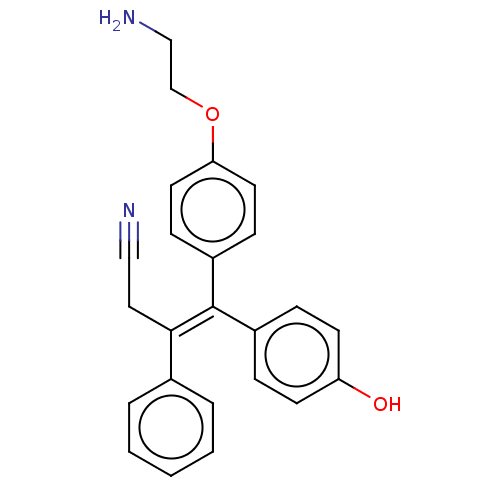 (CHEMBL3422040) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 49 | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Inhibition of human recombinant aromatase using 7-methoxy-trifluoromethylcoumarin as substrate assessed as formation of fluorescent metabolite after ... | J Med Chem 58: 2623-48 (2015) Article DOI: 10.1021/jm501218e BindingDB Entry DOI: 10.7270/Q2TX3H32 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM50081464 (CHEMBL3422032) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 53 | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Inhibition of human recombinant aromatase using 7-methoxy-trifluoromethylcoumarin as substrate assessed as formation of fluorescent metabolite after ... | J Med Chem 58: 2623-48 (2015) Article DOI: 10.1021/jm501218e BindingDB Entry DOI: 10.7270/Q2TX3H32 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM50435005 (CHEMBL2386285) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 102 | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Inhibition of human recombinant aromatase using 7-methoxy-trifluoromethylcoumarin as substrate assessed as formation of fluorescent metabolite after ... | J Med Chem 58: 2623-48 (2015) Article DOI: 10.1021/jm501218e BindingDB Entry DOI: 10.7270/Q2TX3H32 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM50081432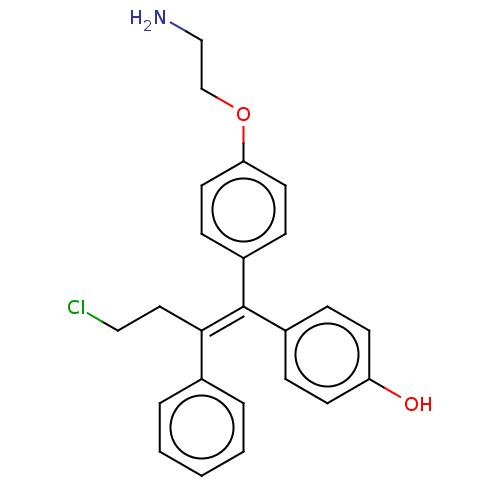 (CHEMBL3422036) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 143 | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Inhibition of human recombinant aromatase using 7-methoxy-trifluoromethylcoumarin as substrate assessed as formation of fluorescent metabolite after ... | J Med Chem 58: 2623-48 (2015) Article DOI: 10.1021/jm501218e BindingDB Entry DOI: 10.7270/Q2TX3H32 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM50081408 (CHEMBL3422042) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 174 | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Inhibition of human recombinant aromatase using 7-methoxy-trifluoromethylcoumarin as substrate assessed as formation of fluorescent metabolite after ... | J Med Chem 58: 2623-48 (2015) Article DOI: 10.1021/jm501218e BindingDB Entry DOI: 10.7270/Q2TX3H32 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 1A2 (Homo sapiens (Human)) | BDBM50435005 (CHEMBL2386285) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 207 | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Inhibition of human recombinant CYP1A2 assessed as metabolism of 3-cyano-7-ethoxycoumarin after 30 mins by fluorescence assay | J Med Chem 58: 2623-48 (2015) Article DOI: 10.1021/jm501218e BindingDB Entry DOI: 10.7270/Q2TX3H32 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 1A2 (Homo sapiens (Human)) | BDBM50081468 (CHEMBL3422028) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 207 | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Inhibition of human recombinant CYP1A2 assessed as metabolism of 3-cyano-7-ethoxycoumarin after 30 mins by fluorescence assay | J Med Chem 58: 2623-48 (2015) Article DOI: 10.1021/jm501218e BindingDB Entry DOI: 10.7270/Q2TX3H32 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM50081430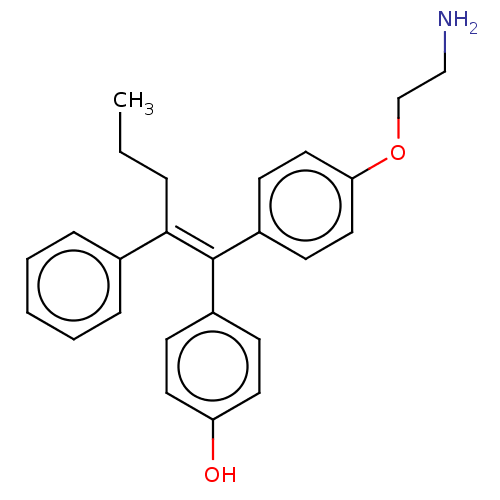 (CHEMBL3422037) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 261 | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Inhibition of human recombinant aromatase using 7-methoxy-trifluoromethylcoumarin as substrate assessed as formation of fluorescent metabolite after ... | J Med Chem 58: 2623-48 (2015) Article DOI: 10.1021/jm501218e BindingDB Entry DOI: 10.7270/Q2TX3H32 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 3A4 (Homo sapiens (Human)) | BDBM50435005 (CHEMBL2386285) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 285 | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Inhibition of human recombinant CYP3A4 assessed as metabolism of 7-benzyloxy-4-trifluoromethylcoumarin to HFC after 30 mins by fluorescence assay | J Med Chem 58: 2623-48 (2015) Article DOI: 10.1021/jm501218e BindingDB Entry DOI: 10.7270/Q2TX3H32 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM50081421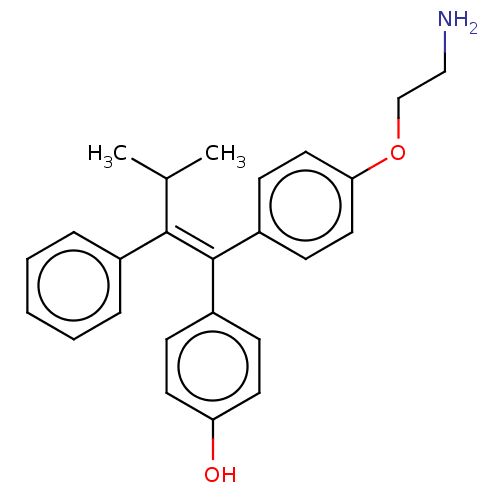 (CHEMBL3422038) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 328 | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Inhibition of human recombinant aromatase using 7-methoxy-trifluoromethylcoumarin as substrate assessed as formation of fluorescent metabolite after ... | J Med Chem 58: 2623-48 (2015) Article DOI: 10.1021/jm501218e BindingDB Entry DOI: 10.7270/Q2TX3H32 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM50081433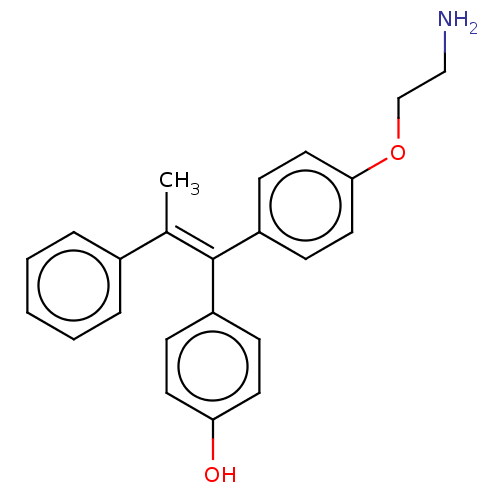 (CHEMBL3422035) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 337 | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Inhibition of human recombinant aromatase using 7-methoxy-trifluoromethylcoumarin as substrate assessed as formation of fluorescent metabolite after ... | J Med Chem 58: 2623-48 (2015) Article DOI: 10.1021/jm501218e BindingDB Entry DOI: 10.7270/Q2TX3H32 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM50081536 (CHEMBL3422052) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 491 | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Inhibition of human recombinant aromatase using 7-methoxy-trifluoromethylcoumarin as substrate assessed as formation of fluorescent metabolite after ... | J Med Chem 58: 2623-48 (2015) Article DOI: 10.1021/jm501218e BindingDB Entry DOI: 10.7270/Q2TX3H32 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM50081469 (CHEMBL3422045) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 493 | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Inhibition of human recombinant aromatase using 7-methoxy-trifluoromethylcoumarin as substrate assessed as formation of fluorescent metabolite after ... | J Med Chem 58: 2623-48 (2015) Article DOI: 10.1021/jm501218e BindingDB Entry DOI: 10.7270/Q2TX3H32 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 3A5 (Homo sapiens (Human)) | BDBM50081468 (CHEMBL3422028) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 556 | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Inhibition of human recombinant CYP3A5 assessed as metabolism of 7-benzyloxy-4-trifluoromethylcoumarin to HFC after 30 mins by fluorescence assay | J Med Chem 58: 2623-48 (2015) Article DOI: 10.1021/jm501218e BindingDB Entry DOI: 10.7270/Q2TX3H32 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM50081403 (CHEMBL3422044) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 699 | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Inhibition of human recombinant aromatase using 7-methoxy-trifluoromethylcoumarin as substrate assessed as formation of fluorescent metabolite after ... | J Med Chem 58: 2623-48 (2015) Article DOI: 10.1021/jm501218e BindingDB Entry DOI: 10.7270/Q2TX3H32 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM50081407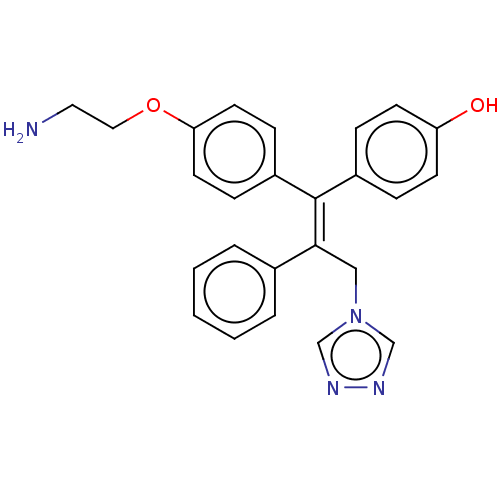 (CHEMBL3422043) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 715 | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Inhibition of human recombinant aromatase using 7-methoxy-trifluoromethylcoumarin as substrate assessed as formation of fluorescent metabolite after ... | J Med Chem 58: 2623-48 (2015) Article DOI: 10.1021/jm501218e BindingDB Entry DOI: 10.7270/Q2TX3H32 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 3A5 (Homo sapiens (Human)) | BDBM50435005 (CHEMBL2386285) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 723 | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Inhibition of human recombinant CYP3A5 assessed as metabolism of 7-benzyloxy-4-trifluoromethylcoumarin to HFC after 30 mins by fluorescence assay | J Med Chem 58: 2623-48 (2015) Article DOI: 10.1021/jm501218e BindingDB Entry DOI: 10.7270/Q2TX3H32 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM50081465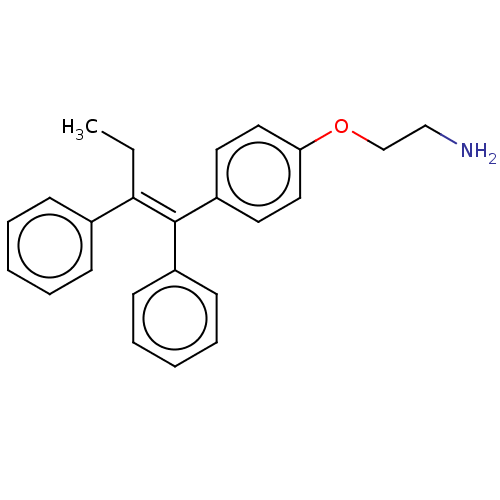 (CHEMBL3422031) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 913 | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Inhibition of human recombinant aromatase using 7-methoxy-trifluoromethylcoumarin as substrate assessed as formation of fluorescent metabolite after ... | J Med Chem 58: 2623-48 (2015) Article DOI: 10.1021/jm501218e BindingDB Entry DOI: 10.7270/Q2TX3H32 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM50081467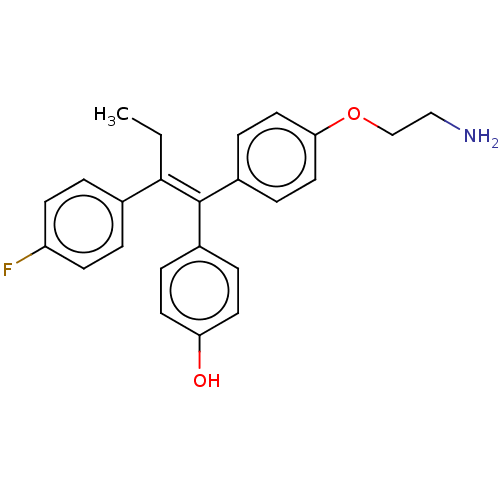 (CHEMBL3422029) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.02E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Inhibition of human recombinant aromatase using 7-methoxy-trifluoromethylcoumarin as substrate assessed as formation of fluorescent metabolite after ... | J Med Chem 58: 2623-48 (2015) Article DOI: 10.1021/jm501218e BindingDB Entry DOI: 10.7270/Q2TX3H32 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM50081530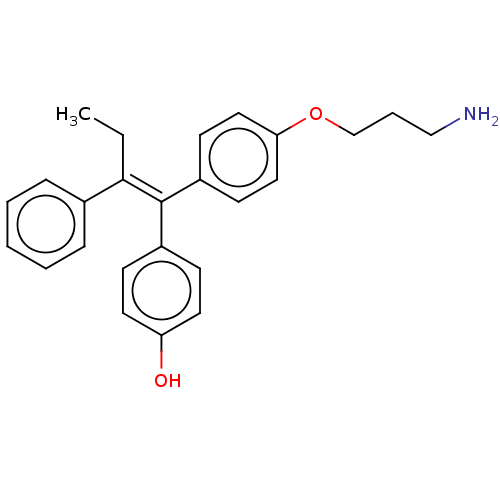 (CHEMBL3422050) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.24E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Inhibition of human recombinant aromatase using 7-methoxy-trifluoromethylcoumarin as substrate assessed as formation of fluorescent metabolite after ... | J Med Chem 58: 2623-48 (2015) Article DOI: 10.1021/jm501218e BindingDB Entry DOI: 10.7270/Q2TX3H32 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM50081466 (CHEMBL3422030) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.32E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Inhibition of human recombinant aromatase using 7-methoxy-trifluoromethylcoumarin as substrate assessed as formation of fluorescent metabolite after ... | J Med Chem 58: 2623-48 (2015) Article DOI: 10.1021/jm501218e BindingDB Entry DOI: 10.7270/Q2TX3H32 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 3A4 (Homo sapiens (Human)) | BDBM50081468 (CHEMBL3422028) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.88E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Inhibition of human recombinant CYP3A4 assessed as metabolism of 7-benzyloxy-4-trifluoromethylcoumarin to HFC after 30 mins by fluorescence assay | J Med Chem 58: 2623-48 (2015) Article DOI: 10.1021/jm501218e BindingDB Entry DOI: 10.7270/Q2TX3H32 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM50081418 (CHEMBL3422039) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.93E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Inhibition of human recombinant aromatase using 7-methoxy-trifluoromethylcoumarin as substrate assessed as formation of fluorescent metabolite after ... | J Med Chem 58: 2623-48 (2015) Article DOI: 10.1021/jm501218e BindingDB Entry DOI: 10.7270/Q2TX3H32 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2D6 (Homo sapiens (Human)) | BDBM50081468 (CHEMBL3422028) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Inhibition of human recombinant CYP2D6 after 30 mins by fluorescence assay | J Med Chem 58: 2623-48 (2015) Article DOI: 10.1021/jm501218e BindingDB Entry DOI: 10.7270/Q2TX3H32 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM50081471 (CHEMBL3422047) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.68E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Inhibition of human recombinant aromatase using 7-methoxy-trifluoromethylcoumarin as substrate assessed as formation of fluorescent metabolite after ... | J Med Chem 58: 2623-48 (2015) Article DOI: 10.1021/jm501218e BindingDB Entry DOI: 10.7270/Q2TX3H32 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM50081462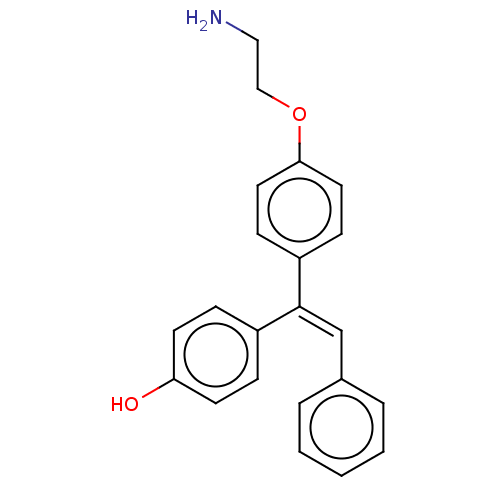 (CHEMBL3422034) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6.29E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Inhibition of human recombinant aromatase using 7-methoxy-trifluoromethylcoumarin as substrate assessed as formation of fluorescent metabolite after ... | J Med Chem 58: 2623-48 (2015) Article DOI: 10.1021/jm501218e BindingDB Entry DOI: 10.7270/Q2TX3H32 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2A6 (Homo sapiens (Human)) | BDBM50435005 (CHEMBL2386285) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6.37E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Inhibition of human recombinant CYP2A6 assessed as metabolism of coumarin to 7-hydroxycoumarin after 30 mins by fluorescence assay | J Med Chem 58: 2623-48 (2015) Article DOI: 10.1021/jm501218e BindingDB Entry DOI: 10.7270/Q2TX3H32 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2A6 (Homo sapiens (Human)) | BDBM50081468 (CHEMBL3422028) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6.37E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Inhibition of human recombinant CYP2A6 assessed as metabolism of coumarin to 7-hydroxycoumarin after 30 mins by fluorescence assay | J Med Chem 58: 2623-48 (2015) Article DOI: 10.1021/jm501218e BindingDB Entry DOI: 10.7270/Q2TX3H32 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM50081535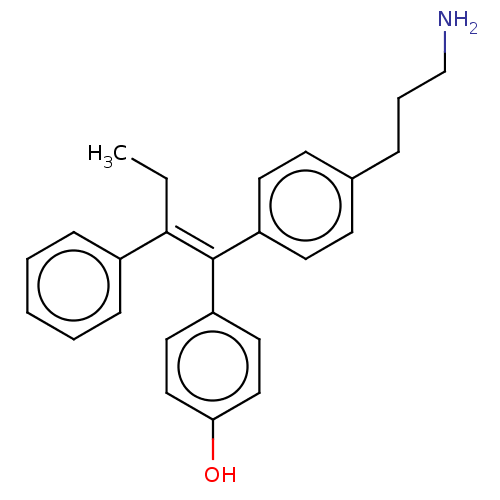 (CHEMBL3422051) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 7.34E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Inhibition of human recombinant aromatase using 7-methoxy-trifluoromethylcoumarin as substrate assessed as formation of fluorescent metabolite after ... | J Med Chem 58: 2623-48 (2015) Article DOI: 10.1021/jm501218e BindingDB Entry DOI: 10.7270/Q2TX3H32 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM50081470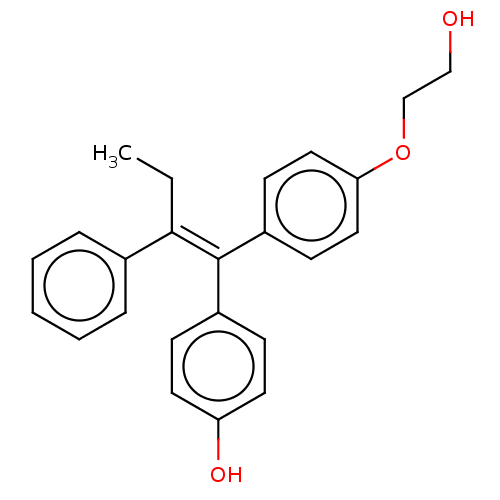 (CHEMBL3422046) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.05E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Inhibition of human recombinant aromatase using 7-methoxy-trifluoromethylcoumarin as substrate assessed as formation of fluorescent metabolite after ... | J Med Chem 58: 2623-48 (2015) Article DOI: 10.1021/jm501218e BindingDB Entry DOI: 10.7270/Q2TX3H32 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor beta (Homo sapiens (Human)) | BDBM50081464 (CHEMBL3422032) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 182 | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Displacement of fluorescein-labeled ES2 from human recombinant ERbeta receptor after 2 hrs by fluorescence polarization assay | J Med Chem 58: 2623-48 (2015) Article DOI: 10.1021/jm501218e BindingDB Entry DOI: 10.7270/Q2TX3H32 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor beta (Homo sapiens (Human)) | BDBM50081536 (CHEMBL3422052) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 293 | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Displacement of fluorescein-labeled ES2 from human recombinant ERbeta receptor after 2 hrs by fluorescence polarization assay | J Med Chem 58: 2623-48 (2015) Article DOI: 10.1021/jm501218e BindingDB Entry DOI: 10.7270/Q2TX3H32 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor beta (Homo sapiens (Human)) | BDBM50435005 (CHEMBL2386285) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 35 | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Displacement of fluorescein-labeled ES2 from human recombinant ERbeta receptor after 2 hrs by fluorescence polarization assay | J Med Chem 58: 2623-48 (2015) Article DOI: 10.1021/jm501218e BindingDB Entry DOI: 10.7270/Q2TX3H32 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor beta (Homo sapiens (Human)) | BDBM50081530 (CHEMBL3422050) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 21 | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Displacement of fluorescein-labeled ES2 from human recombinant ERbeta receptor after 2 hrs by fluorescence polarization assay | J Med Chem 58: 2623-48 (2015) Article DOI: 10.1021/jm501218e BindingDB Entry DOI: 10.7270/Q2TX3H32 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor beta (Homo sapiens (Human)) | BDBM50081403 (CHEMBL3422044) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 58 | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Displacement of fluorescein-labeled ES2 from human recombinant ERbeta receptor after 2 hrs by fluorescence polarization assay | J Med Chem 58: 2623-48 (2015) Article DOI: 10.1021/jm501218e BindingDB Entry DOI: 10.7270/Q2TX3H32 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor beta (Homo sapiens (Human)) | BDBM50081463 (CHEMBL3422033) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 50 | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Displacement of fluorescein-labeled ES2 from human recombinant ERbeta receptor after 2 hrs by fluorescence polarization assay | J Med Chem 58: 2623-48 (2015) Article DOI: 10.1021/jm501218e BindingDB Entry DOI: 10.7270/Q2TX3H32 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor beta (Homo sapiens (Human)) | BDBM50081432 (CHEMBL3422036) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 52 | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Displacement of fluorescein-labeled ES2 from human recombinant ERbeta receptor after 2 hrs by fluorescence polarization assay | J Med Chem 58: 2623-48 (2015) Article DOI: 10.1021/jm501218e BindingDB Entry DOI: 10.7270/Q2TX3H32 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor beta (Homo sapiens (Human)) | BDBM50081407 (CHEMBL3422043) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 48 | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Displacement of fluorescein-labeled ES2 from human recombinant ERbeta receptor after 2 hrs by fluorescence polarization assay | J Med Chem 58: 2623-48 (2015) Article DOI: 10.1021/jm501218e BindingDB Entry DOI: 10.7270/Q2TX3H32 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor (Homo sapiens (Human)) | BDBM50081407 (CHEMBL3422043) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 57 | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Displacement of fluorescein-labeled ES2 from human recombinant ERalpha receptor after 2 hrs by fluorescence polarization assay | J Med Chem 58: 2623-48 (2015) Article DOI: 10.1021/jm501218e BindingDB Entry DOI: 10.7270/Q2TX3H32 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor (Homo sapiens (Human)) | BDBM50435005 (CHEMBL2386285) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 27 | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Displacement of fluorescein-labeled ES2 from human recombinant ERalpha receptor after 2 hrs by fluorescence polarization assay | J Med Chem 58: 2623-48 (2015) Article DOI: 10.1021/jm501218e BindingDB Entry DOI: 10.7270/Q2TX3H32 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor (Homo sapiens (Human)) | BDBM50081408 (CHEMBL3422042) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 290 | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Displacement of fluorescein-labeled ES2 from human recombinant ERalpha receptor after 2 hrs by fluorescence polarization assay | J Med Chem 58: 2623-48 (2015) Article DOI: 10.1021/jm501218e BindingDB Entry DOI: 10.7270/Q2TX3H32 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor (Homo sapiens (Human)) | BDBM50081433 (CHEMBL3422035) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 675 | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Displacement of fluorescein-labeled ES2 from human recombinant ERalpha receptor after 2 hrs by fluorescence polarization assay | J Med Chem 58: 2623-48 (2015) Article DOI: 10.1021/jm501218e BindingDB Entry DOI: 10.7270/Q2TX3H32 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor (Homo sapiens (Human)) | BDBM50081432 (CHEMBL3422036) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 265 | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Displacement of fluorescein-labeled ES2 from human recombinant ERalpha receptor after 2 hrs by fluorescence polarization assay | J Med Chem 58: 2623-48 (2015) Article DOI: 10.1021/jm501218e BindingDB Entry DOI: 10.7270/Q2TX3H32 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor (Homo sapiens (Human)) | BDBM50081536 (CHEMBL3422052) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 1.92E+3 | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Displacement of fluorescein-labeled ES2 from human recombinant ERalpha receptor after 2 hrs by fluorescence polarization assay | J Med Chem 58: 2623-48 (2015) Article DOI: 10.1021/jm501218e BindingDB Entry DOI: 10.7270/Q2TX3H32 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor beta (Homo sapiens (Human)) | BDBM50081462 (CHEMBL3422034) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 20 | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Displacement of fluorescein-labeled ES2 from human recombinant ERbeta receptor after 2 hrs by fluorescence polarization assay | J Med Chem 58: 2623-48 (2015) Article DOI: 10.1021/jm501218e BindingDB Entry DOI: 10.7270/Q2TX3H32 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor (Homo sapiens (Human)) | BDBM50081462 (CHEMBL3422034) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 1.05E+3 | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Displacement of fluorescein-labeled ES2 from human recombinant ERalpha receptor after 2 hrs by fluorescence polarization assay | J Med Chem 58: 2623-48 (2015) Article DOI: 10.1021/jm501218e BindingDB Entry DOI: 10.7270/Q2TX3H32 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor (Homo sapiens (Human)) | BDBM50081430 (CHEMBL3422037) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 384 | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Displacement of fluorescein-labeled ES2 from human recombinant ERalpha receptor after 2 hrs by fluorescence polarization assay | J Med Chem 58: 2623-48 (2015) Article DOI: 10.1021/jm501218e BindingDB Entry DOI: 10.7270/Q2TX3H32 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor beta (Homo sapiens (Human)) | BDBM50081418 (CHEMBL3422039) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 478 | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Displacement of fluorescein-labeled ES2 from human recombinant ERbeta receptor after 2 hrs by fluorescence polarization assay | J Med Chem 58: 2623-48 (2015) Article DOI: 10.1021/jm501218e BindingDB Entry DOI: 10.7270/Q2TX3H32 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor (Homo sapiens (Human)) | BDBM50081467 (CHEMBL3422029) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 88 | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Displacement of fluorescein-labeled ES2 from human recombinant ERalpha receptor after 2 hrs by fluorescence polarization assay | J Med Chem 58: 2623-48 (2015) Article DOI: 10.1021/jm501218e BindingDB Entry DOI: 10.7270/Q2TX3H32 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor (Homo sapiens (Human)) | BDBM50081466 (CHEMBL3422030) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 277 | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Displacement of fluorescein-labeled ES2 from human recombinant ERalpha receptor after 2 hrs by fluorescence polarization assay | J Med Chem 58: 2623-48 (2015) Article DOI: 10.1021/jm501218e BindingDB Entry DOI: 10.7270/Q2TX3H32 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor (Homo sapiens (Human)) | BDBM50081418 (CHEMBL3422039) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 653 | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Displacement of fluorescein-labeled ES2 from human recombinant ERalpha receptor after 2 hrs by fluorescence polarization assay | J Med Chem 58: 2623-48 (2015) Article DOI: 10.1021/jm501218e BindingDB Entry DOI: 10.7270/Q2TX3H32 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor beta (Homo sapiens (Human)) | BDBM50081433 (CHEMBL3422035) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 28 | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Displacement of fluorescein-labeled ES2 from human recombinant ERbeta receptor after 2 hrs by fluorescence polarization assay | J Med Chem 58: 2623-48 (2015) Article DOI: 10.1021/jm501218e BindingDB Entry DOI: 10.7270/Q2TX3H32 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor beta (Homo sapiens (Human)) | BDBM50081408 (CHEMBL3422042) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 519 | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Displacement of fluorescein-labeled ES2 from human recombinant ERbeta receptor after 2 hrs by fluorescence polarization assay | J Med Chem 58: 2623-48 (2015) Article DOI: 10.1021/jm501218e BindingDB Entry DOI: 10.7270/Q2TX3H32 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor beta (Homo sapiens (Human)) | BDBM50081469 (CHEMBL3422045) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 1.35E+3 | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Displacement of fluorescein-labeled ES2 from human recombinant ERbeta receptor after 2 hrs by fluorescence polarization assay | J Med Chem 58: 2623-48 (2015) Article DOI: 10.1021/jm501218e BindingDB Entry DOI: 10.7270/Q2TX3H32 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor (Homo sapiens (Human)) | BDBM50081463 (CHEMBL3422033) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 144 | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Displacement of fluorescein-labeled ES2 from human recombinant ERalpha receptor after 2 hrs by fluorescence polarization assay | J Med Chem 58: 2623-48 (2015) Article DOI: 10.1021/jm501218e BindingDB Entry DOI: 10.7270/Q2TX3H32 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor (Homo sapiens (Human)) | BDBM50081464 (CHEMBL3422032) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 274 | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Displacement of fluorescein-labeled ES2 from human recombinant ERalpha receptor after 2 hrs by fluorescence polarization assay | J Med Chem 58: 2623-48 (2015) Article DOI: 10.1021/jm501218e BindingDB Entry DOI: 10.7270/Q2TX3H32 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor (Homo sapiens (Human)) | BDBM50081403 (CHEMBL3422044) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 176 | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Displacement of fluorescein-labeled ES2 from human recombinant ERalpha receptor after 2 hrs by fluorescence polarization assay | J Med Chem 58: 2623-48 (2015) Article DOI: 10.1021/jm501218e BindingDB Entry DOI: 10.7270/Q2TX3H32 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor (Homo sapiens (Human)) | BDBM50081535 (CHEMBL3422051) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 27 | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Displacement of fluorescein-labeled ES2 from human recombinant ERalpha receptor after 2 hrs by fluorescence polarization assay | J Med Chem 58: 2623-48 (2015) Article DOI: 10.1021/jm501218e BindingDB Entry DOI: 10.7270/Q2TX3H32 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor beta (Homo sapiens (Human)) | BDBM50081468 (CHEMBL3422028) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 9.5 | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Displacement of fluorescein-labeled ES2 from human recombinant ERbeta receptor after 2 hrs by fluorescence polarization assay | J Med Chem 58: 2623-48 (2015) Article DOI: 10.1021/jm501218e BindingDB Entry DOI: 10.7270/Q2TX3H32 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor beta (Homo sapiens (Human)) | BDBM50081467 (CHEMBL3422029) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 49 | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Displacement of fluorescein-labeled ES2 from human recombinant ERbeta receptor after 2 hrs by fluorescence polarization assay | J Med Chem 58: 2623-48 (2015) Article DOI: 10.1021/jm501218e BindingDB Entry DOI: 10.7270/Q2TX3H32 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor beta (Homo sapiens (Human)) | BDBM50081430 (CHEMBL3422037) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 37 | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Displacement of fluorescein-labeled ES2 from human recombinant ERbeta receptor after 2 hrs by fluorescence polarization assay | J Med Chem 58: 2623-48 (2015) Article DOI: 10.1021/jm501218e BindingDB Entry DOI: 10.7270/Q2TX3H32 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor beta (Homo sapiens (Human)) | BDBM50081535 (CHEMBL3422051) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 13 | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Displacement of fluorescein-labeled ES2 from human recombinant ERbeta receptor after 2 hrs by fluorescence polarization assay | J Med Chem 58: 2623-48 (2015) Article DOI: 10.1021/jm501218e BindingDB Entry DOI: 10.7270/Q2TX3H32 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor (Homo sapiens (Human)) | BDBM50081468 (CHEMBL3422028) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 15 | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Displacement of fluorescein-labeled ES2 from human recombinant ERalpha receptor after 2 hrs by fluorescence polarization assay | J Med Chem 58: 2623-48 (2015) Article DOI: 10.1021/jm501218e BindingDB Entry DOI: 10.7270/Q2TX3H32 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor (Homo sapiens (Human)) | BDBM50081469 (CHEMBL3422045) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 3.18E+3 | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Displacement of fluorescein-labeled ES2 from human recombinant ERalpha receptor after 2 hrs by fluorescence polarization assay | J Med Chem 58: 2623-48 (2015) Article DOI: 10.1021/jm501218e BindingDB Entry DOI: 10.7270/Q2TX3H32 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor (Homo sapiens (Human)) | BDBM50081530 (CHEMBL3422050) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 35 | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Displacement of fluorescein-labeled ES2 from human recombinant ERalpha receptor after 2 hrs by fluorescence polarization assay | J Med Chem 58: 2623-48 (2015) Article DOI: 10.1021/jm501218e BindingDB Entry DOI: 10.7270/Q2TX3H32 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor beta (Homo sapiens (Human)) | BDBM50081466 (CHEMBL3422030) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 202 | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Displacement of fluorescein-labeled ES2 from human recombinant ERbeta receptor after 2 hrs by fluorescence polarization assay | J Med Chem 58: 2623-48 (2015) Article DOI: 10.1021/jm501218e BindingDB Entry DOI: 10.7270/Q2TX3H32 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||