

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM34233 (2-Phenyl-benzo[d]isoselenazol-3-one | 2-Phenyl-ben...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 94 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SIB Swiss Institute of Bioinformatics Curated by ChEMBL | Assay Description Inhibition of human recombinant IDO1 using L-tryptophan as substrate preincubated for 5 mins followed by substrate addition measured after 10 mins by... | J Med Chem 58: 9421-37 (2015) Article DOI: 10.1021/acs.jmedchem.5b00326 BindingDB Entry DOI: 10.7270/Q28K7D3X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM81348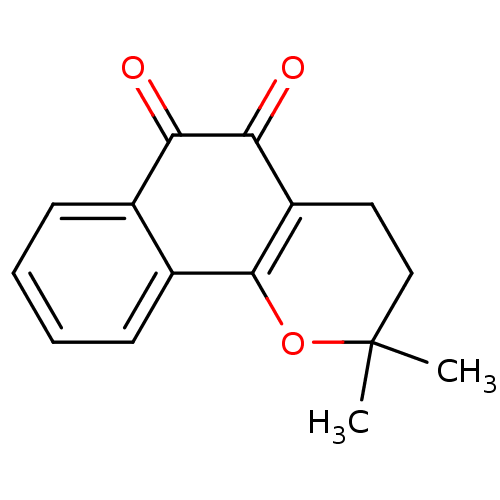 (β-Lapachone (A3) | Beta lapachone | R115 (Rea...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE KEGG PC cid PC sid UniChem Patents | Article PubMed | 100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SIB Swiss Institute of Bioinformatics Curated by ChEMBL | Assay Description Noncompetitive inhibition of human recombinant IDO1 expressed in Escherichia coli by Michaelis-Menton nonlinear regression plot analysis in presence ... | J Med Chem 58: 9421-37 (2015) Article DOI: 10.1021/acs.jmedchem.5b00326 BindingDB Entry DOI: 10.7270/Q28K7D3X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM50183456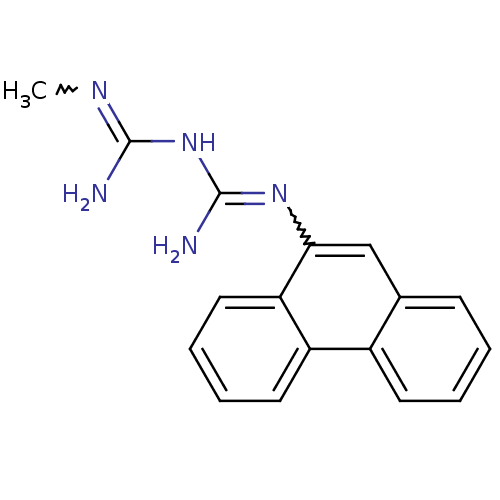 (CHEMBL425403 | N-methyl-N'-9-phenanthrylimidodicar...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | Article PubMed | 1.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SIB Swiss Institute of Bioinformatics Curated by ChEMBL | Assay Description Inhibition of human recombinant IDO1 expressed in yeast IS20-2B using tryptophan as substrate by methylene blue/ascorbate assay | J Med Chem 58: 9421-37 (2015) Article DOI: 10.1021/acs.jmedchem.5b00326 BindingDB Entry DOI: 10.7270/Q28K7D3X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM50279937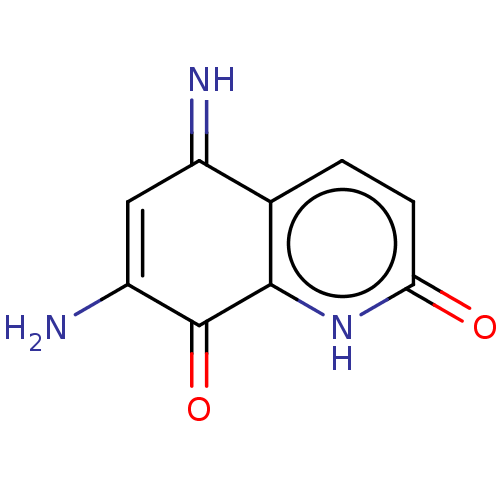 (CHEMBL1985550) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | Article PubMed | 4.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SIB Swiss Institute of Bioinformatics Curated by ChEMBL | Assay Description Inhibition of human recombinant IDO1 using L-tryptophan as substrate after 15 mins by Dixon plot analysis | J Med Chem 58: 9421-37 (2015) Article DOI: 10.1021/acs.jmedchem.5b00326 BindingDB Entry DOI: 10.7270/Q28K7D3X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM36371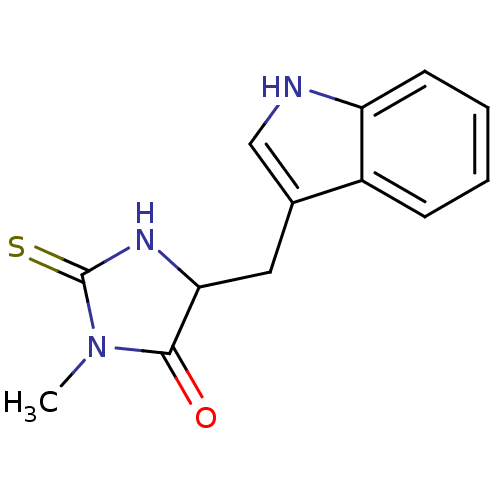 (5-(1H-indol-3-ylmethyl)-3-methyl-2-thioxo-4-Imidaz...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Patents | Article PubMed | 1.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SIB Swiss Institute of Bioinformatics Curated by ChEMBL | Assay Description Competitive inhibition of 6His-tagged human recombinant IDO expressed in Escherichia coli BL21DE3pLys | J Med Chem 58: 9421-37 (2015) Article DOI: 10.1021/acs.jmedchem.5b00326 BindingDB Entry DOI: 10.7270/Q28K7D3X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM50024210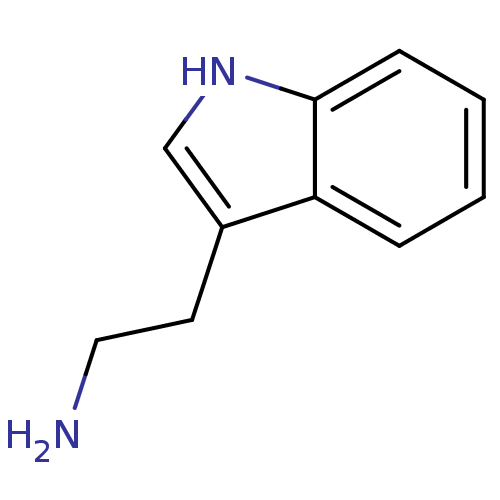 (1H-indole-3-ethanamine | 2-(1H-indol-3-yl)ethanami...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 1.60E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SIB Swiss Institute of Bioinformatics Curated by ChEMBL | Assay Description Noncompetitive inhibition of human recombinant IDO1 expressed in Escherichia coli BL21 by Lineweaver-Burk double-reciprocal plot analysis | J Med Chem 58: 9421-37 (2015) Article DOI: 10.1021/acs.jmedchem.5b00326 BindingDB Entry DOI: 10.7270/Q28K7D3X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM168435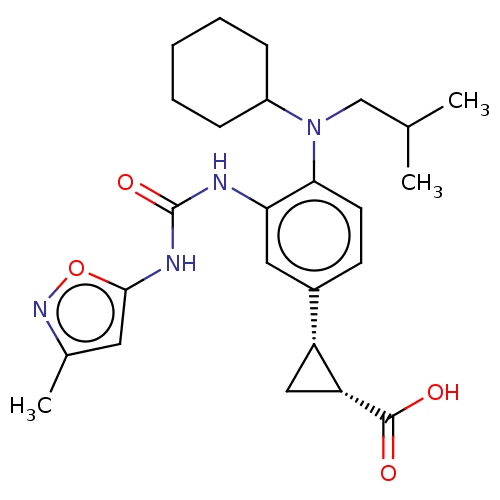 (US9675571, 129) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
SIB Swiss Institute of Bioinformatics Curated by ChEMBL | Assay Description Inhibition of human IDO1 in HEK293 cells after 20 hrs by plate reader assay | J Med Chem 58: 9421-37 (2015) Article DOI: 10.1021/acs.jmedchem.5b00326 BindingDB Entry DOI: 10.7270/Q28K7D3X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM340834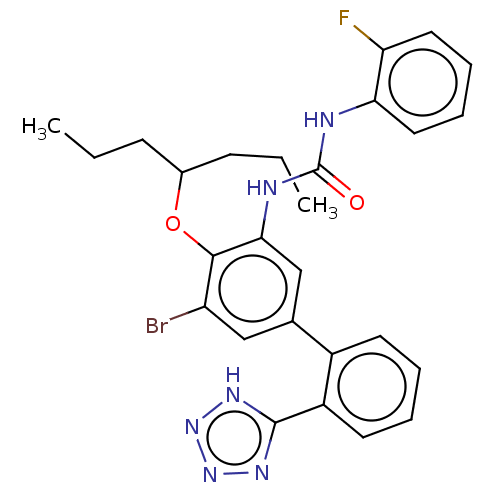 (US9765018, Example 287) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
SIB Swiss Institute of Bioinformatics Curated by ChEMBL | Assay Description Inhibition of human IDO1 in HEK293 cells after 20 hrs by plate reader assay | J Med Chem 58: 9421-37 (2015) Article DOI: 10.1021/acs.jmedchem.5b00326 BindingDB Entry DOI: 10.7270/Q28K7D3X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM317549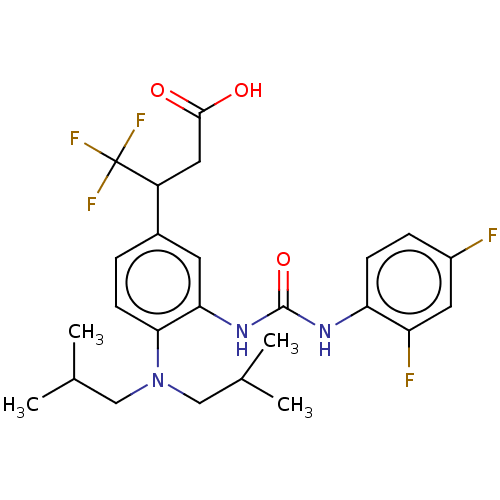 (3-(3-(3-(2,4- difluorophenyl)ureido)-4- (diisobuty...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
SIB Swiss Institute of Bioinformatics Curated by ChEMBL | Assay Description Inhibition of human IDO1 in HEK293 cells after 20 hrs by plate reader assay | J Med Chem 58: 9421-37 (2015) Article DOI: 10.1021/acs.jmedchem.5b00326 BindingDB Entry DOI: 10.7270/Q28K7D3X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM50500137 (CHEMBL3747340) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
SIB Swiss Institute of Bioinformatics Curated by ChEMBL | Assay Description Inhibition of human IDO1 in HEK293 cells after 20 hrs by plate reader assay | J Med Chem 58: 9421-37 (2015) Article DOI: 10.1021/acs.jmedchem.5b00326 BindingDB Entry DOI: 10.7270/Q28K7D3X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM50126143 (Epacadostat | INCB-024360) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | 7.10 | n/a | n/a | n/a | n/a | n/a | n/a |
SIB Swiss Institute of Bioinformatics Curated by ChEMBL | Assay Description Inhibition of IFN-gamma-stimulated IDO1 activity in human HeLa cells using L-tryptophan as substrate after 48 hrs by microplate reader analysis | J Med Chem 58: 9421-37 (2015) Article DOI: 10.1021/acs.jmedchem.5b00326 BindingDB Entry DOI: 10.7270/Q28K7D3X | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM50126143 (Epacadostat | INCB-024360) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | 72 | n/a | n/a | n/a | n/a | n/a | n/a |
SIB Swiss Institute of Bioinformatics Curated by ChEMBL | Assay Description Inhibition of N-terminal His-tagged human recombinant IDO1 expressed in Escherichia coli using D-tryptophan as substrate by methylene blue/ascorbate ... | J Med Chem 58: 9421-37 (2015) Article DOI: 10.1021/acs.jmedchem.5b00326 BindingDB Entry DOI: 10.7270/Q28K7D3X | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM387561 (N3-(3- Chloro-4- fluorophenyl)- 7-(2- morpholino- ...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | <200 | n/a | n/a | n/a | n/a | n/a | n/a |
SIB Swiss Institute of Bioinformatics Curated by ChEMBL | Assay Description Inhibition of N-terminal His-tagged human recombinant IDO1 expressed in Escherichia coli using tryptophan as substrate by UV-visible absorption spect... | J Med Chem 58: 9421-37 (2015) Article DOI: 10.1021/acs.jmedchem.5b00326 BindingDB Entry DOI: 10.7270/Q28K7D3X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM50500142 (CHEMBL3747530) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | <200 | n/a | n/a | n/a | n/a | n/a | n/a |
SIB Swiss Institute of Bioinformatics Curated by ChEMBL | Assay Description Inhibition of N-terminal hexa-Histidine tagged human IDO1 expressed in Escherichia coli using L-tryptophan as substrate after 30 mins by HPLC analysi... | J Med Chem 58: 9421-37 (2015) Article DOI: 10.1021/acs.jmedchem.5b00326 BindingDB Entry DOI: 10.7270/Q28K7D3X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM50500136 (CHEMBL3746040) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 240 | n/a | n/a | n/a | n/a | n/a | n/a |
SIB Swiss Institute of Bioinformatics Curated by ChEMBL | Assay Description Inhibition of N-terminal His-tagged human recombinant IDO1 expressed in Escherichia coli using L-tryptophan as substrate preincubated for 10 mins fol... | J Med Chem 58: 9421-37 (2015) Article DOI: 10.1021/acs.jmedchem.5b00326 BindingDB Entry DOI: 10.7270/Q28K7D3X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM50126144 (CHEMBL3629569 | US10155972, Compound NewLink 1 | U...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | <1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
SIB Swiss Institute of Bioinformatics Curated by ChEMBL | Assay Description Inhibition of C-terminal 6His-tagged human IDO using L-tryptophan as substrate preincubated for 5 mins followed by substrate addition measured after ... | J Med Chem 58: 9421-37 (2015) Article DOI: 10.1021/acs.jmedchem.5b00326 BindingDB Entry DOI: 10.7270/Q28K7D3X | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM317215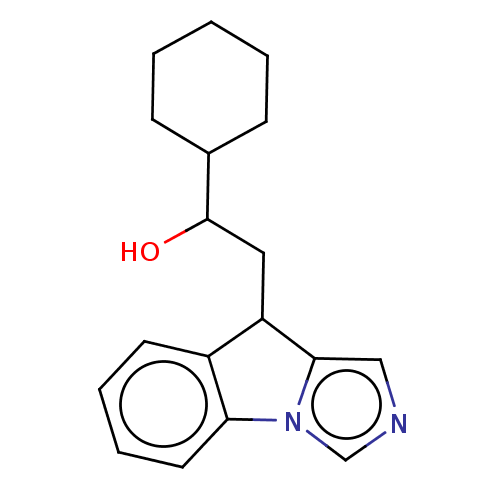 (US9617272, Compound 70 | US9981973, Compound 70) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | KEGG PC cid PC sid UniChem | Article PubMed | n/a | n/a | <1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
SIB Swiss Institute of Bioinformatics Curated by ChEMBL | Assay Description Inhibition of C-terminal 6His-tagged human IDO using L-tryptophan as substrate preincubated for 5 mins followed by substrate addition measured after ... | J Med Chem 58: 9421-37 (2015) Article DOI: 10.1021/acs.jmedchem.5b00326 BindingDB Entry DOI: 10.7270/Q28K7D3X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM50500138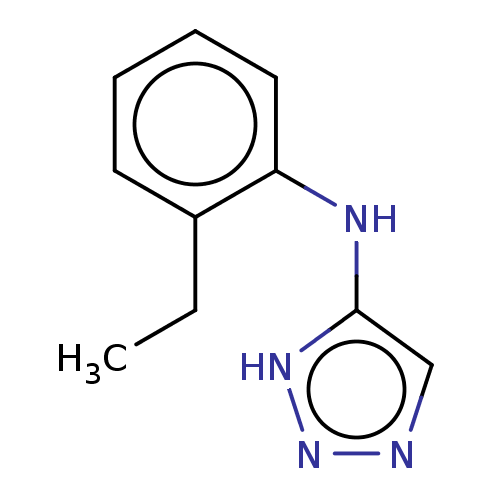 (CHEMBL3745878) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | <1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
SIB Swiss Institute of Bioinformatics Curated by ChEMBL | Assay Description Inhibition of IFN-gamma-stimulated IDO1 activity in human HeLa cells using L-tryptophan as substrate after 48 hrs by spectrophotometric analysis | J Med Chem 58: 9421-37 (2015) Article DOI: 10.1021/acs.jmedchem.5b00326 BindingDB Entry DOI: 10.7270/Q28K7D3X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM50500141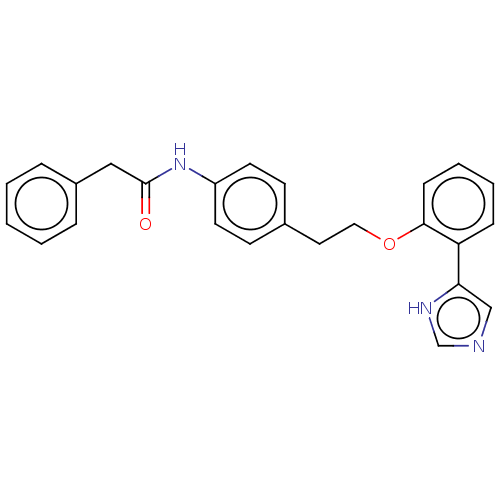 (CHEMBL3746997) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | <1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
SIB Swiss Institute of Bioinformatics Curated by ChEMBL | Assay Description Inhibition of C-terminal 6His-tagged human IDO using L-tryptophan as substrate preincubated for 5 mins followed by substrate addition measured after ... | J Med Chem 58: 9421-37 (2015) Article DOI: 10.1021/acs.jmedchem.5b00326 BindingDB Entry DOI: 10.7270/Q28K7D3X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM50030791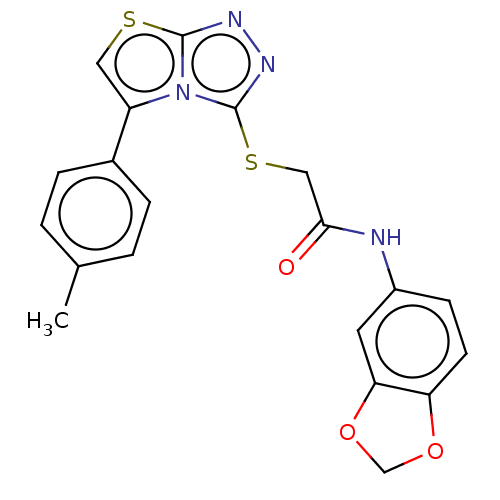 (CHEMBL3342402) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | 3.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
SIB Swiss Institute of Bioinformatics Curated by ChEMBL | Assay Description Inhibition of N-terminal 6His-tagged human recombinant IDO1 expressed in Escherichia coli BL21 using tryptophan as substrate after 90 mins by fluores... | J Med Chem 58: 9421-37 (2015) Article DOI: 10.1021/acs.jmedchem.5b00326 BindingDB Entry DOI: 10.7270/Q28K7D3X | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM50500140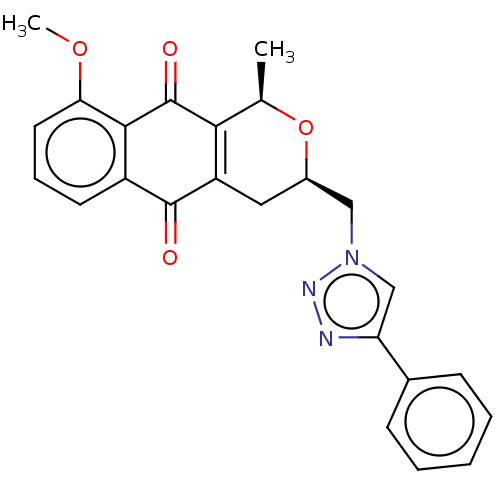 (CHEMBL3103854) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 6.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
SIB Swiss Institute of Bioinformatics Curated by ChEMBL | Assay Description Inhibition of 6His-tagged human recombinant IDO1 expressed in Escherichia coli EC 539 using L-tryptophan as substrate after 1 hr by plate reader anal... | J Med Chem 58: 9421-37 (2015) Article DOI: 10.1021/acs.jmedchem.5b00326 BindingDB Entry DOI: 10.7270/Q28K7D3X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM50500139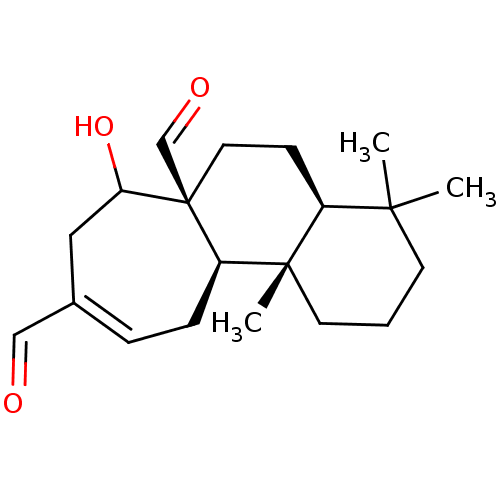 (CHEMBL3745728) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 7.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
SIB Swiss Institute of Bioinformatics Curated by ChEMBL | Assay Description Inhibition of N-terminal His-tagged human recombinant IDO1 expressed in Escherichia coli using L-tryptophan as substrate by methylene blue/ascorbate ... | J Med Chem 58: 9421-37 (2015) Article DOI: 10.1021/acs.jmedchem.5b00326 BindingDB Entry DOI: 10.7270/Q28K7D3X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM50138821 (CHEMBL3747548 | US9617272, Compound 66 | US9981973...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
SIB Swiss Institute of Bioinformatics Curated by ChEMBL | Assay Description Inhibition of C-terminal 6His-tagged human IDO using L-tryptophan as substrate preincubated for 5 mins followed by substrate addition measured after ... | J Med Chem 58: 9421-37 (2015) Article DOI: 10.1021/acs.jmedchem.5b00326 BindingDB Entry DOI: 10.7270/Q28K7D3X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM50146579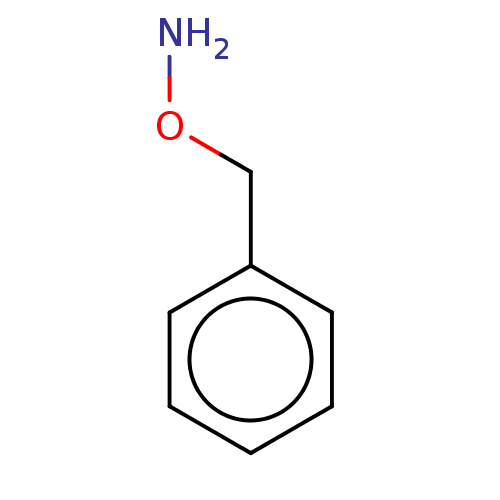 (Benzyl-O-Hydroxylamine | CHEMBL443652 | O-Benzylhy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | <1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
SIB Swiss Institute of Bioinformatics Curated by ChEMBL | Assay Description Inhibition of human IDO using L-tryptophan as substrate preincubated for 5 mins followed by substrate addition measured after 15 mins by spectrophoto... | J Med Chem 58: 9421-37 (2015) Article DOI: 10.1021/acs.jmedchem.5b00326 BindingDB Entry DOI: 10.7270/Q28K7D3X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM50500134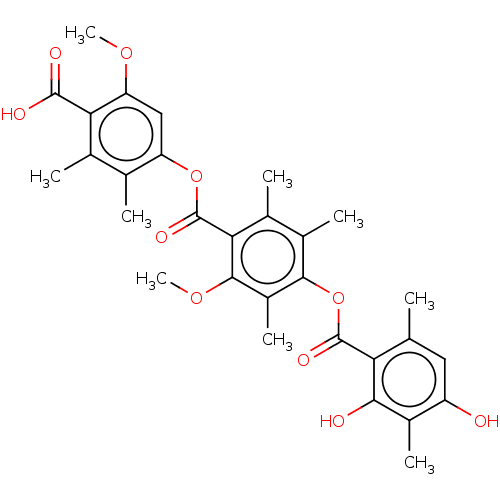 (CHEMBL3747238) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
SIB Swiss Institute of Bioinformatics Curated by ChEMBL | Assay Description Inhibition of IDO (unknown origin) | J Med Chem 58: 9421-37 (2015) Article DOI: 10.1021/acs.jmedchem.5b00326 BindingDB Entry DOI: 10.7270/Q28K7D3X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM50500135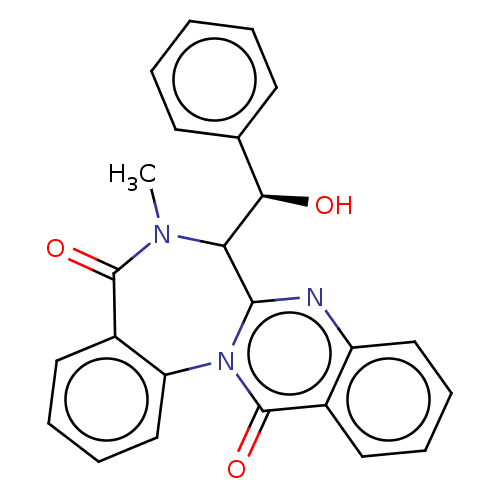 (CHEMBL3745836) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.10E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
SIB Swiss Institute of Bioinformatics Curated by ChEMBL | Assay Description Inhibition of IDO (unknown origin) | J Med Chem 58: 9421-37 (2015) Article DOI: 10.1021/acs.jmedchem.5b00326 BindingDB Entry DOI: 10.7270/Q28K7D3X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||