 Found 18 hits of Enzyme Inhibition Constant Data
Found 18 hits of Enzyme Inhibition Constant Data Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM10880

(AZA | AZA2 | AZM acetazolamide | Acerazolamide, AA...)Show InChI InChI=1S/C4H6N4O3S2/c1-2(9)6-3-7-8-4(12-3)13(5,10)11/h1H3,(H2,5,10,11)(H,6,7,9) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
MMDB
PDB
Article
PubMed
| 370 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AgriIbrahim£e£en University
Curated by ChEMBL
| Assay Description
Inhibition of human erythrocytes CA2 using 4-nitrophenylacetate as substrate by esterase assay |
Bioorg Med Chem Lett 25: 3261-3 (2015)
Article DOI: 10.1016/j.bmcl.2015.05.073
BindingDB Entry DOI: 10.7270/Q2W37Z39 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM26187
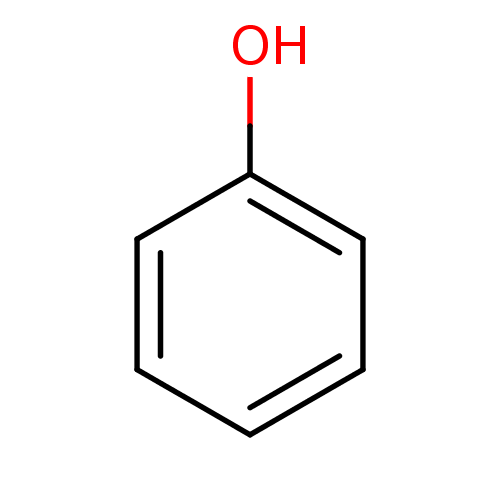
(α-CA inhibitor, 11 | CHEMBL14060 | US9688816,...)Show InChI InChI=1S/C6H6O/c7-6-4-2-1-3-5-6/h1-5,7H | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 5.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AgriIbrahim£e£en University
Curated by ChEMBL
| Assay Description
Inhibition of human erythrocytes CA2 using 4-nitrophenylacetate as substrate by esterase assay |
Bioorg Med Chem Lett 25: 3261-3 (2015)
Article DOI: 10.1016/j.bmcl.2015.05.073
BindingDB Entry DOI: 10.7270/Q2W37Z39 |
More data for this
Ligand-Target Pair | 
3D Structure (docked) |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM26189
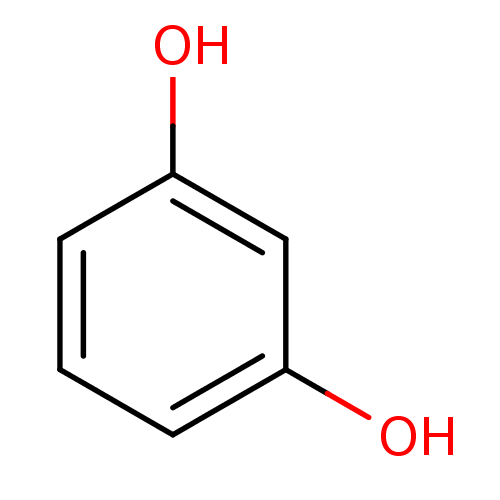
(α-CA inhibitor, 13 | 1,3-Dihydroxybenzene, XI...)Show InChI InChI=1S/C6H6O2/c7-5-2-1-3-6(8)4-5/h1-4,7-8H | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 7.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AgriIbrahim£e£en University
Curated by ChEMBL
| Assay Description
Inhibition of human erythrocytes CA2 using 4-nitrophenylacetate as substrate by esterase assay |
Bioorg Med Chem Lett 25: 3261-3 (2015)
Article DOI: 10.1016/j.bmcl.2015.05.073
BindingDB Entry DOI: 10.7270/Q2W37Z39 |
More data for this
Ligand-Target Pair | 
3D Structure (docked) |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM26188
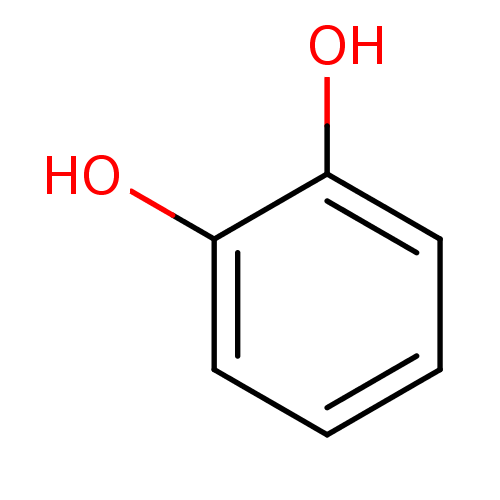
(α-CA inhibitor, 12 | 1,2-Dihydroxybenzene, XI...)Show InChI InChI=1S/C6H6O2/c7-5-3-1-2-4-6(5)8/h1-4,7-8H | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 9.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AgriIbrahim£e£en University
Curated by ChEMBL
| Assay Description
Inhibition of human erythrocytes CA2 using 4-nitrophenylacetate as substrate by esterase assay |
Bioorg Med Chem Lett 25: 3261-3 (2015)
Article DOI: 10.1016/j.bmcl.2015.05.073
BindingDB Entry DOI: 10.7270/Q2W37Z39 |
More data for this
Ligand-Target Pair | 
3D Structure (docked) |
Carbonic anhydrase 1
(Homo sapiens (Human)) | BDBM26187

(α-CA inhibitor, 11 | CHEMBL14060 | US9688816,...)Show InChI InChI=1S/C6H6O/c7-6-4-2-1-3-5-6/h1-5,7H | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 1.02E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AgriIbrahim£e£en University
Curated by ChEMBL
| Assay Description
Inhibition of human erythrocytes CA1 using 4-nitrophenylacetate as substrate by esterase assay |
Bioorg Med Chem Lett 25: 3261-3 (2015)
Article DOI: 10.1016/j.bmcl.2015.05.073
BindingDB Entry DOI: 10.7270/Q2W37Z39 |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 1
(Homo sapiens (Human)) | BDBM50106395

(CHEMBL3601880)Show InChI InChI=1S/C11H9BrN2O4S/c1-7-2-4-8(5-3-7)19(17,18)14-6-9(12)10(15)13-11(14)16/h2-6H,1H3,(H,13,15,16) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 1.08E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AgriIbrahim£e£en University
Curated by ChEMBL
| Assay Description
Inhibition of human erythrocytes CA1 using 4-nitrophenylacetate as substrate by esterase assay |
Bioorg Med Chem Lett 25: 3261-3 (2015)
Article DOI: 10.1016/j.bmcl.2015.05.073
BindingDB Entry DOI: 10.7270/Q2W37Z39 |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM50106395

(CHEMBL3601880)Show InChI InChI=1S/C11H9BrN2O4S/c1-7-2-4-8(5-3-7)19(17,18)14-6-9(12)10(15)13-11(14)16/h2-6H,1H3,(H,13,15,16) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 2.89E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AgriIbrahim£e£en University
Curated by ChEMBL
| Assay Description
Inhibition of human erythrocytes CA2 using 4-nitrophenylacetate as substrate by esterase assay |
Bioorg Med Chem Lett 25: 3261-3 (2015)
Article DOI: 10.1016/j.bmcl.2015.05.073
BindingDB Entry DOI: 10.7270/Q2W37Z39 |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 1
(Homo sapiens (Human)) | BDBM10880

(AZA | AZA2 | AZM acetazolamide | Acerazolamide, AA...)Show InChI InChI=1S/C4H6N4O3S2/c1-2(9)6-3-7-8-4(12-3)13(5,10)11/h1H3,(H2,5,10,11)(H,6,7,9) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
Article
PubMed
| 3.62E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AgriIbrahim£e£en University
Curated by ChEMBL
| Assay Description
Inhibition of human erythrocytes CA1 using 4-nitrophenylacetate as substrate by esterase assay |
Bioorg Med Chem Lett 25: 3261-3 (2015)
Article DOI: 10.1016/j.bmcl.2015.05.073
BindingDB Entry DOI: 10.7270/Q2W37Z39 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Carbonic anhydrase 1
(Homo sapiens (Human)) | BDBM50106411

(CHEBI:16964 | CHEMBL1233360)Show InChI InChI=1S/C5H6N2O3/c8-2-3-1-6-5(10)7-4(3)9/h1,8H,2H2,(H2,6,7,9,10) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| 4.95E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AgriIbrahim£e£en University
Curated by ChEMBL
| Assay Description
Inhibition of human erythrocytes CA1 using 4-nitrophenylacetate as substrate by esterase assay |
Bioorg Med Chem Lett 25: 3261-3 (2015)
Article DOI: 10.1016/j.bmcl.2015.05.073
BindingDB Entry DOI: 10.7270/Q2W37Z39 |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 1
(Homo sapiens (Human)) | BDBM50106396

(CHEBI:74733 | CHEMBL1650614)Show InChI InChI=1S/C5H6N2O2/c1-3-2-4(8)7-5(9)6-3/h2H,1H3,(H2,6,7,8,9) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| 5.78E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AgriIbrahim£e£en University
Curated by ChEMBL
| Assay Description
Inhibition of human erythrocytes CA1 using 4-nitrophenylacetate as substrate by esterase assay |
Bioorg Med Chem Lett 25: 3261-3 (2015)
Article DOI: 10.1016/j.bmcl.2015.05.073
BindingDB Entry DOI: 10.7270/Q2W37Z39 |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM50106397
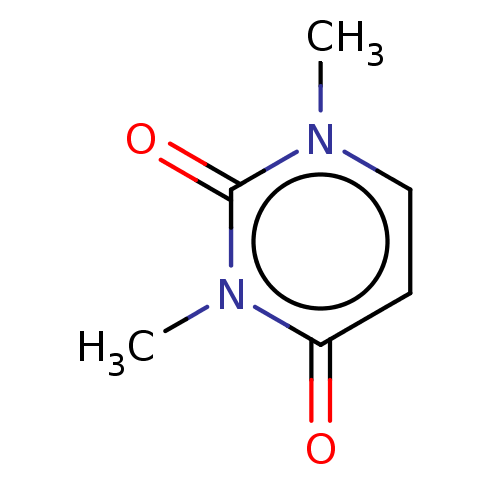
(CHEBI:74763 | CHEMBL11470)Show InChI InChI=1S/C6H8N2O2/c1-7-4-3-5(9)8(2)6(7)10/h3-4H,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.66E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AgriIbrahim£e£en University
Curated by ChEMBL
| Assay Description
Inhibition of human erythrocytes CA2 using 4-nitrophenylacetate as substrate by esterase assay |
Bioorg Med Chem Lett 25: 3261-3 (2015)
Article DOI: 10.1016/j.bmcl.2015.05.073
BindingDB Entry DOI: 10.7270/Q2W37Z39 |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 1
(Homo sapiens (Human)) | BDBM50106397

(CHEBI:74763 | CHEMBL11470)Show InChI InChI=1S/C6H8N2O2/c1-7-4-3-5(9)8(2)6(7)10/h3-4H,1-2H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.16E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AgriIbrahim£e£en University
Curated by ChEMBL
| Assay Description
Inhibition of human erythrocytes CA1 using 4-nitrophenylacetate as substrate by esterase assay |
Bioorg Med Chem Lett 25: 3261-3 (2015)
Article DOI: 10.1016/j.bmcl.2015.05.073
BindingDB Entry DOI: 10.7270/Q2W37Z39 |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 1
(Homo sapiens (Human)) | BDBM50106392
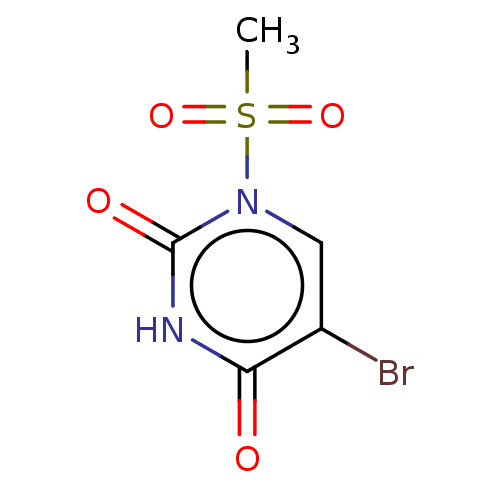
(CHEMBL3601878)Show InChI InChI=1S/C5H5BrN2O4S/c1-13(11,12)8-2-3(6)4(9)7-5(8)10/h2H,1H3,(H,7,9,10) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 4.28E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AgriIbrahim£e£en University
Curated by ChEMBL
| Assay Description
Inhibition of human erythrocytes CA1 using 4-nitrophenylacetate as substrate by esterase assay |
Bioorg Med Chem Lett 25: 3261-3 (2015)
Article DOI: 10.1016/j.bmcl.2015.05.073
BindingDB Entry DOI: 10.7270/Q2W37Z39 |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 1
(Homo sapiens (Human)) | BDBM50106394
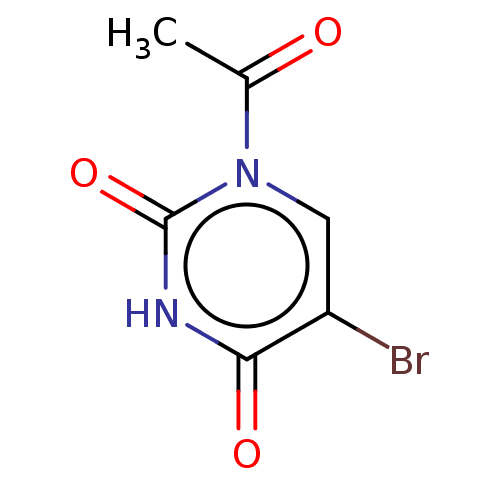
(CHEMBL3601879)Show InChI InChI=1S/C6H5BrN2O3/c1-3(10)9-2-4(7)5(11)8-6(9)12/h2H,1H3,(H,8,11,12) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 4.64E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AgriIbrahim£e£en University
Curated by ChEMBL
| Assay Description
Inhibition of human erythrocytes CA1 using 4-nitrophenylacetate as substrate by esterase assay |
Bioorg Med Chem Lett 25: 3261-3 (2015)
Article DOI: 10.1016/j.bmcl.2015.05.073
BindingDB Entry DOI: 10.7270/Q2W37Z39 |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM50106392

(CHEMBL3601878)Show InChI InChI=1S/C5H5BrN2O4S/c1-13(11,12)8-2-3(6)4(9)7-5(8)10/h2H,1H3,(H,7,9,10) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 6.45E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AgriIbrahim£e£en University
Curated by ChEMBL
| Assay Description
Inhibition of human erythrocytes CA2 using 4-nitrophenylacetate as substrate by esterase assay |
Bioorg Med Chem Lett 25: 3261-3 (2015)
Article DOI: 10.1016/j.bmcl.2015.05.073
BindingDB Entry DOI: 10.7270/Q2W37Z39 |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM50106394

(CHEMBL3601879)Show InChI InChI=1S/C6H5BrN2O3/c1-3(10)9-2-4(7)5(11)8-6(9)12/h2H,1H3,(H,8,11,12) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 7.79E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AgriIbrahim£e£en University
Curated by ChEMBL
| Assay Description
Inhibition of human erythrocytes CA2 using 4-nitrophenylacetate as substrate by esterase assay |
Bioorg Med Chem Lett 25: 3261-3 (2015)
Article DOI: 10.1016/j.bmcl.2015.05.073
BindingDB Entry DOI: 10.7270/Q2W37Z39 |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 1
(Homo sapiens (Human)) | BDBM26189

(α-CA inhibitor, 13 | 1,3-Dihydroxybenzene, XI...)Show InChI InChI=1S/C6H6O2/c7-5-2-1-3-6(8)4-5/h1-4,7-8H | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 7.95E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AgriIbrahim£e£en University
Curated by ChEMBL
| Assay Description
Inhibition of human erythrocytes CA1 using 4-nitrophenylacetate as substrate by esterase assay |
Bioorg Med Chem Lett 25: 3261-3 (2015)
Article DOI: 10.1016/j.bmcl.2015.05.073
BindingDB Entry DOI: 10.7270/Q2W37Z39 |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 1
(Homo sapiens (Human)) | BDBM26188

(α-CA inhibitor, 12 | 1,2-Dihydroxybenzene, XI...)Show InChI InChI=1S/C6H6O2/c7-5-3-1-2-4-6(5)8/h1-4,7-8H | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 4.00E+6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AgriIbrahim£e£en University
Curated by ChEMBL
| Assay Description
Inhibition of human erythrocytes CA1 using 4-nitrophenylacetate as substrate by esterase assay |
Bioorg Med Chem Lett 25: 3261-3 (2015)
Article DOI: 10.1016/j.bmcl.2015.05.073
BindingDB Entry DOI: 10.7270/Q2W37Z39 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data