

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cholinesterase (Equus caballus (Horse)) | BDBM50119275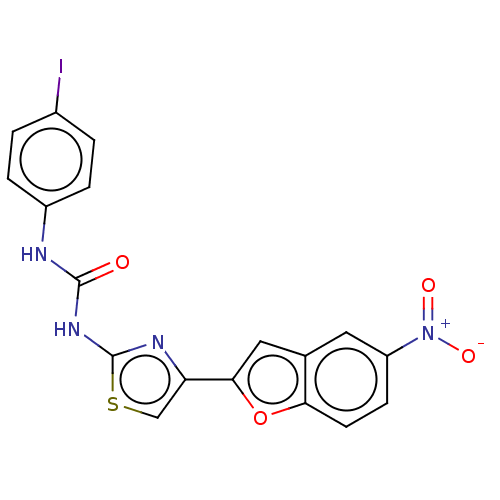 (CHEMBL3617399) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.03E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Bezmialem Vakif University Curated by ChEMBL | Assay Description Inhibition of horse serum butyrylcholinesterase using butyrylthiocholine iodide as substrate preincubated for 15 mins followed by substrate addition ... | Eur J Med Chem 102: 80-92 (2015) Article DOI: 10.1016/j.ejmech.2015.07.005 BindingDB Entry DOI: 10.7270/Q2JM2CFX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50119106 (CHEMBL3617394) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.26E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Bezmialem Vakif University Curated by ChEMBL | Assay Description Inhibition of horse serum butyrylcholinesterase using butyrylthiocholine iodide as substrate preincubated for 15 mins followed by substrate addition ... | Eur J Med Chem 102: 80-92 (2015) Article DOI: 10.1016/j.ejmech.2015.07.005 BindingDB Entry DOI: 10.7270/Q2JM2CFX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM10404 ((1S,12S,14R)-9-methoxy-4-methyl-11-oxa-4-azatetrac...) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 2.41E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Bezmialem Vakif University Curated by ChEMBL | Assay Description Inhibition of electric eel acetylcholinesterase using acetylthiocholine iodide as substrate preincubated for 15 mins followed by substrate addition b... | Eur J Med Chem 102: 80-92 (2015) Article DOI: 10.1016/j.ejmech.2015.07.005 BindingDB Entry DOI: 10.7270/Q2JM2CFX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50119081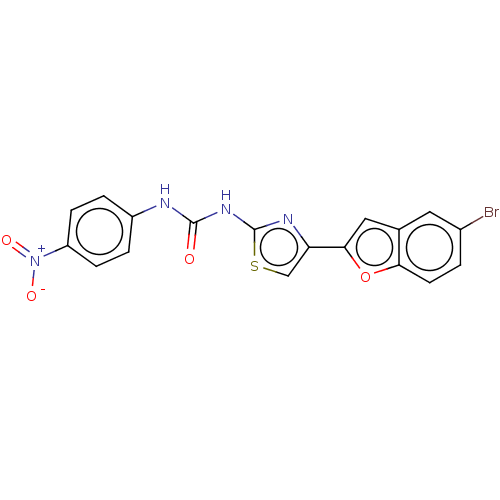 (CHEMBL3617385) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.12E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Bezmialem Vakif University Curated by ChEMBL | Assay Description Inhibition of horse serum butyrylcholinesterase using butyrylthiocholine iodide as substrate preincubated for 15 mins followed by substrate addition ... | Eur J Med Chem 102: 80-92 (2015) Article DOI: 10.1016/j.ejmech.2015.07.005 BindingDB Entry DOI: 10.7270/Q2JM2CFX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50118820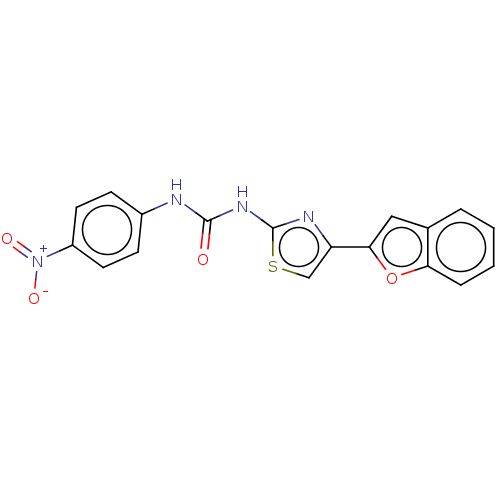 (CHEMBL3617369) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.44E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Bezmialem Vakif University Curated by ChEMBL | Assay Description Inhibition of horse serum butyrylcholinesterase using butyrylthiocholine iodide as substrate preincubated for 15 mins followed by substrate addition ... | Eur J Med Chem 102: 80-92 (2015) Article DOI: 10.1016/j.ejmech.2015.07.005 BindingDB Entry DOI: 10.7270/Q2JM2CFX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50119098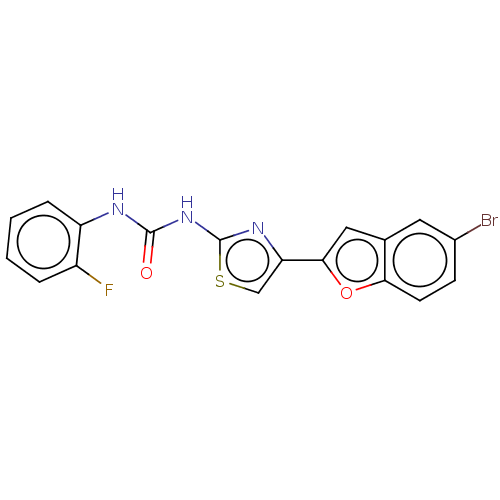 (CHEMBL3617386) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3.85E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Bezmialem Vakif University Curated by ChEMBL | Assay Description Inhibition of electric eel acetylcholinesterase using acetylthiocholine iodide as substrate preincubated for 15 mins followed by substrate addition b... | Eur J Med Chem 102: 80-92 (2015) Article DOI: 10.1016/j.ejmech.2015.07.005 BindingDB Entry DOI: 10.7270/Q2JM2CFX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50119106 (CHEMBL3617394) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.42E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Bezmialem Vakif University Curated by ChEMBL | Assay Description Inhibition of electric eel acetylcholinesterase using acetylthiocholine iodide as substrate preincubated for 15 mins followed by substrate addition b... | Eur J Med Chem 102: 80-92 (2015) Article DOI: 10.1016/j.ejmech.2015.07.005 BindingDB Entry DOI: 10.7270/Q2JM2CFX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50119081 (CHEMBL3617385) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.78E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Bezmialem Vakif University Curated by ChEMBL | Assay Description Inhibition of electric eel acetylcholinesterase using acetylthiocholine iodide as substrate preincubated for 15 mins followed by substrate addition b... | Eur J Med Chem 102: 80-92 (2015) Article DOI: 10.1016/j.ejmech.2015.07.005 BindingDB Entry DOI: 10.7270/Q2JM2CFX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50119072 (CHEMBL3617383) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 5.12E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Bezmialem Vakif University Curated by ChEMBL | Assay Description Inhibition of horse serum butyrylcholinesterase using butyrylthiocholine iodide as substrate preincubated for 15 mins followed by substrate addition ... | Eur J Med Chem 102: 80-92 (2015) Article DOI: 10.1016/j.ejmech.2015.07.005 BindingDB Entry DOI: 10.7270/Q2JM2CFX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50118820 (CHEMBL3617369) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.23E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Bezmialem Vakif University Curated by ChEMBL | Assay Description Inhibition of electric eel acetylcholinesterase using acetylthiocholine iodide as substrate preincubated for 15 mins followed by substrate addition b... | Eur J Med Chem 102: 80-92 (2015) Article DOI: 10.1016/j.ejmech.2015.07.005 BindingDB Entry DOI: 10.7270/Q2JM2CFX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50119275 (CHEMBL3617399) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.53E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Bezmialem Vakif University Curated by ChEMBL | Assay Description Inhibition of electric eel acetylcholinesterase using acetylthiocholine iodide as substrate preincubated for 15 mins followed by substrate addition b... | Eur J Med Chem 102: 80-92 (2015) Article DOI: 10.1016/j.ejmech.2015.07.005 BindingDB Entry DOI: 10.7270/Q2JM2CFX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50118960 (CHEMBL3617381) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.65E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Bezmialem Vakif University Curated by ChEMBL | Assay Description Inhibition of horse serum butyrylcholinesterase using butyrylthiocholine iodide as substrate preincubated for 15 mins followed by substrate addition ... | Eur J Med Chem 102: 80-92 (2015) Article DOI: 10.1016/j.ejmech.2015.07.005 BindingDB Entry DOI: 10.7270/Q2JM2CFX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50119104 (CHEMBL3617392) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 6.42E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Bezmialem Vakif University Curated by ChEMBL | Assay Description Inhibition of electric eel acetylcholinesterase using acetylthiocholine iodide as substrate preincubated for 15 mins followed by substrate addition b... | Eur J Med Chem 102: 80-92 (2015) Article DOI: 10.1016/j.ejmech.2015.07.005 BindingDB Entry DOI: 10.7270/Q2JM2CFX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50118817 (CHEMBL3617372) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 7.45E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Bezmialem Vakif University Curated by ChEMBL | Assay Description Inhibition of horse serum butyrylcholinesterase using butyrylthiocholine iodide as substrate preincubated for 15 mins followed by substrate addition ... | Eur J Med Chem 102: 80-92 (2015) Article DOI: 10.1016/j.ejmech.2015.07.005 BindingDB Entry DOI: 10.7270/Q2JM2CFX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50119101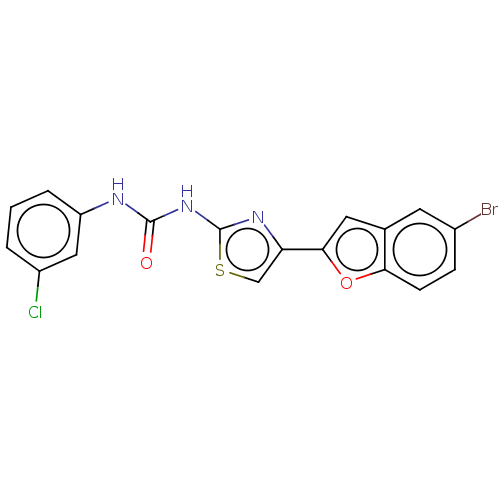 (CHEMBL3617389) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 7.93E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Bezmialem Vakif University Curated by ChEMBL | Assay Description Inhibition of electric eel acetylcholinesterase using acetylthiocholine iodide as substrate preincubated for 15 mins followed by substrate addition b... | Eur J Med Chem 102: 80-92 (2015) Article DOI: 10.1016/j.ejmech.2015.07.005 BindingDB Entry DOI: 10.7270/Q2JM2CFX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50119072 (CHEMBL3617383) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 8.82E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Bezmialem Vakif University Curated by ChEMBL | Assay Description Inhibition of electric eel acetylcholinesterase using acetylthiocholine iodide as substrate preincubated for 15 mins followed by substrate addition b... | Eur J Med Chem 102: 80-92 (2015) Article DOI: 10.1016/j.ejmech.2015.07.005 BindingDB Entry DOI: 10.7270/Q2JM2CFX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50119098 (CHEMBL3617386) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 9.25E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Bezmialem Vakif University Curated by ChEMBL | Assay Description Inhibition of horse serum butyrylcholinesterase using butyrylthiocholine iodide as substrate preincubated for 15 mins followed by substrate addition ... | Eur J Med Chem 102: 80-92 (2015) Article DOI: 10.1016/j.ejmech.2015.07.005 BindingDB Entry DOI: 10.7270/Q2JM2CFX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50118816 (CHEMBL3617373) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 9.67E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Bezmialem Vakif University Curated by ChEMBL | Assay Description Inhibition of electric eel acetylcholinesterase using acetylthiocholine iodide as substrate preincubated for 15 mins followed by substrate addition b... | Eur J Med Chem 102: 80-92 (2015) Article DOI: 10.1016/j.ejmech.2015.07.005 BindingDB Entry DOI: 10.7270/Q2JM2CFX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50118890 (CHEMBL3617365) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.04E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Bezmialem Vakif University Curated by ChEMBL | Assay Description Inhibition of electric eel acetylcholinesterase using acetylthiocholine iodide as substrate preincubated for 15 mins followed by substrate addition b... | Eur J Med Chem 102: 80-92 (2015) Article DOI: 10.1016/j.ejmech.2015.07.005 BindingDB Entry DOI: 10.7270/Q2JM2CFX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50119103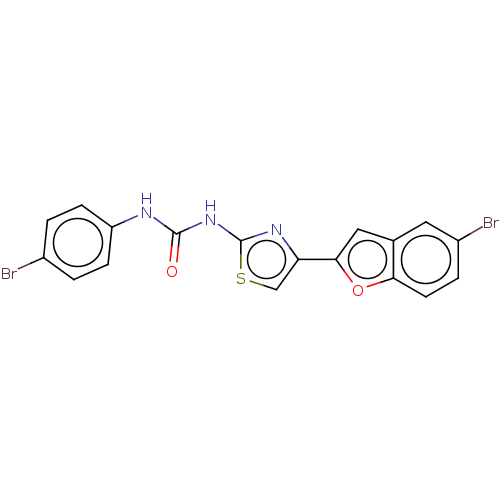 (CHEMBL3617391) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.19E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Bezmialem Vakif University Curated by ChEMBL | Assay Description Inhibition of electric eel acetylcholinesterase using acetylthiocholine iodide as substrate preincubated for 15 mins followed by substrate addition b... | Eur J Med Chem 102: 80-92 (2015) Article DOI: 10.1016/j.ejmech.2015.07.005 BindingDB Entry DOI: 10.7270/Q2JM2CFX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50118958 (CHEMBL3617379) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.40E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Bezmialem Vakif University Curated by ChEMBL | Assay Description Inhibition of horse serum butyrylcholinesterase using butyrylthiocholine iodide as substrate preincubated for 15 mins followed by substrate addition ... | Eur J Med Chem 102: 80-92 (2015) Article DOI: 10.1016/j.ejmech.2015.07.005 BindingDB Entry DOI: 10.7270/Q2JM2CFX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM10404 ((1S,12S,14R)-9-methoxy-4-methyl-11-oxa-4-azatetrac...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 1.74E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Bezmialem Vakif University Curated by ChEMBL | Assay Description Inhibition of horse serum butyrylcholinesterase using butyrylthiocholine iodide as substrate preincubated for 15 mins followed by substrate addition ... | Eur J Med Chem 102: 80-92 (2015) Article DOI: 10.1016/j.ejmech.2015.07.005 BindingDB Entry DOI: 10.7270/Q2JM2CFX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50119075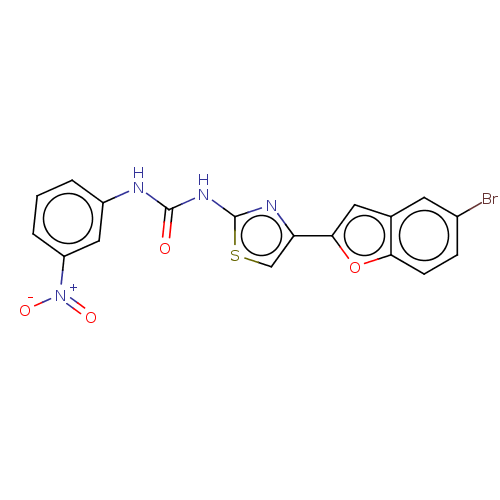 (CHEMBL3617384) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.75E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Bezmialem Vakif University Curated by ChEMBL | Assay Description Inhibition of electric eel acetylcholinesterase using acetylthiocholine iodide as substrate preincubated for 15 mins followed by substrate addition b... | Eur J Med Chem 102: 80-92 (2015) Article DOI: 10.1016/j.ejmech.2015.07.005 BindingDB Entry DOI: 10.7270/Q2JM2CFX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50118818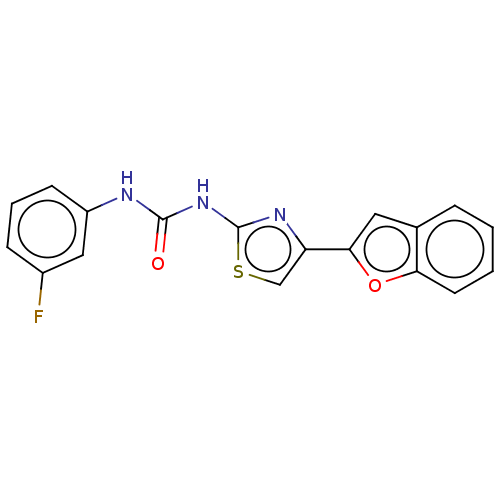 (CHEMBL3617371) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.80E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Bezmialem Vakif University Curated by ChEMBL | Assay Description Inhibition of horse serum butyrylcholinesterase using butyrylthiocholine iodide as substrate preincubated for 15 mins followed by substrate addition ... | Eur J Med Chem 102: 80-92 (2015) Article DOI: 10.1016/j.ejmech.2015.07.005 BindingDB Entry DOI: 10.7270/Q2JM2CFX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50118889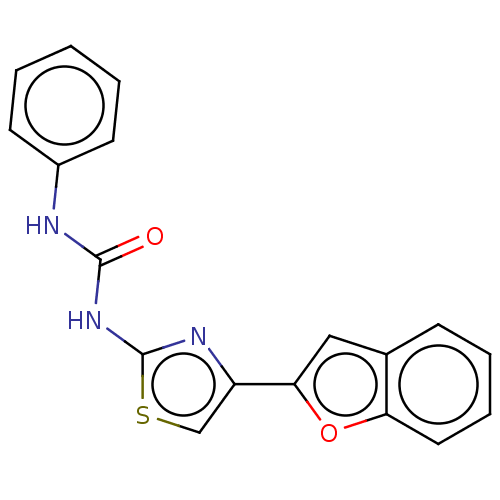 (CHEMBL3617366) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.13E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Bezmialem Vakif University Curated by ChEMBL | Assay Description Inhibition of horse serum butyrylcholinesterase using butyrylthiocholine iodide as substrate preincubated for 15 mins followed by substrate addition ... | Eur J Med Chem 102: 80-92 (2015) Article DOI: 10.1016/j.ejmech.2015.07.005 BindingDB Entry DOI: 10.7270/Q2JM2CFX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50118956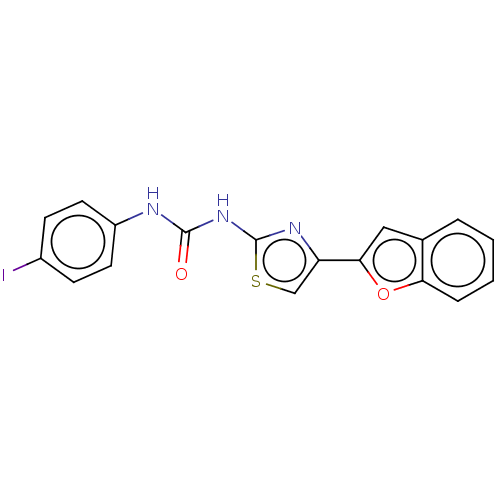 (CHEMBL3617377) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.14E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Bezmialem Vakif University Curated by ChEMBL | Assay Description Inhibition of electric eel acetylcholinesterase using acetylthiocholine iodide as substrate preincubated for 15 mins followed by substrate addition b... | Eur J Med Chem 102: 80-92 (2015) Article DOI: 10.1016/j.ejmech.2015.07.005 BindingDB Entry DOI: 10.7270/Q2JM2CFX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50118816 (CHEMBL3617373) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.15E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Bezmialem Vakif University Curated by ChEMBL | Assay Description Inhibition of horse serum butyrylcholinesterase using butyrylthiocholine iodide as substrate preincubated for 15 mins followed by substrate addition ... | Eur J Med Chem 102: 80-92 (2015) Article DOI: 10.1016/j.ejmech.2015.07.005 BindingDB Entry DOI: 10.7270/Q2JM2CFX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50119102 (CHEMBL3617390) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.22E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Bezmialem Vakif University Curated by ChEMBL | Assay Description Inhibition of electric eel acetylcholinesterase using acetylthiocholine iodide as substrate preincubated for 15 mins followed by substrate addition b... | Eur J Med Chem 102: 80-92 (2015) Article DOI: 10.1016/j.ejmech.2015.07.005 BindingDB Entry DOI: 10.7270/Q2JM2CFX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50118963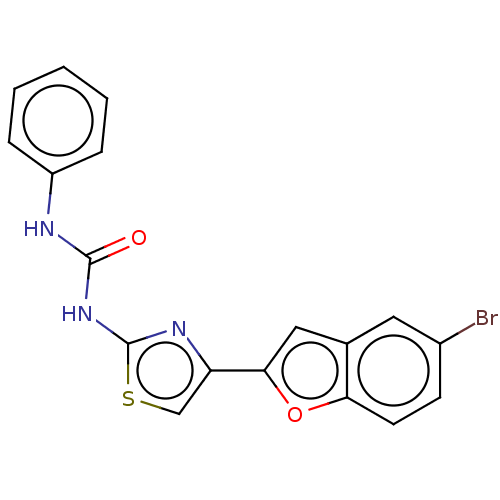 (CHEMBL3617382) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.34E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Bezmialem Vakif University Curated by ChEMBL | Assay Description Inhibition of electric eel acetylcholinesterase using acetylthiocholine iodide as substrate preincubated for 15 mins followed by substrate addition b... | Eur J Med Chem 102: 80-92 (2015) Article DOI: 10.1016/j.ejmech.2015.07.005 BindingDB Entry DOI: 10.7270/Q2JM2CFX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50118889 (CHEMBL3617366) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.47E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Bezmialem Vakif University Curated by ChEMBL | Assay Description Inhibition of electric eel acetylcholinesterase using acetylthiocholine iodide as substrate preincubated for 15 mins followed by substrate addition b... | Eur J Med Chem 102: 80-92 (2015) Article DOI: 10.1016/j.ejmech.2015.07.005 BindingDB Entry DOI: 10.7270/Q2JM2CFX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50119107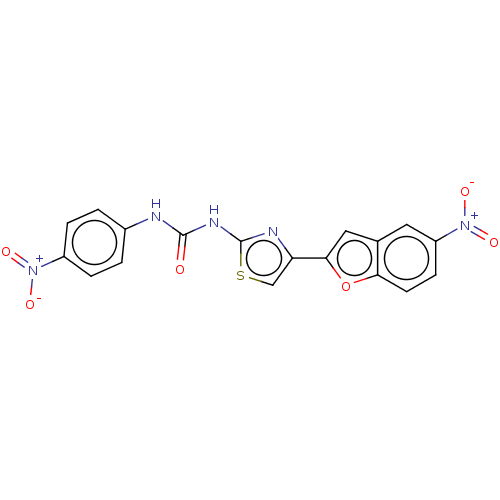 (CHEMBL3617395) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.52E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Bezmialem Vakif University Curated by ChEMBL | Assay Description Inhibition of electric eel acetylcholinesterase using acetylthiocholine iodide as substrate preincubated for 15 mins followed by substrate addition b... | Eur J Med Chem 102: 80-92 (2015) Article DOI: 10.1016/j.ejmech.2015.07.005 BindingDB Entry DOI: 10.7270/Q2JM2CFX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50119107 (CHEMBL3617395) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.56E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Bezmialem Vakif University Curated by ChEMBL | Assay Description Inhibition of horse serum butyrylcholinesterase using butyrylthiocholine iodide as substrate preincubated for 15 mins followed by substrate addition ... | Eur J Med Chem 102: 80-92 (2015) Article DOI: 10.1016/j.ejmech.2015.07.005 BindingDB Entry DOI: 10.7270/Q2JM2CFX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50119101 (CHEMBL3617389) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.63E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Bezmialem Vakif University Curated by ChEMBL | Assay Description Inhibition of horse serum butyrylcholinesterase using butyrylthiocholine iodide as substrate preincubated for 15 mins followed by substrate addition ... | Eur J Med Chem 102: 80-92 (2015) Article DOI: 10.1016/j.ejmech.2015.07.005 BindingDB Entry DOI: 10.7270/Q2JM2CFX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50119109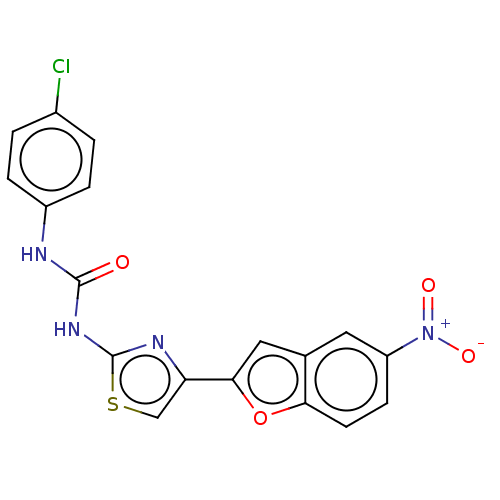 (CHEMBL3617397) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.66E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Bezmialem Vakif University Curated by ChEMBL | Assay Description Inhibition of horse serum butyrylcholinesterase using butyrylthiocholine iodide as substrate preincubated for 15 mins followed by substrate addition ... | Eur J Med Chem 102: 80-92 (2015) Article DOI: 10.1016/j.ejmech.2015.07.005 BindingDB Entry DOI: 10.7270/Q2JM2CFX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50118821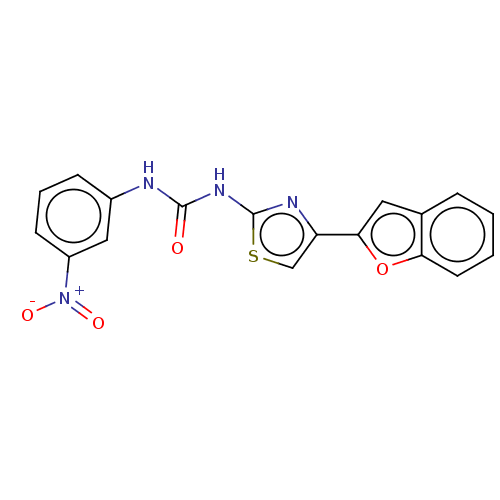 (CHEMBL3617368) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.68E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Bezmialem Vakif University Curated by ChEMBL | Assay Description Inhibition of horse serum butyrylcholinesterase using butyrylthiocholine iodide as substrate preincubated for 15 mins followed by substrate addition ... | Eur J Med Chem 102: 80-92 (2015) Article DOI: 10.1016/j.ejmech.2015.07.005 BindingDB Entry DOI: 10.7270/Q2JM2CFX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50119170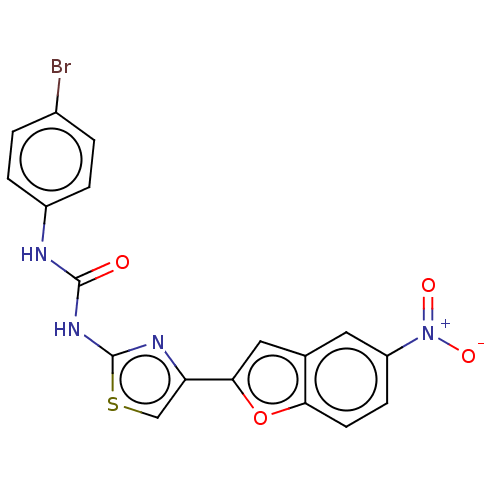 (CHEMBL3617398) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.82E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Bezmialem Vakif University Curated by ChEMBL | Assay Description Inhibition of electric eel acetylcholinesterase using acetylthiocholine iodide as substrate preincubated for 15 mins followed by substrate addition b... | Eur J Med Chem 102: 80-92 (2015) Article DOI: 10.1016/j.ejmech.2015.07.005 BindingDB Entry DOI: 10.7270/Q2JM2CFX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50119099 (CHEMBL3617387) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.94E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Bezmialem Vakif University Curated by ChEMBL | Assay Description Inhibition of electric eel acetylcholinesterase using acetylthiocholine iodide as substrate preincubated for 15 mins followed by substrate addition b... | Eur J Med Chem 102: 80-92 (2015) Article DOI: 10.1016/j.ejmech.2015.07.005 BindingDB Entry DOI: 10.7270/Q2JM2CFX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50118958 (CHEMBL3617379) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.94E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Bezmialem Vakif University Curated by ChEMBL | Assay Description Inhibition of electric eel acetylcholinesterase using acetylthiocholine iodide as substrate preincubated for 15 mins followed by substrate addition b... | Eur J Med Chem 102: 80-92 (2015) Article DOI: 10.1016/j.ejmech.2015.07.005 BindingDB Entry DOI: 10.7270/Q2JM2CFX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50119109 (CHEMBL3617397) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.06E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Bezmialem Vakif University Curated by ChEMBL | Assay Description Inhibition of electric eel acetylcholinesterase using acetylthiocholine iodide as substrate preincubated for 15 mins followed by substrate addition b... | Eur J Med Chem 102: 80-92 (2015) Article DOI: 10.1016/j.ejmech.2015.07.005 BindingDB Entry DOI: 10.7270/Q2JM2CFX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50118819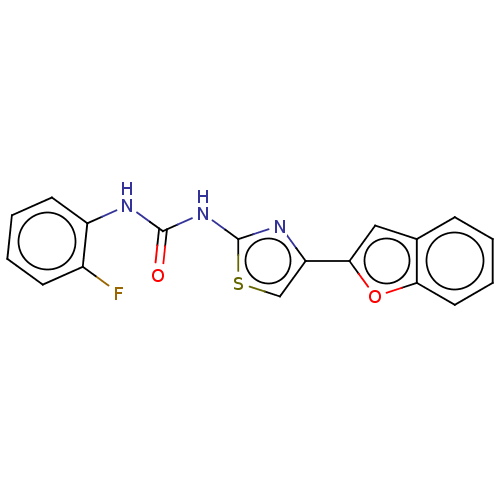 (CHEMBL3617370) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.09E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Bezmialem Vakif University Curated by ChEMBL | Assay Description Inhibition of electric eel acetylcholinesterase using acetylthiocholine iodide as substrate preincubated for 15 mins followed by substrate addition b... | Eur J Med Chem 102: 80-92 (2015) Article DOI: 10.1016/j.ejmech.2015.07.005 BindingDB Entry DOI: 10.7270/Q2JM2CFX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50118959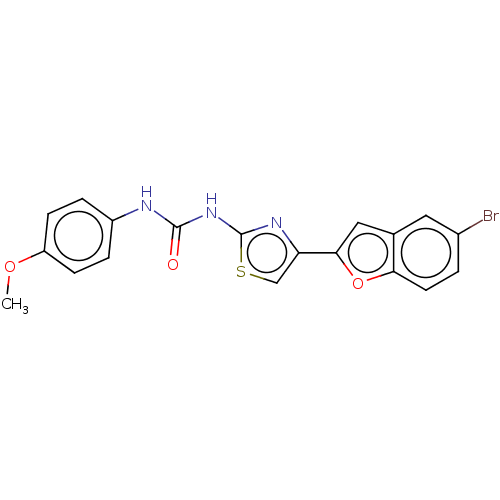 (CHEMBL3617380) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3.10E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Bezmialem Vakif University Curated by ChEMBL | Assay Description Inhibition of horse serum butyrylcholinesterase using butyrylthiocholine iodide as substrate preincubated for 15 mins followed by substrate addition ... | Eur J Med Chem 102: 80-92 (2015) Article DOI: 10.1016/j.ejmech.2015.07.005 BindingDB Entry DOI: 10.7270/Q2JM2CFX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50118960 (CHEMBL3617381) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.25E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Bezmialem Vakif University Curated by ChEMBL | Assay Description Inhibition of electric eel acetylcholinesterase using acetylthiocholine iodide as substrate preincubated for 15 mins followed by substrate addition b... | Eur J Med Chem 102: 80-92 (2015) Article DOI: 10.1016/j.ejmech.2015.07.005 BindingDB Entry DOI: 10.7270/Q2JM2CFX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50118959 (CHEMBL3617380) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3.28E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Bezmialem Vakif University Curated by ChEMBL | Assay Description Inhibition of electric eel acetylcholinesterase using acetylthiocholine iodide as substrate preincubated for 15 mins followed by substrate addition b... | Eur J Med Chem 102: 80-92 (2015) Article DOI: 10.1016/j.ejmech.2015.07.005 BindingDB Entry DOI: 10.7270/Q2JM2CFX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50118797 (CHEMBL3617376) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.28E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Bezmialem Vakif University Curated by ChEMBL | Assay Description Inhibition of electric eel acetylcholinesterase using acetylthiocholine iodide as substrate preincubated for 15 mins followed by substrate addition b... | Eur J Med Chem 102: 80-92 (2015) Article DOI: 10.1016/j.ejmech.2015.07.005 BindingDB Entry DOI: 10.7270/Q2JM2CFX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50119105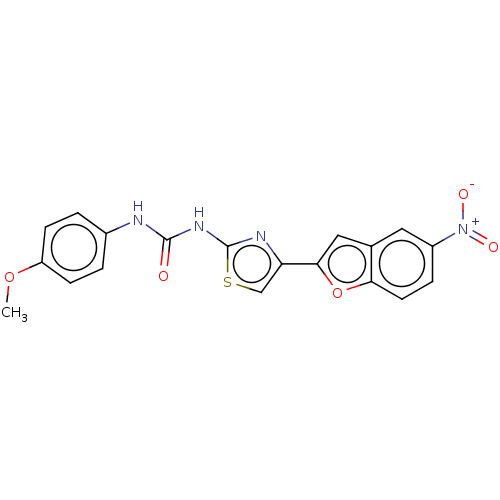 (CHEMBL3617393) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.41E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Bezmialem Vakif University Curated by ChEMBL | Assay Description Inhibition of electric eel acetylcholinesterase using acetylthiocholine iodide as substrate preincubated for 15 mins followed by substrate addition b... | Eur J Med Chem 102: 80-92 (2015) Article DOI: 10.1016/j.ejmech.2015.07.005 BindingDB Entry DOI: 10.7270/Q2JM2CFX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50119170 (CHEMBL3617398) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.45E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Bezmialem Vakif University Curated by ChEMBL | Assay Description Inhibition of horse serum butyrylcholinesterase using butyrylthiocholine iodide as substrate preincubated for 15 mins followed by substrate addition ... | Eur J Med Chem 102: 80-92 (2015) Article DOI: 10.1016/j.ejmech.2015.07.005 BindingDB Entry DOI: 10.7270/Q2JM2CFX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50118963 (CHEMBL3617382) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Bezmialem Vakif University Curated by ChEMBL | Assay Description Inhibition of horse serum butyrylcholinesterase using butyrylthiocholine iodide as substrate preincubated for 15 mins followed by substrate addition ... | Eur J Med Chem 102: 80-92 (2015) Article DOI: 10.1016/j.ejmech.2015.07.005 BindingDB Entry DOI: 10.7270/Q2JM2CFX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50119100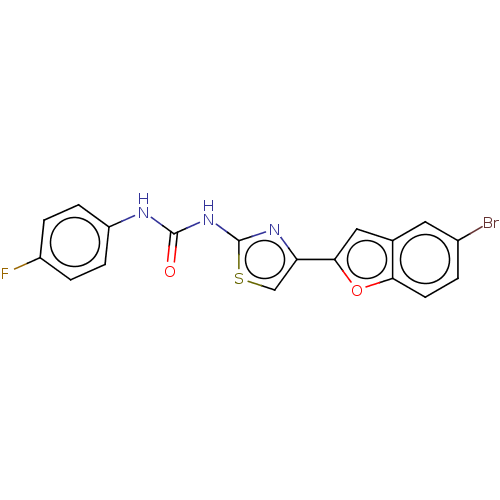 (CHEMBL3617388) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.56E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Bezmialem Vakif University Curated by ChEMBL | Assay Description Inhibition of electric eel acetylcholinesterase using acetylthiocholine iodide as substrate preincubated for 15 mins followed by substrate addition b... | Eur J Med Chem 102: 80-92 (2015) Article DOI: 10.1016/j.ejmech.2015.07.005 BindingDB Entry DOI: 10.7270/Q2JM2CFX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50119103 (CHEMBL3617391) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.58E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Bezmialem Vakif University Curated by ChEMBL | Assay Description Inhibition of horse serum butyrylcholinesterase using butyrylthiocholine iodide as substrate preincubated for 15 mins followed by substrate addition ... | Eur J Med Chem 102: 80-92 (2015) Article DOI: 10.1016/j.ejmech.2015.07.005 BindingDB Entry DOI: 10.7270/Q2JM2CFX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50119102 (CHEMBL3617390) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.69E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Bezmialem Vakif University Curated by ChEMBL | Assay Description Inhibition of horse serum butyrylcholinesterase using butyrylthiocholine iodide as substrate preincubated for 15 mins followed by substrate addition ... | Eur J Med Chem 102: 80-92 (2015) Article DOI: 10.1016/j.ejmech.2015.07.005 BindingDB Entry DOI: 10.7270/Q2JM2CFX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50118815 (CHEMBL3617374) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.86E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Bezmialem Vakif University Curated by ChEMBL | Assay Description Inhibition of horse serum butyrylcholinesterase using butyrylthiocholine iodide as substrate preincubated for 15 mins followed by substrate addition ... | Eur J Med Chem 102: 80-92 (2015) Article DOI: 10.1016/j.ejmech.2015.07.005 BindingDB Entry DOI: 10.7270/Q2JM2CFX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50119099 (CHEMBL3617387) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.02E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Bezmialem Vakif University Curated by ChEMBL | Assay Description Inhibition of horse serum butyrylcholinesterase using butyrylthiocholine iodide as substrate preincubated for 15 mins followed by substrate addition ... | Eur J Med Chem 102: 80-92 (2015) Article DOI: 10.1016/j.ejmech.2015.07.005 BindingDB Entry DOI: 10.7270/Q2JM2CFX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50119108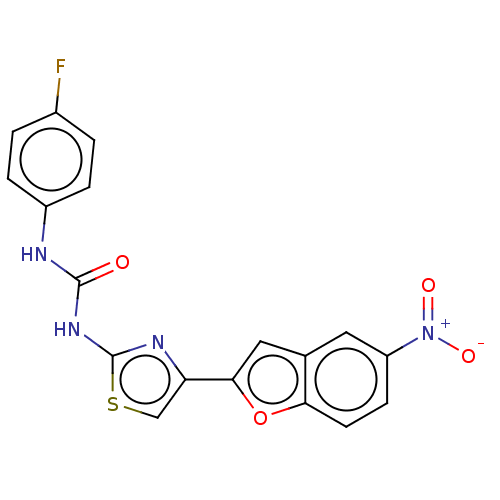 (CHEMBL3617396) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 4.11E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Bezmialem Vakif University Curated by ChEMBL | Assay Description Inhibition of electric eel acetylcholinesterase using acetylthiocholine iodide as substrate preincubated for 15 mins followed by substrate addition b... | Eur J Med Chem 102: 80-92 (2015) Article DOI: 10.1016/j.ejmech.2015.07.005 BindingDB Entry DOI: 10.7270/Q2JM2CFX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50118821 (CHEMBL3617368) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.12E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Bezmialem Vakif University Curated by ChEMBL | Assay Description Inhibition of electric eel acetylcholinesterase using acetylthiocholine iodide as substrate preincubated for 15 mins followed by substrate addition b... | Eur J Med Chem 102: 80-92 (2015) Article DOI: 10.1016/j.ejmech.2015.07.005 BindingDB Entry DOI: 10.7270/Q2JM2CFX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50118956 (CHEMBL3617377) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.13E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Bezmialem Vakif University Curated by ChEMBL | Assay Description Inhibition of horse serum butyrylcholinesterase using butyrylthiocholine iodide as substrate preincubated for 15 mins followed by substrate addition ... | Eur J Med Chem 102: 80-92 (2015) Article DOI: 10.1016/j.ejmech.2015.07.005 BindingDB Entry DOI: 10.7270/Q2JM2CFX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50118819 (CHEMBL3617370) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.17E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Bezmialem Vakif University Curated by ChEMBL | Assay Description Inhibition of horse serum butyrylcholinesterase using butyrylthiocholine iodide as substrate preincubated for 15 mins followed by substrate addition ... | Eur J Med Chem 102: 80-92 (2015) Article DOI: 10.1016/j.ejmech.2015.07.005 BindingDB Entry DOI: 10.7270/Q2JM2CFX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50118861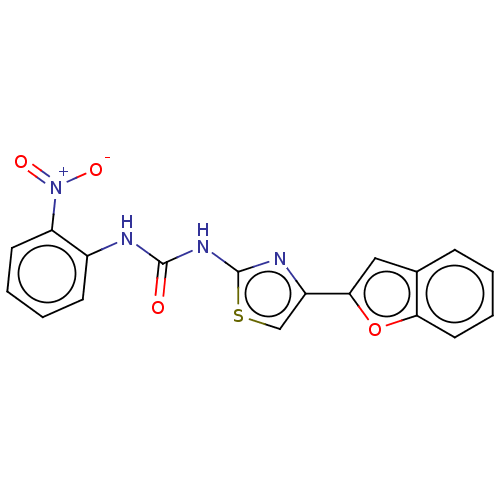 (CHEMBL3617367) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 4.19E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Bezmialem Vakif University Curated by ChEMBL | Assay Description Inhibition of electric eel acetylcholinesterase using acetylthiocholine iodide as substrate preincubated for 15 mins followed by substrate addition b... | Eur J Med Chem 102: 80-92 (2015) Article DOI: 10.1016/j.ejmech.2015.07.005 BindingDB Entry DOI: 10.7270/Q2JM2CFX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50118815 (CHEMBL3617374) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.26E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Bezmialem Vakif University Curated by ChEMBL | Assay Description Inhibition of electric eel acetylcholinesterase using acetylthiocholine iodide as substrate preincubated for 15 mins followed by substrate addition b... | Eur J Med Chem 102: 80-92 (2015) Article DOI: 10.1016/j.ejmech.2015.07.005 BindingDB Entry DOI: 10.7270/Q2JM2CFX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50119104 (CHEMBL3617392) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 4.36E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Bezmialem Vakif University Curated by ChEMBL | Assay Description Inhibition of horse serum butyrylcholinesterase using butyrylthiocholine iodide as substrate preincubated for 15 mins followed by substrate addition ... | Eur J Med Chem 102: 80-92 (2015) Article DOI: 10.1016/j.ejmech.2015.07.005 BindingDB Entry DOI: 10.7270/Q2JM2CFX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50119108 (CHEMBL3617396) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 4.76E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Bezmialem Vakif University Curated by ChEMBL | Assay Description Inhibition of horse serum butyrylcholinesterase using butyrylthiocholine iodide as substrate preincubated for 15 mins followed by substrate addition ... | Eur J Med Chem 102: 80-92 (2015) Article DOI: 10.1016/j.ejmech.2015.07.005 BindingDB Entry DOI: 10.7270/Q2JM2CFX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50118911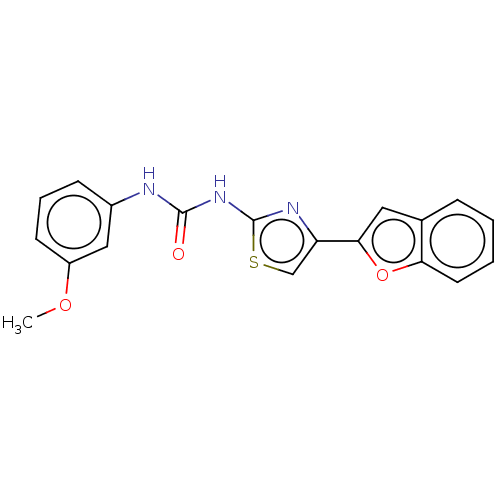 (CHEMBL3617363) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.89E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Bezmialem Vakif University Curated by ChEMBL | Assay Description Inhibition of electric eel acetylcholinesterase using acetylthiocholine iodide as substrate preincubated for 15 mins followed by substrate addition b... | Eur J Med Chem 102: 80-92 (2015) Article DOI: 10.1016/j.ejmech.2015.07.005 BindingDB Entry DOI: 10.7270/Q2JM2CFX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50118818 (CHEMBL3617371) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.90E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Bezmialem Vakif University Curated by ChEMBL | Assay Description Inhibition of electric eel acetylcholinesterase using acetylthiocholine iodide as substrate preincubated for 15 mins followed by substrate addition b... | Eur J Med Chem 102: 80-92 (2015) Article DOI: 10.1016/j.ejmech.2015.07.005 BindingDB Entry DOI: 10.7270/Q2JM2CFX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50119105 (CHEMBL3617393) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.92E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Bezmialem Vakif University Curated by ChEMBL | Assay Description Inhibition of horse serum butyrylcholinesterase using butyrylthiocholine iodide as substrate preincubated for 15 mins followed by substrate addition ... | Eur J Med Chem 102: 80-92 (2015) Article DOI: 10.1016/j.ejmech.2015.07.005 BindingDB Entry DOI: 10.7270/Q2JM2CFX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50118814 (CHEMBL3617375) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Bezmialem Vakif University Curated by ChEMBL | Assay Description Inhibition of electric eel acetylcholinesterase using acetylthiocholine iodide as substrate preincubated for 15 mins followed by substrate addition b... | Eur J Med Chem 102: 80-92 (2015) Article DOI: 10.1016/j.ejmech.2015.07.005 BindingDB Entry DOI: 10.7270/Q2JM2CFX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50118895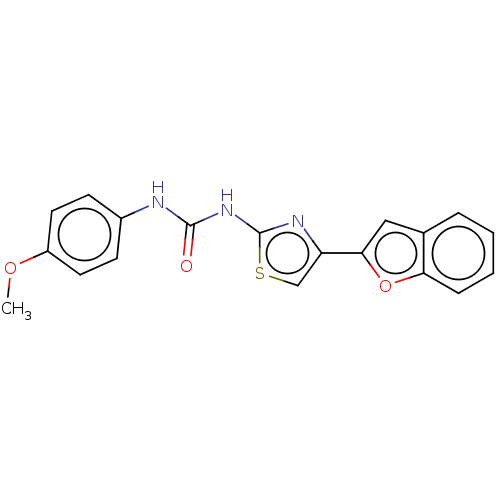 (CHEMBL3617364) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.25E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Bezmialem Vakif University Curated by ChEMBL | Assay Description Inhibition of electric eel acetylcholinesterase using acetylthiocholine iodide as substrate preincubated for 15 mins followed by substrate addition b... | Eur J Med Chem 102: 80-92 (2015) Article DOI: 10.1016/j.ejmech.2015.07.005 BindingDB Entry DOI: 10.7270/Q2JM2CFX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50118817 (CHEMBL3617372) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Bezmialem Vakif University Curated by ChEMBL | Assay Description Inhibition of electric eel acetylcholinesterase using acetylthiocholine iodide as substrate preincubated for 15 mins followed by substrate addition b... | Eur J Med Chem 102: 80-92 (2015) Article DOI: 10.1016/j.ejmech.2015.07.005 BindingDB Entry DOI: 10.7270/Q2JM2CFX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50118797 (CHEMBL3617376) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.52E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Bezmialem Vakif University Curated by ChEMBL | Assay Description Inhibition of horse serum butyrylcholinesterase using butyrylthiocholine iodide as substrate preincubated for 15 mins followed by substrate addition ... | Eur J Med Chem 102: 80-92 (2015) Article DOI: 10.1016/j.ejmech.2015.07.005 BindingDB Entry DOI: 10.7270/Q2JM2CFX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50118890 (CHEMBL3617365) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 5.63E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Bezmialem Vakif University Curated by ChEMBL | Assay Description Inhibition of horse serum butyrylcholinesterase using butyrylthiocholine iodide as substrate preincubated for 15 mins followed by substrate addition ... | Eur J Med Chem 102: 80-92 (2015) Article DOI: 10.1016/j.ejmech.2015.07.005 BindingDB Entry DOI: 10.7270/Q2JM2CFX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50118911 (CHEMBL3617363) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.96E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Bezmialem Vakif University Curated by ChEMBL | Assay Description Inhibition of horse serum butyrylcholinesterase using butyrylthiocholine iodide as substrate preincubated for 15 mins followed by substrate addition ... | Eur J Med Chem 102: 80-92 (2015) Article DOI: 10.1016/j.ejmech.2015.07.005 BindingDB Entry DOI: 10.7270/Q2JM2CFX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50118925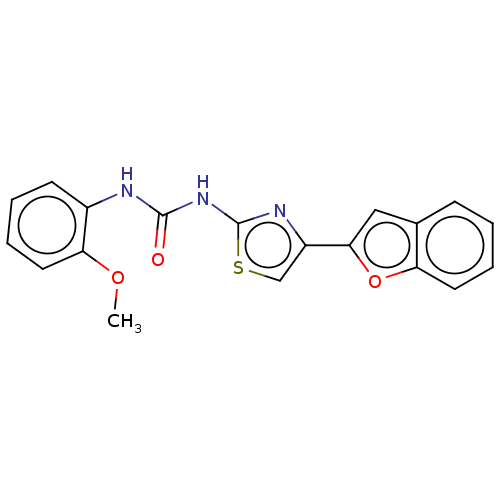 (CHEMBL3617362) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 6.10E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Bezmialem Vakif University Curated by ChEMBL | Assay Description Inhibition of electric eel acetylcholinesterase using acetylthiocholine iodide as substrate preincubated for 15 mins followed by substrate addition b... | Eur J Med Chem 102: 80-92 (2015) Article DOI: 10.1016/j.ejmech.2015.07.005 BindingDB Entry DOI: 10.7270/Q2JM2CFX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50119100 (CHEMBL3617388) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6.43E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Bezmialem Vakif University Curated by ChEMBL | Assay Description Inhibition of horse serum butyrylcholinesterase using butyrylthiocholine iodide as substrate preincubated for 15 mins followed by substrate addition ... | Eur J Med Chem 102: 80-92 (2015) Article DOI: 10.1016/j.ejmech.2015.07.005 BindingDB Entry DOI: 10.7270/Q2JM2CFX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50118895 (CHEMBL3617364) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 7.43E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Bezmialem Vakif University Curated by ChEMBL | Assay Description Inhibition of horse serum butyrylcholinesterase using butyrylthiocholine iodide as substrate preincubated for 15 mins followed by substrate addition ... | Eur J Med Chem 102: 80-92 (2015) Article DOI: 10.1016/j.ejmech.2015.07.005 BindingDB Entry DOI: 10.7270/Q2JM2CFX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50118814 (CHEMBL3617375) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 7.56E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Bezmialem Vakif University Curated by ChEMBL | Assay Description Inhibition of horse serum butyrylcholinesterase using butyrylthiocholine iodide as substrate preincubated for 15 mins followed by substrate addition ... | Eur J Med Chem 102: 80-92 (2015) Article DOI: 10.1016/j.ejmech.2015.07.005 BindingDB Entry DOI: 10.7270/Q2JM2CFX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50118957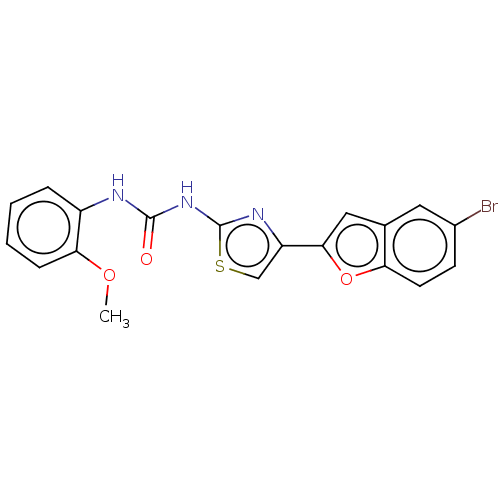 (CHEMBL3617378) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | 7.89E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Bezmialem Vakif University Curated by ChEMBL | Assay Description Inhibition of electric eel acetylcholinesterase using acetylthiocholine iodide as substrate preincubated for 15 mins followed by substrate addition b... | Eur J Med Chem 102: 80-92 (2015) Article DOI: 10.1016/j.ejmech.2015.07.005 BindingDB Entry DOI: 10.7270/Q2JM2CFX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50119075 (CHEMBL3617384) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 7.90E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Bezmialem Vakif University Curated by ChEMBL | Assay Description Inhibition of horse serum butyrylcholinesterase using butyrylthiocholine iodide as substrate preincubated for 15 mins followed by substrate addition ... | Eur J Med Chem 102: 80-92 (2015) Article DOI: 10.1016/j.ejmech.2015.07.005 BindingDB Entry DOI: 10.7270/Q2JM2CFX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50118861 (CHEMBL3617367) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 7.99E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Bezmialem Vakif University Curated by ChEMBL | Assay Description Inhibition of horse serum butyrylcholinesterase using butyrylthiocholine iodide as substrate preincubated for 15 mins followed by substrate addition ... | Eur J Med Chem 102: 80-92 (2015) Article DOI: 10.1016/j.ejmech.2015.07.005 BindingDB Entry DOI: 10.7270/Q2JM2CFX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50118925 (CHEMBL3617362) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 8.13E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Bezmialem Vakif University Curated by ChEMBL | Assay Description Inhibition of horse serum butyrylcholinesterase using butyrylthiocholine iodide as substrate preincubated for 15 mins followed by substrate addition ... | Eur J Med Chem 102: 80-92 (2015) Article DOI: 10.1016/j.ejmech.2015.07.005 BindingDB Entry DOI: 10.7270/Q2JM2CFX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50118957 (CHEMBL3617378) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | 1.54E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Bezmialem Vakif University Curated by ChEMBL | Assay Description Inhibition of horse serum butyrylcholinesterase using butyrylthiocholine iodide as substrate preincubated for 15 mins followed by substrate addition ... | Eur J Med Chem 102: 80-92 (2015) Article DOI: 10.1016/j.ejmech.2015.07.005 BindingDB Entry DOI: 10.7270/Q2JM2CFX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||