

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cocaine esterase (Homo sapiens (Human)) | BDBM50130903 (CHEMBL3632950) | NCI pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.76E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Liaoning University of Traditional Chinese Medicine Curated by ChEMBL | Assay Description Fixed inhibition of human carboxylesterase 2 using 4-benzoyl-N-butyl-1,8-naphthalimide as substrate by Dixon and Lineweaver-Burk plot analysis | J Nat Prod 78: 2372-80 (2015) Article DOI: 10.1021/acs.jnatprod.5b00321 BindingDB Entry DOI: 10.7270/Q20K2BDR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cocaine esterase (Homo sapiens (Human)) | BDBM50130903 (CHEMBL3632950) | NCI pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.02E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Liaoning University of Traditional Chinese Medicine Curated by ChEMBL | Assay Description Inhibition of human carboxylesterase 2 using 4-benzoyl-N-butyl-1,8-naphthalimide as substrate by fluorescence assay | J Nat Prod 78: 2372-80 (2015) Article DOI: 10.1021/acs.jnatprod.5b00321 BindingDB Entry DOI: 10.7270/Q20K2BDR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cocaine esterase (Homo sapiens (Human)) | BDBM50130915 (CHEBI:3122 | CHEMBL1231178) | NCI pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | 4.03E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Liaoning University of Traditional Chinese Medicine Curated by ChEMBL | Assay Description Inhibition of human carboxylesterase 2 using 4-benzoyl-N-butyl-1,8-naphthalimide as substrate by fluorescence assay | J Nat Prod 78: 2372-80 (2015) Article DOI: 10.1021/acs.jnatprod.5b00321 BindingDB Entry DOI: 10.7270/Q20K2BDR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cocaine esterase (Homo sapiens (Human)) | BDBM50130901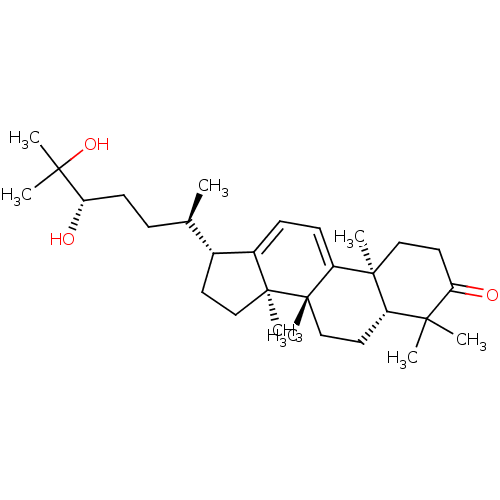 (CHEMBL3632948) | NCI pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.58E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Liaoning University of Traditional Chinese Medicine Curated by ChEMBL | Assay Description Inhibition of human carboxylesterase 2 using 4-benzoyl-N-butyl-1,8-naphthalimide as substrate by fluorescence assay | J Nat Prod 78: 2372-80 (2015) Article DOI: 10.1021/acs.jnatprod.5b00321 BindingDB Entry DOI: 10.7270/Q20K2BDR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cocaine esterase (Homo sapiens (Human)) | BDBM50130908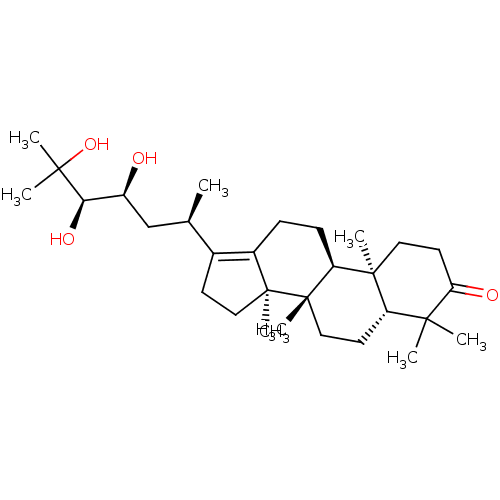 (CHEMBL3632954) | NCI pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.63E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Liaoning University of Traditional Chinese Medicine Curated by ChEMBL | Assay Description Inhibition of human carboxylesterase 2 using 4-benzoyl-N-butyl-1,8-naphthalimide as substrate by fluorescence assay | J Nat Prod 78: 2372-80 (2015) Article DOI: 10.1021/acs.jnatprod.5b00321 BindingDB Entry DOI: 10.7270/Q20K2BDR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cocaine esterase (Homo sapiens (Human)) | BDBM50130884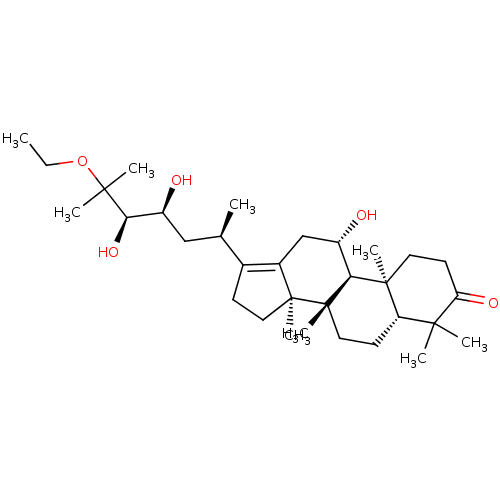 (CHEMBL3632946) | NCI pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.72E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Liaoning University of Traditional Chinese Medicine Curated by ChEMBL | Assay Description Inhibition of human carboxylesterase 2 using 4-benzoyl-N-butyl-1,8-naphthalimide as substrate by fluorescence assay | J Nat Prod 78: 2372-80 (2015) Article DOI: 10.1021/acs.jnatprod.5b00321 BindingDB Entry DOI: 10.7270/Q20K2BDR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cocaine esterase (Homo sapiens (Human)) | BDBM50130919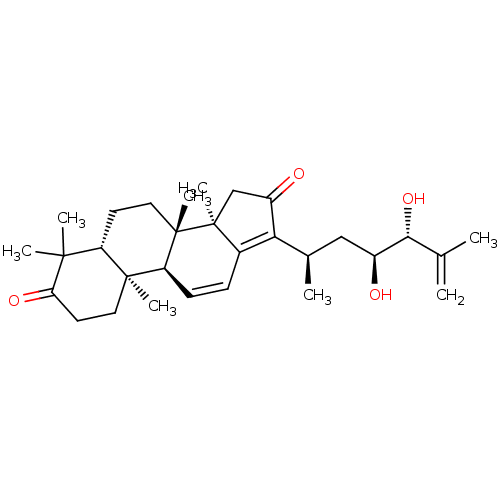 (CHEMBL3632942) | NCI pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 8.68E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Liaoning University of Traditional Chinese Medicine Curated by ChEMBL | Assay Description Inhibition of human carboxylesterase 2 using 4-benzoyl-N-butyl-1,8-naphthalimide as substrate by fluorescence assay | J Nat Prod 78: 2372-80 (2015) Article DOI: 10.1021/acs.jnatprod.5b00321 BindingDB Entry DOI: 10.7270/Q20K2BDR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cocaine esterase (Homo sapiens (Human)) | BDBM50130922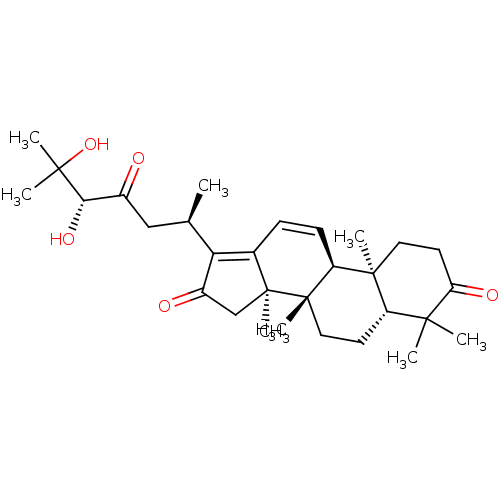 (CHEMBL3632941) | NCI pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.01E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Liaoning University of Traditional Chinese Medicine Curated by ChEMBL | Assay Description Inhibition of human carboxylesterase 2 using 4-benzoyl-N-butyl-1,8-naphthalimide as substrate by fluorescence assay | J Nat Prod 78: 2372-80 (2015) Article DOI: 10.1021/acs.jnatprod.5b00321 BindingDB Entry DOI: 10.7270/Q20K2BDR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cocaine esterase (Homo sapiens (Human)) | BDBM50130905 (CHEMBL3632952) | NCI pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.08E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Liaoning University of Traditional Chinese Medicine Curated by ChEMBL | Assay Description Inhibition of human carboxylesterase 2 using 4-benzoyl-N-butyl-1,8-naphthalimide as substrate by fluorescence assay | J Nat Prod 78: 2372-80 (2015) Article DOI: 10.1021/acs.jnatprod.5b00321 BindingDB Entry DOI: 10.7270/Q20K2BDR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cocaine esterase (Homo sapiens (Human)) | BDBM50130913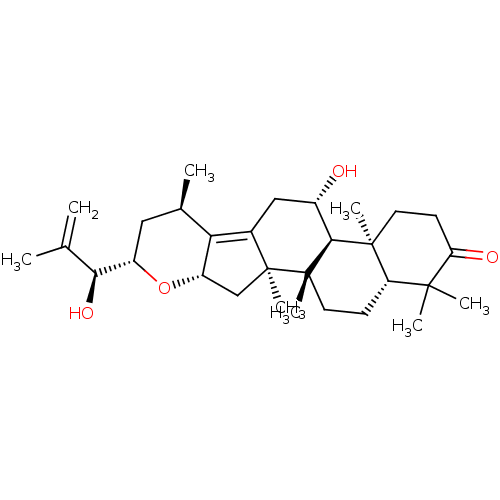 (CHEMBL3632958) | NCI pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.09E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Liaoning University of Traditional Chinese Medicine Curated by ChEMBL | Assay Description Inhibition of human carboxylesterase 2 using 4-benzoyl-N-butyl-1,8-naphthalimide as substrate by fluorescence assay | J Nat Prod 78: 2372-80 (2015) Article DOI: 10.1021/acs.jnatprod.5b00321 BindingDB Entry DOI: 10.7270/Q20K2BDR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cocaine esterase (Homo sapiens (Human)) | BDBM50130911 (CHEMBL3632956) | NCI pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.73E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Liaoning University of Traditional Chinese Medicine Curated by ChEMBL | Assay Description Inhibition of human carboxylesterase 2 using 4-benzoyl-N-butyl-1,8-naphthalimide as substrate by fluorescence assay | J Nat Prod 78: 2372-80 (2015) Article DOI: 10.1021/acs.jnatprod.5b00321 BindingDB Entry DOI: 10.7270/Q20K2BDR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cocaine esterase (Homo sapiens (Human)) | BDBM50130894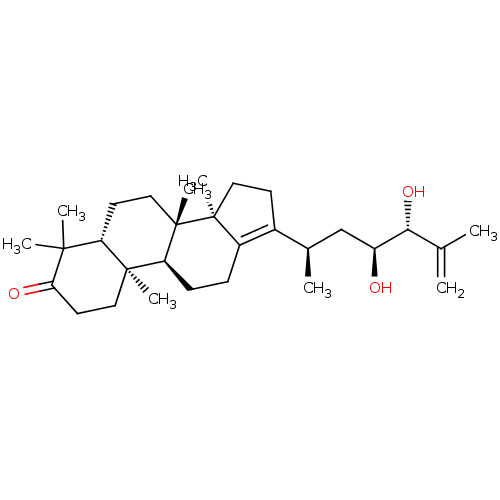 (CHEMBL3632627) | NCI pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.74E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Liaoning University of Traditional Chinese Medicine Curated by ChEMBL | Assay Description Inhibition of human carboxylesterase 2 using 4-benzoyl-N-butyl-1,8-naphthalimide as substrate by fluorescence assay | J Nat Prod 78: 2372-80 (2015) Article DOI: 10.1021/acs.jnatprod.5b00321 BindingDB Entry DOI: 10.7270/Q20K2BDR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cocaine esterase (Homo sapiens (Human)) | BDBM50130914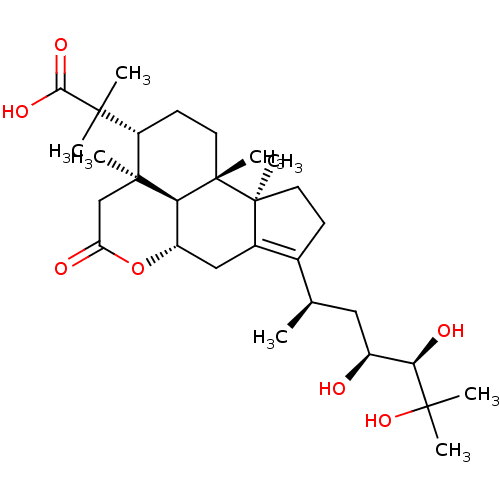 (CHEMBL3632959) | NCI pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.09E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Liaoning University of Traditional Chinese Medicine Curated by ChEMBL | Assay Description Inhibition of human carboxylesterase 2 using 4-benzoyl-N-butyl-1,8-naphthalimide as substrate by fluorescence assay | J Nat Prod 78: 2372-80 (2015) Article DOI: 10.1021/acs.jnatprod.5b00321 BindingDB Entry DOI: 10.7270/Q20K2BDR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cocaine esterase (Homo sapiens (Human)) | BDBM50130906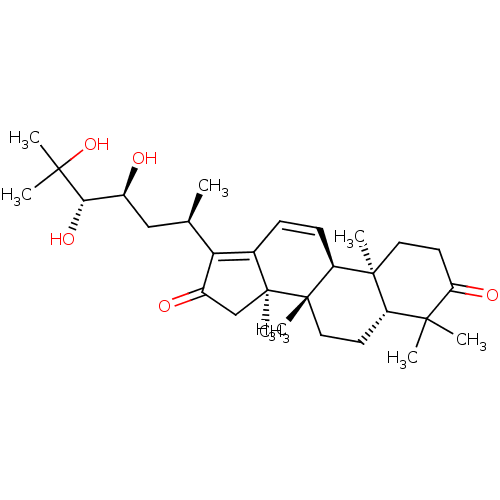 (CHEMBL3632953) | NCI pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.27E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Liaoning University of Traditional Chinese Medicine Curated by ChEMBL | Assay Description Inhibition of human carboxylesterase 2 using 4-benzoyl-N-butyl-1,8-naphthalimide as substrate by fluorescence assay | J Nat Prod 78: 2372-80 (2015) Article DOI: 10.1021/acs.jnatprod.5b00321 BindingDB Entry DOI: 10.7270/Q20K2BDR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cocaine esterase (Homo sapiens (Human)) | BDBM50130912 (CHEMBL3632957) | NCI pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.43E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Liaoning University of Traditional Chinese Medicine Curated by ChEMBL | Assay Description Inhibition of human carboxylesterase 2 using 4-benzoyl-N-butyl-1,8-naphthalimide as substrate by fluorescence assay | J Nat Prod 78: 2372-80 (2015) Article DOI: 10.1021/acs.jnatprod.5b00321 BindingDB Entry DOI: 10.7270/Q20K2BDR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cocaine esterase (Homo sapiens (Human)) | BDBM50130900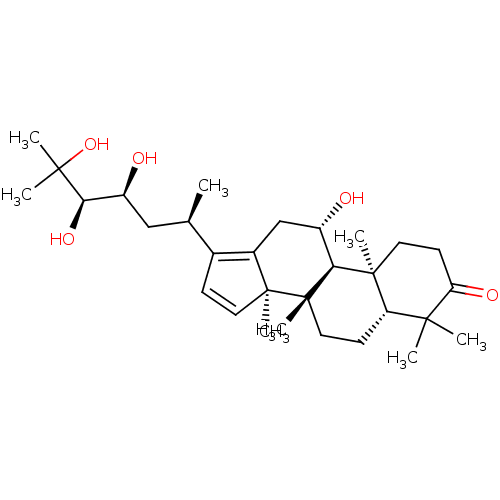 (CHEMBL3632947) | NCI pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 2.57E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Liaoning University of Traditional Chinese Medicine Curated by ChEMBL | Assay Description Inhibition of human carboxylesterase 2 using 4-benzoyl-N-butyl-1,8-naphthalimide as substrate by fluorescence assay | J Nat Prod 78: 2372-80 (2015) Article DOI: 10.1021/acs.jnatprod.5b00321 BindingDB Entry DOI: 10.7270/Q20K2BDR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cocaine esterase (Homo sapiens (Human)) | BDBM50130918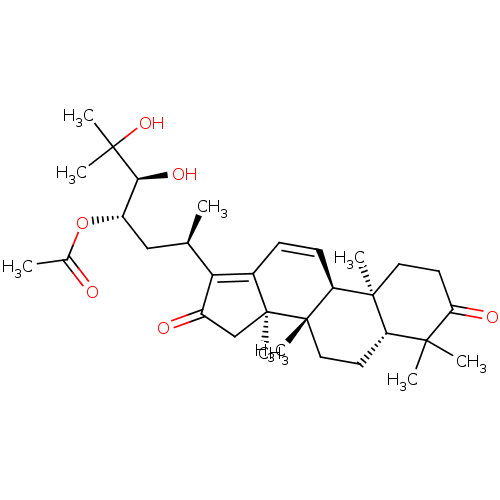 (CHEMBL3632943) | NCI pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.34E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Liaoning University of Traditional Chinese Medicine Curated by ChEMBL | Assay Description Inhibition of human carboxylesterase 2 using 4-benzoyl-N-butyl-1,8-naphthalimide as substrate by fluorescence assay | J Nat Prod 78: 2372-80 (2015) Article DOI: 10.1021/acs.jnatprod.5b00321 BindingDB Entry DOI: 10.7270/Q20K2BDR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cocaine esterase (Homo sapiens (Human)) | BDBM50130909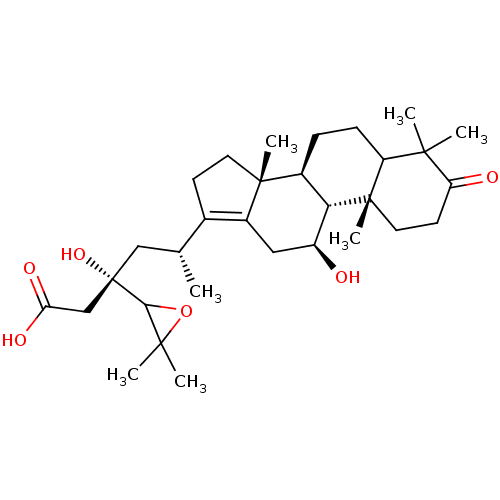 (CHEMBL2074634) | NCI pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.62E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Liaoning University of Traditional Chinese Medicine Curated by ChEMBL | Assay Description Inhibition of human carboxylesterase 2 using 4-benzoyl-N-butyl-1,8-naphthalimide as substrate by fluorescence assay | J Nat Prod 78: 2372-80 (2015) Article DOI: 10.1021/acs.jnatprod.5b00321 BindingDB Entry DOI: 10.7270/Q20K2BDR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cocaine esterase (Homo sapiens (Human)) | BDBM50130910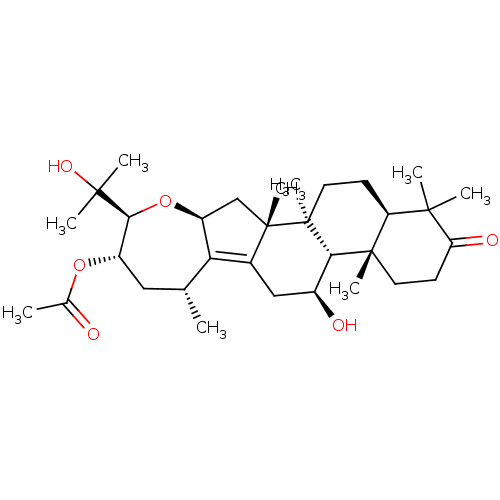 (CHEMBL3632955) | NCI pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.11E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Liaoning University of Traditional Chinese Medicine Curated by ChEMBL | Assay Description Inhibition of human carboxylesterase 2 using 4-benzoyl-N-butyl-1,8-naphthalimide as substrate by fluorescence assay | J Nat Prod 78: 2372-80 (2015) Article DOI: 10.1021/acs.jnatprod.5b00321 BindingDB Entry DOI: 10.7270/Q20K2BDR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cocaine esterase (Homo sapiens (Human)) | BDBM50130902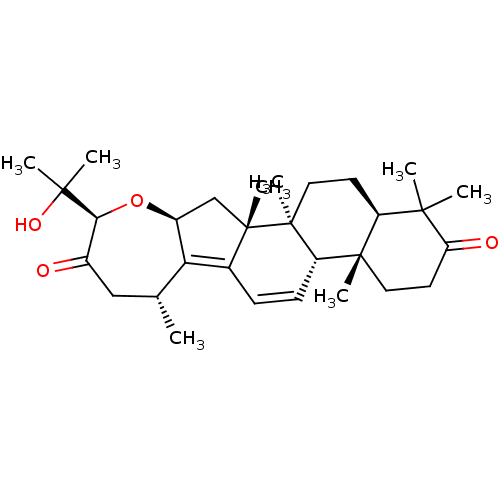 (CHEMBL3632949) | NCI pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.88E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Liaoning University of Traditional Chinese Medicine Curated by ChEMBL | Assay Description Inhibition of human carboxylesterase 2 using 4-benzoyl-N-butyl-1,8-naphthalimide as substrate by fluorescence assay | J Nat Prod 78: 2372-80 (2015) Article DOI: 10.1021/acs.jnatprod.5b00321 BindingDB Entry DOI: 10.7270/Q20K2BDR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cocaine esterase (Homo sapiens (Human)) | BDBM50130904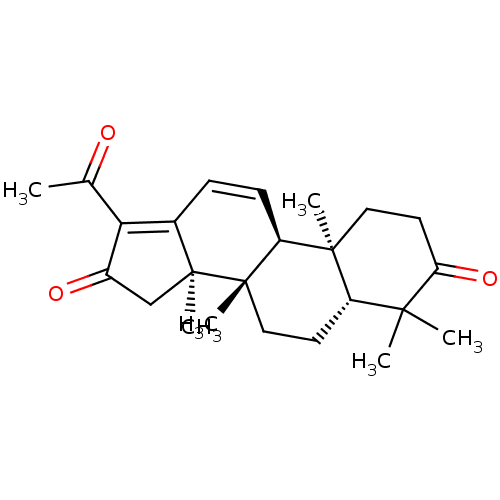 (CHEMBL3632951) | NCI pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 7.16E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Liaoning University of Traditional Chinese Medicine Curated by ChEMBL | Assay Description Inhibition of human carboxylesterase 2 using 4-benzoyl-N-butyl-1,8-naphthalimide as substrate by fluorescence assay | J Nat Prod 78: 2372-80 (2015) Article DOI: 10.1021/acs.jnatprod.5b00321 BindingDB Entry DOI: 10.7270/Q20K2BDR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cocaine esterase (Homo sapiens (Human)) | BDBM50130916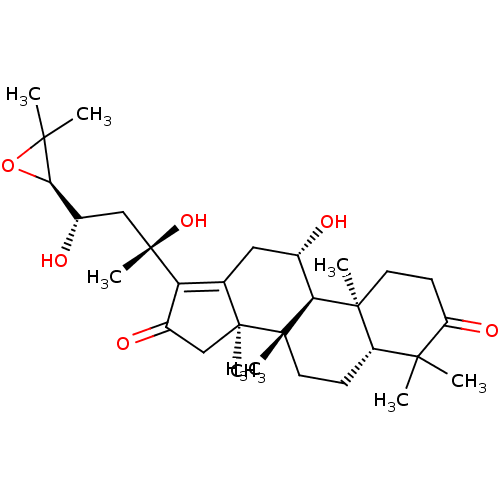 (CHEMBL3632945) | NCI pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Liaoning University of Traditional Chinese Medicine Curated by ChEMBL | Assay Description Inhibition of human carboxylesterase 2 using 4-benzoyl-N-butyl-1,8-naphthalimide as substrate by fluorescence assay | J Nat Prod 78: 2372-80 (2015) Article DOI: 10.1021/acs.jnatprod.5b00321 BindingDB Entry DOI: 10.7270/Q20K2BDR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cocaine esterase (Homo sapiens (Human)) | BDBM50130917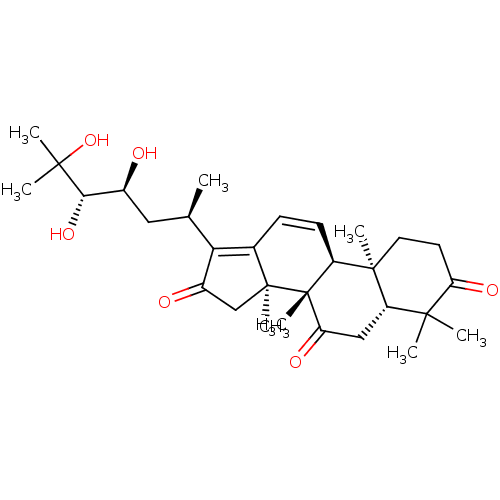 (CHEMBL3632944) | NCI pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Liaoning University of Traditional Chinese Medicine Curated by ChEMBL | Assay Description Inhibition of human carboxylesterase 2 using 4-benzoyl-N-butyl-1,8-naphthalimide as substrate by fluorescence assay | J Nat Prod 78: 2372-80 (2015) Article DOI: 10.1021/acs.jnatprod.5b00321 BindingDB Entry DOI: 10.7270/Q20K2BDR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cocaine esterase (Homo sapiens (Human)) | BDBM50130907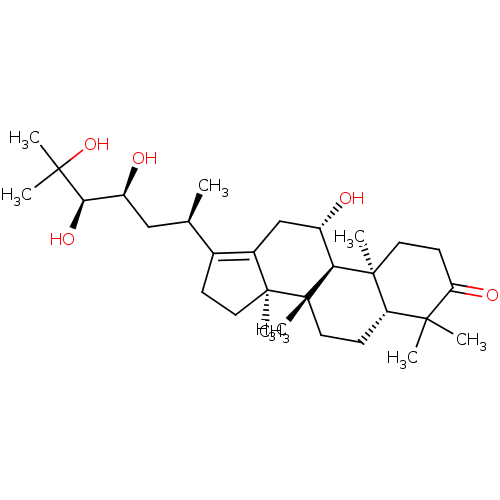 (ALISOL A) | NCI pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE KEGG PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Liaoning University of Traditional Chinese Medicine Curated by ChEMBL | Assay Description Inhibition of human carboxylesterase 2 using 4-benzoyl-N-butyl-1,8-naphthalimide as substrate by fluorescence assay | J Nat Prod 78: 2372-80 (2015) Article DOI: 10.1021/acs.jnatprod.5b00321 BindingDB Entry DOI: 10.7270/Q20K2BDR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||