

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50128528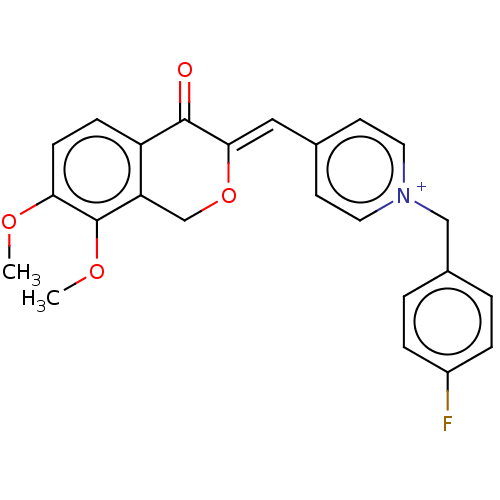 (CHEMBL3628617) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 8.90 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of electric eel AChE using thiocholine iodide as substrate by Ellman's method | Bioorg Med Chem Lett 25: 5212-6 (2015) Article DOI: 10.1016/j.bmcl.2015.09.063 BindingDB Entry DOI: 10.7270/Q2B56MKS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50128545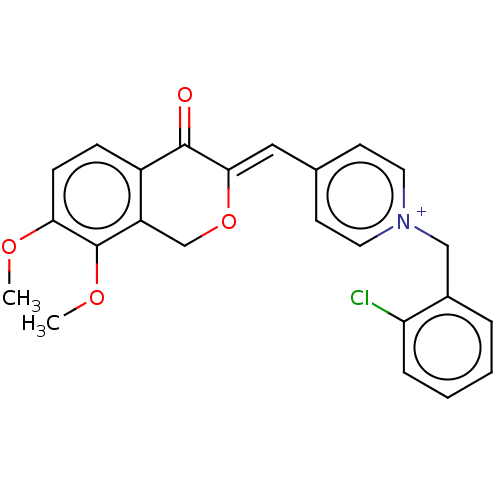 (CHEMBL3628621) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of electric eel AChE using thiocholine iodide as substrate by Ellman's method | Bioorg Med Chem Lett 25: 5212-6 (2015) Article DOI: 10.1016/j.bmcl.2015.09.063 BindingDB Entry DOI: 10.7270/Q2B56MKS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50128566 (CHEMBL3628615) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 21 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of electric eel AChE using thiocholine iodide as substrate by Ellman's method | Bioorg Med Chem Lett 25: 5212-6 (2015) Article DOI: 10.1016/j.bmcl.2015.09.063 BindingDB Entry DOI: 10.7270/Q2B56MKS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50128558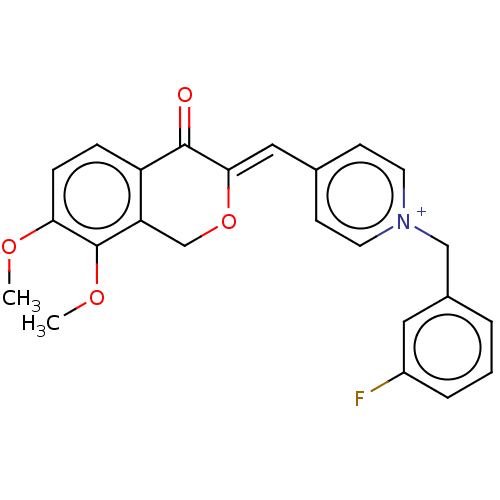 (CHEMBL3628616) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 26 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of electric eel AChE using thiocholine iodide as substrate by Ellman's method | Bioorg Med Chem Lett 25: 5212-6 (2015) Article DOI: 10.1016/j.bmcl.2015.09.063 BindingDB Entry DOI: 10.7270/Q2B56MKS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50128553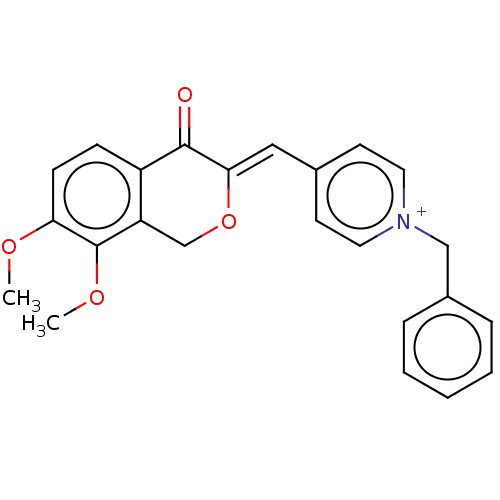 (CHEMBL3628614) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 45 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of electric eel AChE using thiocholine iodide as substrate by Ellman's method | Bioorg Med Chem Lett 25: 5212-6 (2015) Article DOI: 10.1016/j.bmcl.2015.09.063 BindingDB Entry DOI: 10.7270/Q2B56MKS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM8960 ((+/-)-2-[(1-benzylpiperidin-4-yl)methyl]-5,6-dimet...) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 60 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of electric eel AChE using thiocholine iodide as substrate by Ellman's method | Bioorg Med Chem Lett 25: 5212-6 (2015) Article DOI: 10.1016/j.bmcl.2015.09.063 BindingDB Entry DOI: 10.7270/Q2B56MKS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50128549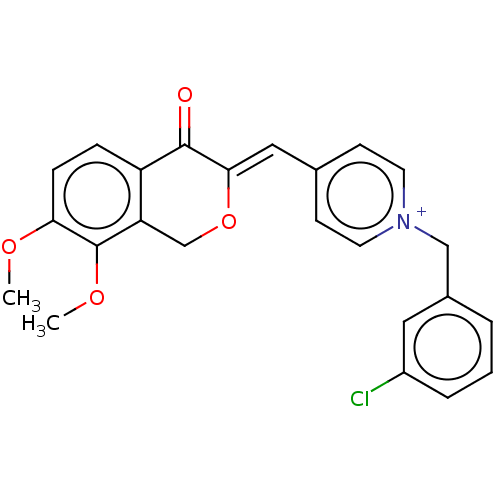 (CHEMBL3628622) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 70 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of electric eel AChE using thiocholine iodide as substrate by Ellman's method | Bioorg Med Chem Lett 25: 5212-6 (2015) Article DOI: 10.1016/j.bmcl.2015.09.063 BindingDB Entry DOI: 10.7270/Q2B56MKS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50128526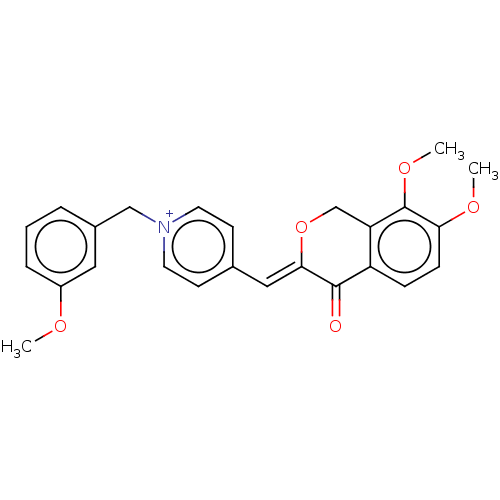 (CHEMBL3628619) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 441 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of electric eel AChE using thiocholine iodide as substrate by Ellman's method | Bioorg Med Chem Lett 25: 5212-6 (2015) Article DOI: 10.1016/j.bmcl.2015.09.063 BindingDB Entry DOI: 10.7270/Q2B56MKS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50128541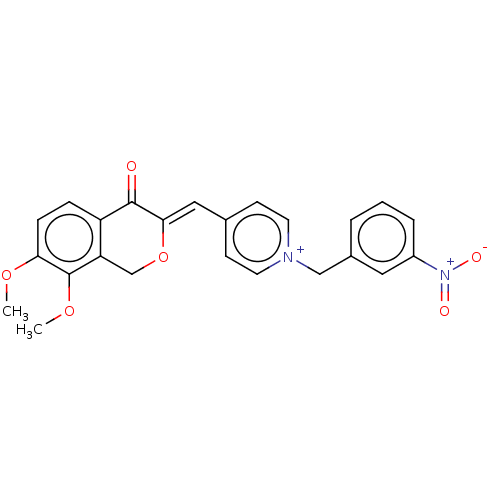 (CHEMBL3628625) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 456 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of electric eel AChE using thiocholine iodide as substrate by Ellman's method | Bioorg Med Chem Lett 25: 5212-6 (2015) Article DOI: 10.1016/j.bmcl.2015.09.063 BindingDB Entry DOI: 10.7270/Q2B56MKS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50128559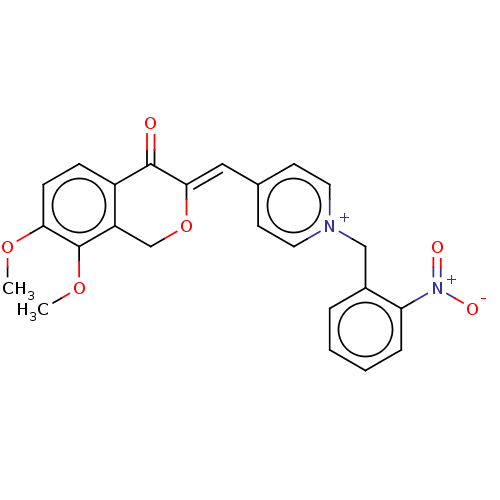 (CHEMBL3628624) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 503 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of electric eel AChE using thiocholine iodide as substrate by Ellman's method | Bioorg Med Chem Lett 25: 5212-6 (2015) Article DOI: 10.1016/j.bmcl.2015.09.063 BindingDB Entry DOI: 10.7270/Q2B56MKS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50128525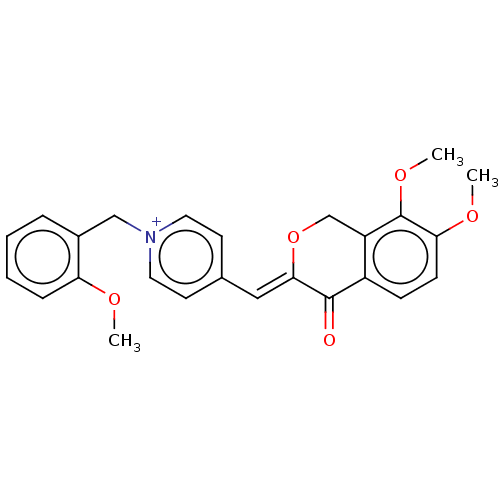 (CHEMBL3628618) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 640 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of electric eel AChE using thiocholine iodide as substrate by Ellman's method | Bioorg Med Chem Lett 25: 5212-6 (2015) Article DOI: 10.1016/j.bmcl.2015.09.063 BindingDB Entry DOI: 10.7270/Q2B56MKS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50128562 (CHEMBL3628623) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 646 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of electric eel AChE using thiocholine iodide as substrate by Ellman's method | Bioorg Med Chem Lett 25: 5212-6 (2015) Article DOI: 10.1016/j.bmcl.2015.09.063 BindingDB Entry DOI: 10.7270/Q2B56MKS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50128527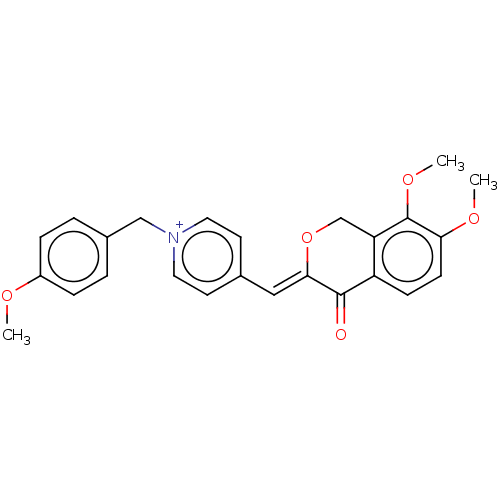 (CHEMBL3628620) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.19E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of electric eel AChE using thiocholine iodide as substrate by Ellman's method | Bioorg Med Chem Lett 25: 5212-6 (2015) Article DOI: 10.1016/j.bmcl.2015.09.063 BindingDB Entry DOI: 10.7270/Q2B56MKS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50128528 (CHEMBL3628617) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.07E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of equine serum BuChE using thiocholine iodide as substrate by Ellman's method | Bioorg Med Chem Lett 25: 5212-6 (2015) Article DOI: 10.1016/j.bmcl.2015.09.063 BindingDB Entry DOI: 10.7270/Q2B56MKS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50128526 (CHEMBL3628619) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.06E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of equine serum BuChE using thiocholine iodide as substrate by Ellman's method | Bioorg Med Chem Lett 25: 5212-6 (2015) Article DOI: 10.1016/j.bmcl.2015.09.063 BindingDB Entry DOI: 10.7270/Q2B56MKS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50128524 (CHEMBL3628626) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.55E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of electric eel AChE using thiocholine iodide as substrate by Ellman's method | Bioorg Med Chem Lett 25: 5212-6 (2015) Article DOI: 10.1016/j.bmcl.2015.09.063 BindingDB Entry DOI: 10.7270/Q2B56MKS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50128562 (CHEMBL3628623) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 3.56E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of equine serum BuChE using thiocholine iodide as substrate by Ellman's method | Bioorg Med Chem Lett 25: 5212-6 (2015) Article DOI: 10.1016/j.bmcl.2015.09.063 BindingDB Entry DOI: 10.7270/Q2B56MKS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM8960 ((+/-)-2-[(1-benzylpiperidin-4-yl)methyl]-5,6-dimet...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 3.62E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of equine serum BuChE using thiocholine iodide as substrate by Ellman's method | Bioorg Med Chem Lett 25: 5212-6 (2015) Article DOI: 10.1016/j.bmcl.2015.09.063 BindingDB Entry DOI: 10.7270/Q2B56MKS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50128545 (CHEMBL3628621) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3.64E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of equine serum BuChE using thiocholine iodide as substrate by Ellman's method | Bioorg Med Chem Lett 25: 5212-6 (2015) Article DOI: 10.1016/j.bmcl.2015.09.063 BindingDB Entry DOI: 10.7270/Q2B56MKS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50128527 (CHEMBL3628620) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.33E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of equine serum BuChE using thiocholine iodide as substrate by Ellman's method | Bioorg Med Chem Lett 25: 5212-6 (2015) Article DOI: 10.1016/j.bmcl.2015.09.063 BindingDB Entry DOI: 10.7270/Q2B56MKS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50128566 (CHEMBL3628615) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.41E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of equine serum BuChE using thiocholine iodide as substrate by Ellman's method | Bioorg Med Chem Lett 25: 5212-6 (2015) Article DOI: 10.1016/j.bmcl.2015.09.063 BindingDB Entry DOI: 10.7270/Q2B56MKS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50128553 (CHEMBL3628614) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 6.87E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of equine serum BuChE using thiocholine iodide as substrate by Ellman's method | Bioorg Med Chem Lett 25: 5212-6 (2015) Article DOI: 10.1016/j.bmcl.2015.09.063 BindingDB Entry DOI: 10.7270/Q2B56MKS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50128525 (CHEMBL3628618) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 8.79E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of equine serum BuChE using thiocholine iodide as substrate by Ellman's method | Bioorg Med Chem Lett 25: 5212-6 (2015) Article DOI: 10.1016/j.bmcl.2015.09.063 BindingDB Entry DOI: 10.7270/Q2B56MKS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50128558 (CHEMBL3628616) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 9.44E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of equine serum BuChE using thiocholine iodide as substrate by Ellman's method | Bioorg Med Chem Lett 25: 5212-6 (2015) Article DOI: 10.1016/j.bmcl.2015.09.063 BindingDB Entry DOI: 10.7270/Q2B56MKS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50128549 (CHEMBL3628622) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 9.92E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of equine serum BuChE using thiocholine iodide as substrate by Ellman's method | Bioorg Med Chem Lett 25: 5212-6 (2015) Article DOI: 10.1016/j.bmcl.2015.09.063 BindingDB Entry DOI: 10.7270/Q2B56MKS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50128559 (CHEMBL3628624) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.09E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of equine serum BuChE using thiocholine iodide as substrate by Ellman's method | Bioorg Med Chem Lett 25: 5212-6 (2015) Article DOI: 10.1016/j.bmcl.2015.09.063 BindingDB Entry DOI: 10.7270/Q2B56MKS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50128541 (CHEMBL3628625) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.92E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of equine serum BuChE using thiocholine iodide as substrate by Ellman's method | Bioorg Med Chem Lett 25: 5212-6 (2015) Article DOI: 10.1016/j.bmcl.2015.09.063 BindingDB Entry DOI: 10.7270/Q2B56MKS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50128524 (CHEMBL3628626) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of equine serum BuChE using thiocholine iodide as substrate by Ellman's method | Bioorg Med Chem Lett 25: 5212-6 (2015) Article DOI: 10.1016/j.bmcl.2015.09.063 BindingDB Entry DOI: 10.7270/Q2B56MKS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||