

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Bifunctional epoxide hydrolase 2 (Homo sapiens (Human)) | BDBM50133707 (CHEMBL3633681) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.10 | n/a | n/a | n/a | n/a | n/a | n/a |
University of California at Davis Curated by ChEMBL | Assay Description Inhibition of recombinant human soluble epoxide hydrolase using CMNPC as substrate by kinetic fluorescent assay | Bioorg Med Chem Lett 25: 5514-9 (2015) Article DOI: 10.1016/j.bmcl.2015.10.066 BindingDB Entry DOI: 10.7270/Q28P62C1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional epoxide hydrolase 2 (Homo sapiens (Human)) | BDBM50133694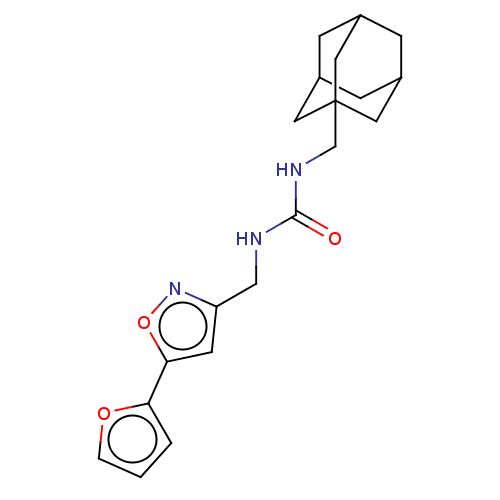 (CHEMBL3633668) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.90 | n/a | n/a | n/a | n/a | n/a | n/a |
University of California at Davis Curated by ChEMBL | Assay Description Inhibition of recombinant human soluble epoxide hydrolase using CMNPC as substrate by kinetic fluorescent assay | Bioorg Med Chem Lett 25: 5514-9 (2015) Article DOI: 10.1016/j.bmcl.2015.10.066 BindingDB Entry DOI: 10.7270/Q28P62C1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional epoxide hydrolase 2 (Homo sapiens (Human)) | BDBM50133695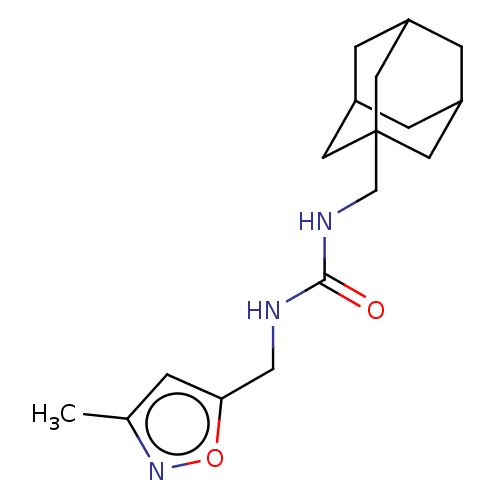 (CHEMBL3633669) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6.70 | n/a | n/a | n/a | n/a | n/a | n/a |
University of California at Davis Curated by ChEMBL | Assay Description Inhibition of recombinant human soluble epoxide hydrolase using CMNPC as substrate by kinetic fluorescent assay | Bioorg Med Chem Lett 25: 5514-9 (2015) Article DOI: 10.1016/j.bmcl.2015.10.066 BindingDB Entry DOI: 10.7270/Q28P62C1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional epoxide hydrolase 2 (Homo sapiens (Human)) | BDBM50133699 (CHEMBL3633673) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
University of California at Davis Curated by ChEMBL | Assay Description Inhibition of recombinant human soluble epoxide hydrolase using CMNPC as substrate by kinetic fluorescent assay | Bioorg Med Chem Lett 25: 5514-9 (2015) Article DOI: 10.1016/j.bmcl.2015.10.066 BindingDB Entry DOI: 10.7270/Q28P62C1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional epoxide hydrolase 2 (Homo sapiens (Human)) | BDBM50133693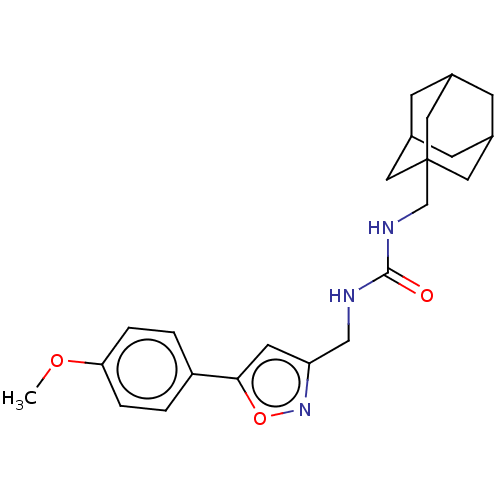 (CHEMBL3633667) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
University of California at Davis Curated by ChEMBL | Assay Description Inhibition of recombinant human soluble epoxide hydrolase using CMNPC as substrate by kinetic fluorescent assay | Bioorg Med Chem Lett 25: 5514-9 (2015) Article DOI: 10.1016/j.bmcl.2015.10.066 BindingDB Entry DOI: 10.7270/Q28P62C1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional epoxide hydrolase 2 (Homo sapiens (Human)) | BDBM50133706 (CHEMBL3633680) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
University of California at Davis Curated by ChEMBL | Assay Description Inhibition of recombinant human soluble epoxide hydrolase using CMNPC as substrate by kinetic fluorescent assay | Bioorg Med Chem Lett 25: 5514-9 (2015) Article DOI: 10.1016/j.bmcl.2015.10.066 BindingDB Entry DOI: 10.7270/Q28P62C1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional epoxide hydrolase 2 (Homo sapiens (Human)) | BDBM50133692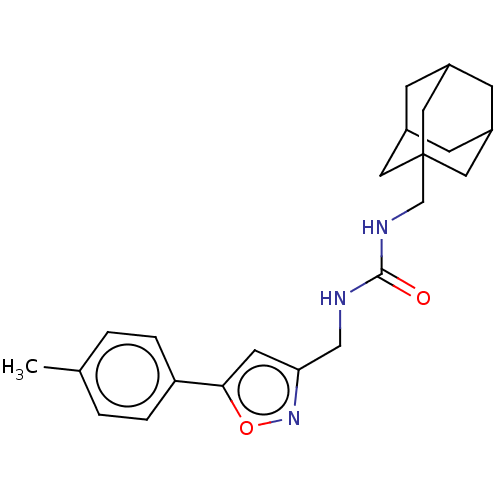 (CHEMBL3633666) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
University of California at Davis Curated by ChEMBL | Assay Description Inhibition of recombinant human soluble epoxide hydrolase using CMNPC as substrate by kinetic fluorescent assay | Bioorg Med Chem Lett 25: 5514-9 (2015) Article DOI: 10.1016/j.bmcl.2015.10.066 BindingDB Entry DOI: 10.7270/Q28P62C1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional epoxide hydrolase 2 (Homo sapiens (Human)) | BDBM50133698 (CHEMBL3633672) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 17 | n/a | n/a | n/a | n/a | n/a | n/a |
University of California at Davis Curated by ChEMBL | Assay Description Inhibition of recombinant human soluble epoxide hydrolase using CMNPC as substrate by kinetic fluorescent assay | Bioorg Med Chem Lett 25: 5514-9 (2015) Article DOI: 10.1016/j.bmcl.2015.10.066 BindingDB Entry DOI: 10.7270/Q28P62C1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional epoxide hydrolase 2 (Homo sapiens (Human)) | BDBM50133709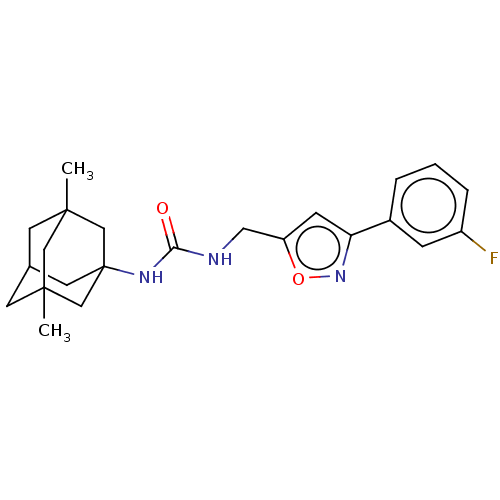 (CHEMBL3633683) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 17 | n/a | n/a | n/a | n/a | n/a | n/a |
University of California at Davis Curated by ChEMBL | Assay Description Inhibition of recombinant human soluble epoxide hydrolase using CMNPC as substrate by kinetic fluorescent assay | Bioorg Med Chem Lett 25: 5514-9 (2015) Article DOI: 10.1016/j.bmcl.2015.10.066 BindingDB Entry DOI: 10.7270/Q28P62C1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional epoxide hydrolase 2 (Homo sapiens (Human)) | BDBM50133705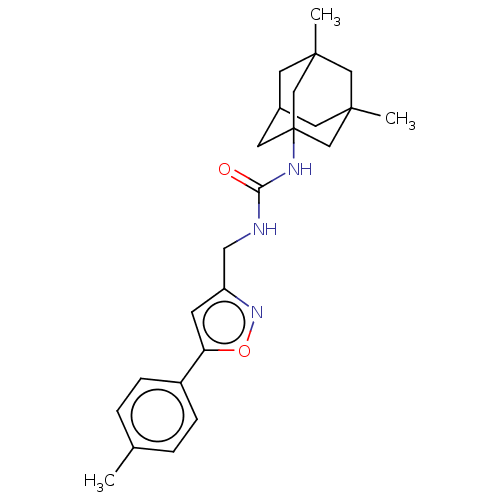 (CHEMBL3633679) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 26 | n/a | n/a | n/a | n/a | n/a | n/a |
University of California at Davis Curated by ChEMBL | Assay Description Inhibition of recombinant human soluble epoxide hydrolase using CMNPC as substrate by kinetic fluorescent assay | Bioorg Med Chem Lett 25: 5514-9 (2015) Article DOI: 10.1016/j.bmcl.2015.10.066 BindingDB Entry DOI: 10.7270/Q28P62C1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional epoxide hydrolase 2 (Homo sapiens (Human)) | BDBM50133696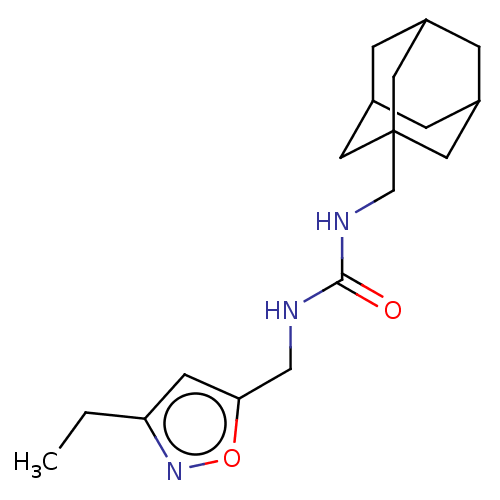 (CHEMBL3633670) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 29 | n/a | n/a | n/a | n/a | n/a | n/a |
University of California at Davis Curated by ChEMBL | Assay Description Inhibition of recombinant human soluble epoxide hydrolase using CMNPC as substrate by kinetic fluorescent assay | Bioorg Med Chem Lett 25: 5514-9 (2015) Article DOI: 10.1016/j.bmcl.2015.10.066 BindingDB Entry DOI: 10.7270/Q28P62C1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional epoxide hydrolase 2 (Homo sapiens (Human)) | BDBM50133697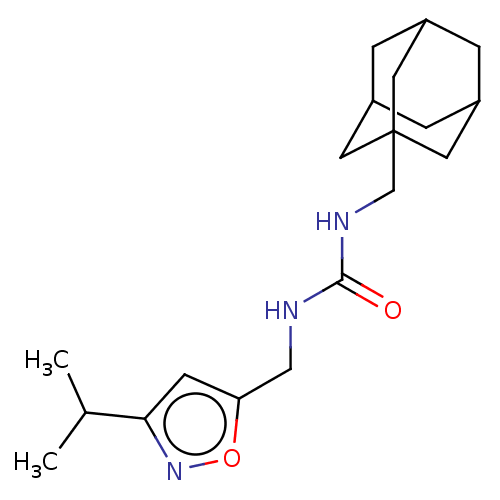 (CHEMBL3633671) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 45 | n/a | n/a | n/a | n/a | n/a | n/a |
University of California at Davis Curated by ChEMBL | Assay Description Inhibition of recombinant human soluble epoxide hydrolase using CMNPC as substrate by kinetic fluorescent assay | Bioorg Med Chem Lett 25: 5514-9 (2015) Article DOI: 10.1016/j.bmcl.2015.10.066 BindingDB Entry DOI: 10.7270/Q28P62C1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional epoxide hydrolase 2 (Homo sapiens (Human)) | BDBM50133704 (CHEMBL3633678) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 91 | n/a | n/a | n/a | n/a | n/a | n/a |
University of California at Davis Curated by ChEMBL | Assay Description Inhibition of recombinant human soluble epoxide hydrolase using CMNPC as substrate by kinetic fluorescent assay | Bioorg Med Chem Lett 25: 5514-9 (2015) Article DOI: 10.1016/j.bmcl.2015.10.066 BindingDB Entry DOI: 10.7270/Q28P62C1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional epoxide hydrolase 2 (Homo sapiens (Human)) | BDBM50133708 (CHEMBL3633682) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 123 | n/a | n/a | n/a | n/a | n/a | n/a |
University of California at Davis Curated by ChEMBL | Assay Description Inhibition of recombinant human soluble epoxide hydrolase using CMNPC as substrate by kinetic fluorescent assay | Bioorg Med Chem Lett 25: 5514-9 (2015) Article DOI: 10.1016/j.bmcl.2015.10.066 BindingDB Entry DOI: 10.7270/Q28P62C1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional epoxide hydrolase 2 (Homo sapiens (Human)) | BDBM50133703 (CHEMBL3633677) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 191 | n/a | n/a | n/a | n/a | n/a | n/a |
University of California at Davis Curated by ChEMBL | Assay Description Inhibition of recombinant human soluble epoxide hydrolase using CMNPC as substrate by kinetic fluorescent assay | Bioorg Med Chem Lett 25: 5514-9 (2015) Article DOI: 10.1016/j.bmcl.2015.10.066 BindingDB Entry DOI: 10.7270/Q28P62C1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional epoxide hydrolase 2 (Homo sapiens (Human)) | BDBM50133702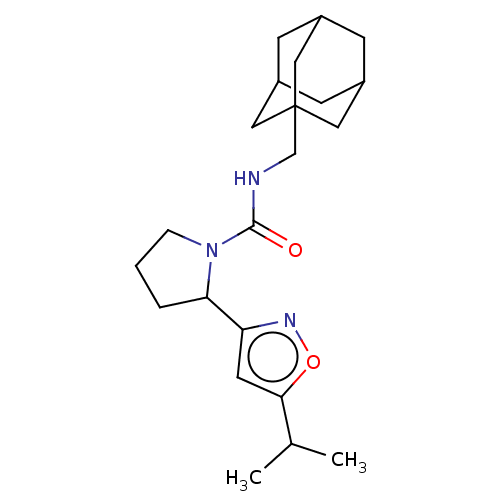 (CHEMBL3633676) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 247 | n/a | n/a | n/a | n/a | n/a | n/a |
University of California at Davis Curated by ChEMBL | Assay Description Inhibition of recombinant human soluble epoxide hydrolase using CMNPC as substrate by kinetic fluorescent assay | Bioorg Med Chem Lett 25: 5514-9 (2015) Article DOI: 10.1016/j.bmcl.2015.10.066 BindingDB Entry DOI: 10.7270/Q28P62C1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional epoxide hydrolase 2 (Homo sapiens (Human)) | BDBM50133701 (CHEMBL3633675) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 524 | n/a | n/a | n/a | n/a | n/a | n/a |
University of California at Davis Curated by ChEMBL | Assay Description Inhibition of recombinant human soluble epoxide hydrolase using CMNPC as substrate by kinetic fluorescent assay | Bioorg Med Chem Lett 25: 5514-9 (2015) Article DOI: 10.1016/j.bmcl.2015.10.066 BindingDB Entry DOI: 10.7270/Q28P62C1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional epoxide hydrolase 2 (Homo sapiens (Human)) | BDBM50133700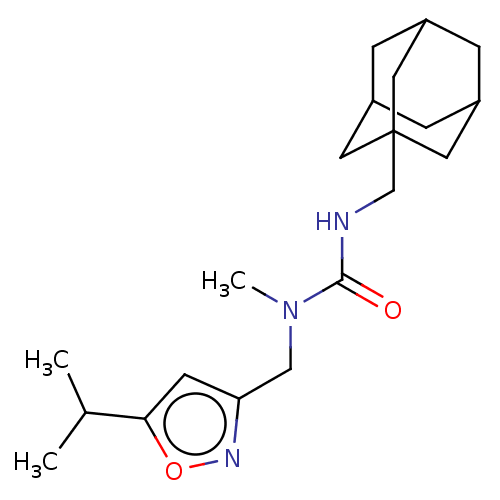 (CHEMBL3633674) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 576 | n/a | n/a | n/a | n/a | n/a | n/a |
University of California at Davis Curated by ChEMBL | Assay Description Inhibition of recombinant human soluble epoxide hydrolase using CMNPC as substrate by kinetic fluorescent assay | Bioorg Med Chem Lett 25: 5514-9 (2015) Article DOI: 10.1016/j.bmcl.2015.10.066 BindingDB Entry DOI: 10.7270/Q28P62C1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||