 Found 42 hits of Enzyme Inhibition Constant Data
Found 42 hits of Enzyme Inhibition Constant Data Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Diacylglycerol O-acyltransferase 2
(Homo sapiens (Human)) | BDBM50499290
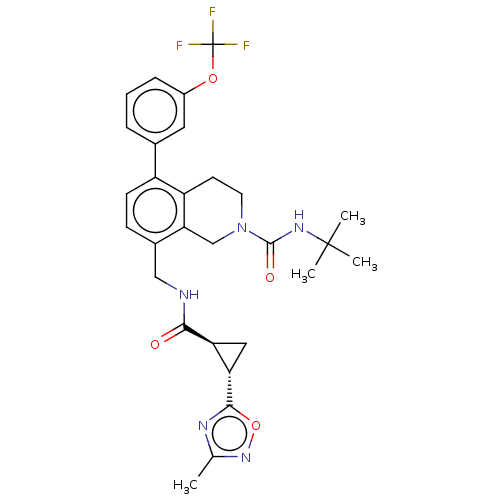
(CHEMBL3734797)Show SMILES Cc1noc(n1)[C@H]1C[C@@H]1C(=O)NCc1ccc(-c2cccc(OC(F)(F)F)c2)c2CCN(Cc12)C(=O)NC(C)(C)C |r| Show InChI InChI=1S/C29H32F3N5O4/c1-16-34-26(41-36-16)23-13-22(23)25(38)33-14-18-8-9-20(17-6-5-7-19(12-17)40-29(30,31)32)21-10-11-37(15-24(18)21)27(39)35-28(2,3)4/h5-9,12,22-23H,10-11,13-15H2,1-4H3,(H,33,38)(H,35,39)/t22-,23-/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck & Co., Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human DGAT2 expressed in Sf9 cell membranes assessed as triolein formation by LC/MS/MS analysis using oleoyl-CoA as substrate |
J Med Chem 58: 9345-53 (2015)
Article DOI: 10.1021/acs.jmedchem.5b01345
BindingDB Entry DOI: 10.7270/Q28W3H9F |
More data for this
Ligand-Target Pair | |
Diacylglycerol O-acyltransferase 2
(Homo sapiens (Human)) | BDBM50499298
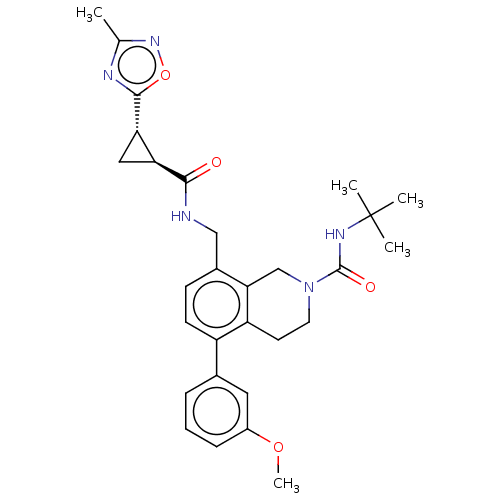
(CHEMBL3734847)Show SMILES COc1cccc(c1)-c1ccc(CNC(=O)[C@H]2C[C@@H]2c2nc(C)no2)c2CN(CCc12)C(=O)NC(C)(C)C |r| Show InChI InChI=1S/C29H35N5O4/c1-17-31-27(38-33-17)24-14-23(24)26(35)30-15-19-9-10-21(18-7-6-8-20(13-18)37-5)22-11-12-34(16-25(19)22)28(36)32-29(2,3)4/h6-10,13,23-24H,11-12,14-16H2,1-5H3,(H,30,35)(H,32,36)/t23-,24-/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck & Co., Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human DGAT2 expressed in Sf9 cell membranes assessed as triolein formation by LC/MS/MS analysis using oleoyl-CoA as substrate |
J Med Chem 58: 9345-53 (2015)
Article DOI: 10.1021/acs.jmedchem.5b01345
BindingDB Entry DOI: 10.7270/Q28W3H9F |
More data for this
Ligand-Target Pair | |
Diacylglycerol O-acyltransferase 2
(Homo sapiens (Human)) | BDBM50499292
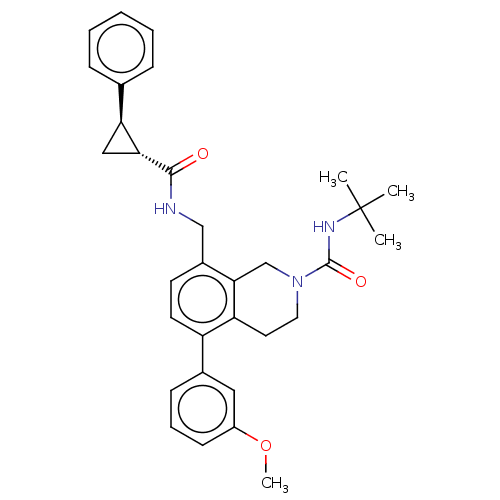
(CHEMBL3734764)Show SMILES COc1cccc(c1)-c1ccc(CNC(=O)[C@@H]2C[C@H]2c2ccccc2)c2CN(CCc12)C(=O)NC(C)(C)C |r| Show InChI InChI=1S/C32H37N3O3/c1-32(2,3)34-31(37)35-16-15-26-25(22-11-8-12-24(17-22)38-4)14-13-23(29(26)20-35)19-33-30(36)28-18-27(28)21-9-6-5-7-10-21/h5-14,17,27-28H,15-16,18-20H2,1-4H3,(H,33,36)(H,34,37)/t27-,28+/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck & Co., Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human DGAT2 expressed in Sf9 cell membranes assessed as triolein formation by LC/MS/MS analysis using oleoyl-CoA as substrate |
J Med Chem 58: 9345-53 (2015)
Article DOI: 10.1021/acs.jmedchem.5b01345
BindingDB Entry DOI: 10.7270/Q28W3H9F |
More data for this
Ligand-Target Pair | |
Alpha-1,6-mannosyl-glycoprotein 2-beta-N-acetylglucosaminyltransferase
(Homo sapiens (Human)) | BDBM50499286

(CHEMBL3735388)Show SMILES COc1cccc(c1)-c1ccc(CNC(=O)[C@@H]2C[C@H]2c2ccccc2)c2CN(CCc12)C(C)=O |r| Show InChI InChI=1S/C29H30N2O3/c1-19(32)31-14-13-25-24(21-9-6-10-23(15-21)34-2)12-11-22(28(25)18-31)17-30-29(33)27-16-26(27)20-7-4-3-5-8-20/h3-12,15,26-27H,13-14,16-18H2,1-2H3,(H,30,33)/t26-,27+/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 23 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck & Co., Inc.
Curated by ChEMBL
| Assay Description
Inhibition of MGAT2 (unknown origin) by CPM assay |
J Med Chem 58: 9345-53 (2015)
Article DOI: 10.1021/acs.jmedchem.5b01345
BindingDB Entry DOI: 10.7270/Q28W3H9F |
More data for this
Ligand-Target Pair | |
Diacylglycerol O-acyltransferase 2
(Homo sapiens (Human)) | BDBM50499289

(CHEMBL3735235)Show SMILES COc1cccc(c1)-c1ccc(CNC(=O)[C@@H]2C[C@H]2c2ccccc2)c2CN(CCc12)C(=O)OC(C)(C)C |r| Show InChI InChI=1S/C32H36N2O4/c1-32(2,3)38-31(36)34-16-15-26-25(22-11-8-12-24(17-22)37-4)14-13-23(29(26)20-34)19-33-30(35)28-18-27(28)21-9-6-5-7-10-21/h5-14,17,27-28H,15-16,18-20H2,1-4H3,(H,33,35)/t27-,28+/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 60 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck & Co., Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human DGAT2 expressed in Sf9 cell membranes assessed as triolein formation by LC/MS/MS analysis using oleoyl-CoA as substrate |
J Med Chem 58: 9345-53 (2015)
Article DOI: 10.1021/acs.jmedchem.5b01345
BindingDB Entry DOI: 10.7270/Q28W3H9F |
More data for this
Ligand-Target Pair | |
Diacylglycerol O-acyltransferase 2
(Homo sapiens (Human)) | BDBM360598

(US9828369, Example 155 | methyl 3-(8-((3-(2,3- dic...)Show SMILES COC(=O)c1cccc(c1)-c1ccc(CNC(=O)Nc2cccc(Cl)c2Cl)c2cnccc12 Show InChI InChI=1S/C25H19Cl2N3O3/c1-33-24(31)16-5-2-4-15(12-16)18-9-8-17(20-14-28-11-10-19(18)20)13-29-25(32)30-22-7-3-6-21(26)23(22)27/h2-12,14H,13H2,1H3,(H2,29,30,32) | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 120 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck & Co., Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human DGAT2 expressed in Sf9 cell membranes assessed as triolein formation by LC/MS/MS analysis using oleoyl-CoA as substrate |
J Med Chem 58: 9345-53 (2015)
Article DOI: 10.1021/acs.jmedchem.5b01345
BindingDB Entry DOI: 10.7270/Q28W3H9F |
More data for this
Ligand-Target Pair | |
Diacylglycerol O-acyltransferase 2
(Homo sapiens (Human)) | BDBM50499288
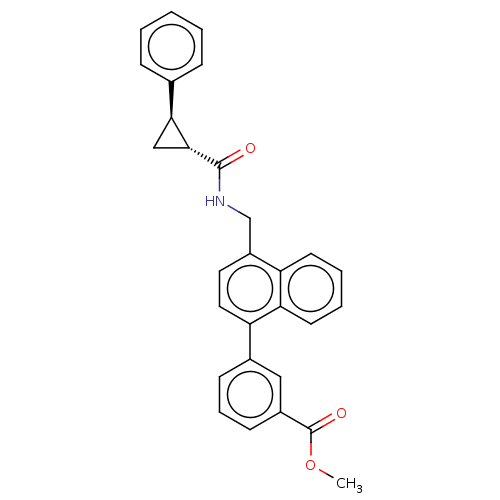
(CHEMBL3736485)Show SMILES COC(=O)c1cccc(c1)-c1ccc(CNC(=O)[C@@H]2C[C@H]2c2ccccc2)c2ccccc12 |r| Show InChI InChI=1S/C29H25NO3/c1-33-29(32)21-11-7-10-20(16-21)24-15-14-22(23-12-5-6-13-25(23)24)18-30-28(31)27-17-26(27)19-8-3-2-4-9-19/h2-16,26-27H,17-18H2,1H3,(H,30,31)/t26-,27+/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 140 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck & Co., Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human DGAT2 expressed in Sf9 cell membranes assessed as triolein formation by LC/MS/MS analysis using oleoyl-CoA as substrate |
J Med Chem 58: 9345-53 (2015)
Article DOI: 10.1021/acs.jmedchem.5b01345
BindingDB Entry DOI: 10.7270/Q28W3H9F |
More data for this
Ligand-Target Pair | |
Diacylglycerol O-acyltransferase 2
(Homo sapiens (Human)) | BDBM360543
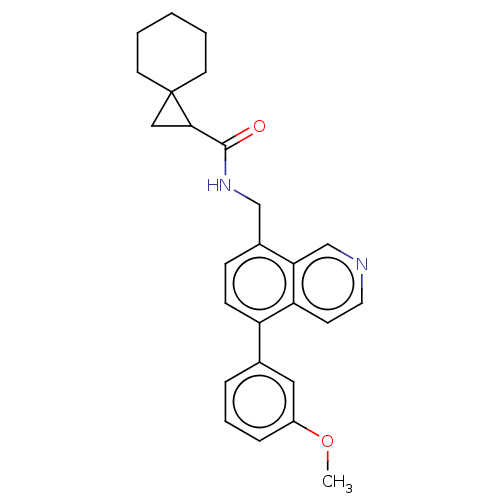
(N-((5-(3-methoxyphenyl)isoquinolin- 8-yl)methyl)sp...)Show SMILES COc1cccc(c1)-c1ccc(CNC(=O)C2CC22CCCCC2)c2cnccc12 Show InChI InChI=1S/C26H28N2O2/c1-30-20-7-5-6-18(14-20)21-9-8-19(23-17-27-13-10-22(21)23)16-28-25(29)24-15-26(24)11-3-2-4-12-26/h5-10,13-14,17,24H,2-4,11-12,15-16H2,1H3,(H,28,29) | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 180 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck & Co., Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human DGAT2 expressed in Sf9 cell membranes assessed as triolein formation by LC/MS/MS analysis using oleoyl-CoA as substrate |
J Med Chem 58: 9345-53 (2015)
Article DOI: 10.1021/acs.jmedchem.5b01345
BindingDB Entry DOI: 10.7270/Q28W3H9F |
More data for this
Ligand-Target Pair | |
Diacylglycerol O-acyltransferase 2
(Homo sapiens (Human)) | BDBM50499297
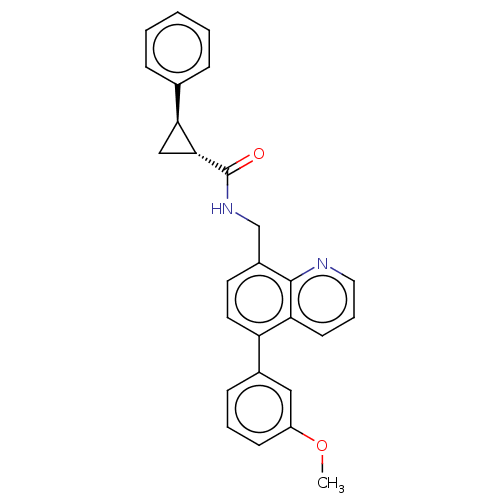
(CHEMBL3736366)Show SMILES COc1cccc(c1)-c1ccc(CNC(=O)[C@@H]2C[C@H]2c2ccccc2)c2ncccc12 |r| Show InChI InChI=1S/C27H24N2O2/c1-31-21-10-5-9-19(15-21)22-13-12-20(26-23(22)11-6-14-28-26)17-29-27(30)25-16-24(25)18-7-3-2-4-8-18/h2-15,24-25H,16-17H2,1H3,(H,29,30)/t24-,25+/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 300 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck & Co., Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human DGAT2 expressed in Sf9 cell membranes assessed as triolein formation by LC/MS/MS analysis using oleoyl-CoA as substrate |
J Med Chem 58: 9345-53 (2015)
Article DOI: 10.1021/acs.jmedchem.5b01345
BindingDB Entry DOI: 10.7270/Q28W3H9F |
More data for this
Ligand-Target Pair | |
Alpha-1,6-mannosyl-glycoprotein 2-beta-N-acetylglucosaminyltransferase
(Homo sapiens (Human)) | BDBM50499297

(CHEMBL3736366)Show SMILES COc1cccc(c1)-c1ccc(CNC(=O)[C@@H]2C[C@H]2c2ccccc2)c2ncccc12 |r| Show InChI InChI=1S/C27H24N2O2/c1-31-21-10-5-9-19(15-21)22-13-12-20(26-23(22)11-6-14-28-26)17-29-27(30)25-16-24(25)18-7-3-2-4-8-18/h2-15,24-25H,16-17H2,1H3,(H,29,30)/t24-,25+/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 400 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck & Co., Inc.
Curated by ChEMBL
| Assay Description
Inhibition of MGAT2 (unknown origin) by CPM assay |
J Med Chem 58: 9345-53 (2015)
Article DOI: 10.1021/acs.jmedchem.5b01345
BindingDB Entry DOI: 10.7270/Q28W3H9F |
More data for this
Ligand-Target Pair | |
Diacylglycerol O-acyltransferase 2
(Homo sapiens (Human)) | BDBM50499295

(CHEMBL3735591)Show SMILES COc1cccc(c1)-c1ccc(NC(=O)[C@@H]2C[C@H]2c2nc(C)no2)c2cnccc12 |r| Show InChI InChI=1S/C23H20N4O3/c1-13-25-23(30-27-13)19-11-18(19)22(28)26-21-7-6-16(17-8-9-24-12-20(17)21)14-4-3-5-15(10-14)29-2/h3-10,12,18-19H,11H2,1-2H3,(H,26,28)/t18-,19-/m1/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 500 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck & Co., Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human DGAT2 expressed in Sf9 cell membranes assessed as triolein formation by LC/MS/MS analysis using oleoyl-CoA as substrate |
J Med Chem 58: 9345-53 (2015)
Article DOI: 10.1021/acs.jmedchem.5b01345
BindingDB Entry DOI: 10.7270/Q28W3H9F |
More data for this
Ligand-Target Pair | |
Diacylglycerol O-acyltransferase 2
(Homo sapiens (Human)) | BDBM50499285
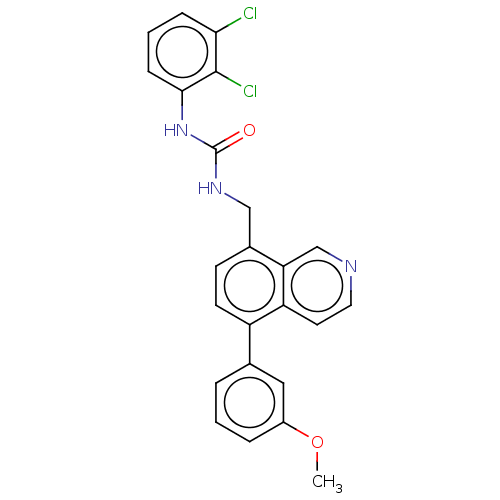
(CHEMBL3736092)Show SMILES COc1cccc(c1)-c1ccc(CNC(=O)Nc2cccc(Cl)c2Cl)c2cnccc12 Show InChI InChI=1S/C24H19Cl2N3O2/c1-31-17-5-2-4-15(12-17)18-9-8-16(20-14-27-11-10-19(18)20)13-28-24(30)29-22-7-3-6-21(25)23(22)26/h2-12,14H,13H2,1H3,(H2,28,29,30) | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 540 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck & Co., Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human DGAT2 expressed in Sf9 cell membranes assessed as triolein formation by LC/MS/MS analysis using oleoyl-CoA as substrate |
J Med Chem 58: 9345-53 (2015)
Article DOI: 10.1021/acs.jmedchem.5b01345
BindingDB Entry DOI: 10.7270/Q28W3H9F |
More data for this
Ligand-Target Pair | |
Diacylglycerol O-acyltransferase 2
(Homo sapiens (Human)) | BDBM50499287
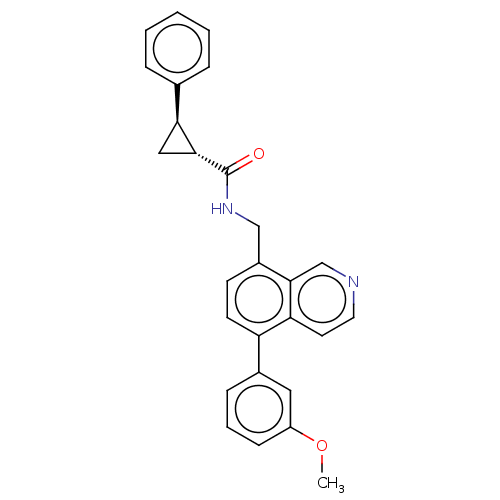
(CHEMBL3734770)Show SMILES COc1cccc(c1)-c1ccc(CNC(=O)[C@@H]2C[C@H]2c2ccccc2)c2cnccc12 |r| Show InChI InChI=1S/C27H24N2O2/c1-31-21-9-5-8-19(14-21)22-11-10-20(26-17-28-13-12-23(22)26)16-29-27(30)25-15-24(25)18-6-3-2-4-7-18/h2-14,17,24-25H,15-16H2,1H3,(H,29,30)/t24-,25+/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 600 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck & Co., Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human DGAT2 expressed in Sf9 cell membranes assessed as triolein formation by LC/MS/MS analysis using oleoyl-CoA as substrate |
J Med Chem 58: 9345-53 (2015)
Article DOI: 10.1021/acs.jmedchem.5b01345
BindingDB Entry DOI: 10.7270/Q28W3H9F |
More data for this
Ligand-Target Pair | |
Alpha-1,6-mannosyl-glycoprotein 2-beta-N-acetylglucosaminyltransferase
(Homo sapiens (Human)) | BDBM50499285

(CHEMBL3736092)Show SMILES COc1cccc(c1)-c1ccc(CNC(=O)Nc2cccc(Cl)c2Cl)c2cnccc12 Show InChI InChI=1S/C24H19Cl2N3O2/c1-31-17-5-2-4-15(12-17)18-9-8-16(20-14-27-11-10-19(18)20)13-28-24(30)29-22-7-3-6-21(25)23(22)26/h2-12,14H,13H2,1H3,(H2,28,29,30) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 760 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck & Co., Inc.
Curated by ChEMBL
| Assay Description
Inhibition of MGAT2 (unknown origin) by CPM assay |
J Med Chem 58: 9345-53 (2015)
Article DOI: 10.1021/acs.jmedchem.5b01345
BindingDB Entry DOI: 10.7270/Q28W3H9F |
More data for this
Ligand-Target Pair | |
Voltage-dependent L-type calcium channel subunit alpha-1F
(Homo sapiens (Human)) | BDBM50499290

(CHEMBL3734797)Show SMILES Cc1noc(n1)[C@H]1C[C@@H]1C(=O)NCc1ccc(-c2cccc(OC(F)(F)F)c2)c2CCN(Cc12)C(=O)NC(C)(C)C |r| Show InChI InChI=1S/C29H32F3N5O4/c1-16-34-26(41-36-16)23-13-22(23)25(38)33-14-18-8-9-20(17-6-5-7-19(12-17)40-29(30,31)32)21-10-11-37(15-24(18)21)27(39)35-28(2,3)4/h5-9,12,22-23H,10-11,13-15H2,1-4H3,(H,33,38)(H,35,39)/t22-,23-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 800 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck & Co., Inc.
Curated by ChEMBL
| Assay Description
Inhibition of voltage-gated calcium channel (unknown origin) |
J Med Chem 58: 9345-53 (2015)
Article DOI: 10.1021/acs.jmedchem.5b01345
BindingDB Entry DOI: 10.7270/Q28W3H9F |
More data for this
Ligand-Target Pair | |
Alpha-1,6-mannosyl-glycoprotein 2-beta-N-acetylglucosaminyltransferase
(Homo sapiens (Human)) | BDBM50499287

(CHEMBL3734770)Show SMILES COc1cccc(c1)-c1ccc(CNC(=O)[C@@H]2C[C@H]2c2ccccc2)c2cnccc12 |r| Show InChI InChI=1S/C27H24N2O2/c1-31-21-9-5-8-19(14-21)22-11-10-20(26-17-28-13-12-23(22)26)16-29-27(30)25-15-24(25)18-6-3-2-4-7-18/h2-14,17,24-25H,15-16H2,1H3,(H,29,30)/t24-,25+/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 800 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck & Co., Inc.
Curated by ChEMBL
| Assay Description
Inhibition of MGAT2 (unknown origin) by CPM assay |
J Med Chem 58: 9345-53 (2015)
Article DOI: 10.1021/acs.jmedchem.5b01345
BindingDB Entry DOI: 10.7270/Q28W3H9F |
More data for this
Ligand-Target Pair | |
Alpha-1,6-mannosyl-glycoprotein 2-beta-N-acetylglucosaminyltransferase
(Homo sapiens (Human)) | BDBM50499289

(CHEMBL3735235)Show SMILES COc1cccc(c1)-c1ccc(CNC(=O)[C@@H]2C[C@H]2c2ccccc2)c2CN(CCc12)C(=O)OC(C)(C)C |r| Show InChI InChI=1S/C32H36N2O4/c1-32(2,3)38-31(36)34-16-15-26-25(22-11-8-12-24(17-22)37-4)14-13-23(29(26)20-34)19-33-30(35)28-18-27(28)21-9-6-5-7-10-21/h5-14,17,27-28H,15-16,18-20H2,1-4H3,(H,33,35)/t27-,28+/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.44E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck & Co., Inc.
Curated by ChEMBL
| Assay Description
Inhibition of MGAT2 (unknown origin) by CPM assay |
J Med Chem 58: 9345-53 (2015)
Article DOI: 10.1021/acs.jmedchem.5b01345
BindingDB Entry DOI: 10.7270/Q28W3H9F |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C9
(Homo sapiens (Human)) | BDBM50499290

(CHEMBL3734797)Show SMILES Cc1noc(n1)[C@H]1C[C@@H]1C(=O)NCc1ccc(-c2cccc(OC(F)(F)F)c2)c2CCN(Cc12)C(=O)NC(C)(C)C |r| Show InChI InChI=1S/C29H32F3N5O4/c1-16-34-26(41-36-16)23-13-22(23)25(38)33-14-18-8-9-20(17-6-5-7-19(12-17)40-29(30,31)32)21-10-11-37(15-24(18)21)27(39)35-28(2,3)4/h5-9,12,22-23H,10-11,13-15H2,1-4H3,(H,33,38)(H,35,39)/t22-,23-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck & Co., Inc.
Curated by ChEMBL
| Assay Description
Inhibition of CYP2C9 (unknown origin) |
J Med Chem 58: 9345-53 (2015)
Article DOI: 10.1021/acs.jmedchem.5b01345
BindingDB Entry DOI: 10.7270/Q28W3H9F |
More data for this
Ligand-Target Pair | |
Diacylglycerol O-acyltransferase 2
(Homo sapiens (Human)) | BDBM50499296
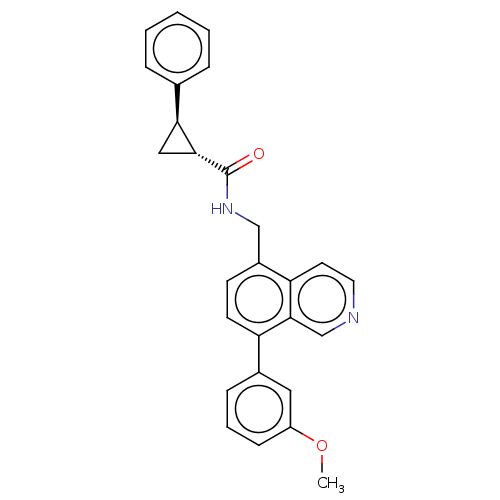
(CHEMBL3736151)Show SMILES COc1cccc(c1)-c1ccc(CNC(=O)[C@@H]2C[C@H]2c2ccccc2)c2ccncc12 |r| Show InChI InChI=1S/C27H24N2O2/c1-31-21-9-5-8-19(14-21)22-11-10-20(23-12-13-28-17-26(22)23)16-29-27(30)25-15-24(25)18-6-3-2-4-7-18/h2-14,17,24-25H,15-16H2,1H3,(H,29,30)/t24-,25+/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck & Co., Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human DGAT2 expressed in Sf9 cell membranes assessed as triolein formation by LC/MS/MS analysis using oleoyl-CoA as substrate |
J Med Chem 58: 9345-53 (2015)
Article DOI: 10.1021/acs.jmedchem.5b01345
BindingDB Entry DOI: 10.7270/Q28W3H9F |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2D6
(Homo sapiens (Human)) | BDBM50499290

(CHEMBL3734797)Show SMILES Cc1noc(n1)[C@H]1C[C@@H]1C(=O)NCc1ccc(-c2cccc(OC(F)(F)F)c2)c2CCN(Cc12)C(=O)NC(C)(C)C |r| Show InChI InChI=1S/C29H32F3N5O4/c1-16-34-26(41-36-16)23-13-22(23)25(38)33-14-18-8-9-20(17-6-5-7-19(12-17)40-29(30,31)32)21-10-11-37(15-24(18)21)27(39)35-28(2,3)4/h5-9,12,22-23H,10-11,13-15H2,1-4H3,(H,33,38)(H,35,39)/t22-,23-/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck & Co., Inc.
Curated by ChEMBL
| Assay Description
Inhibition of CYP2D6 (unknown origin) |
J Med Chem 58: 9345-53 (2015)
Article DOI: 10.1021/acs.jmedchem.5b01345
BindingDB Entry DOI: 10.7270/Q28W3H9F |
More data for this
Ligand-Target Pair | |
Diacylglycerol O-acyltransferase 2
(Homo sapiens (Human)) | BDBM50499291
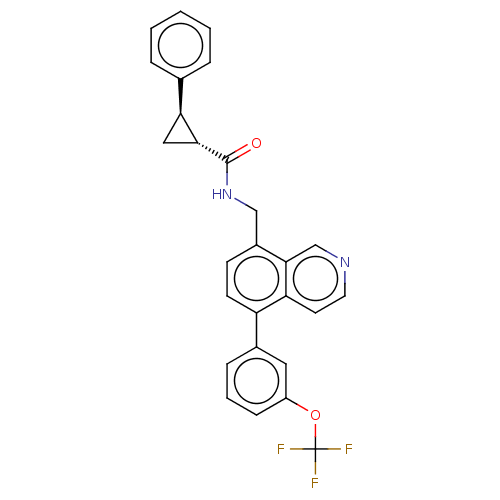
(CHEMBL3735666)Show SMILES FC(F)(F)Oc1cccc(c1)-c1ccc(CNC(=O)[C@@H]2C[C@H]2c2ccccc2)c2cnccc12 |r| Show InChI InChI=1S/C27H21F3N2O2/c28-27(29,30)34-20-8-4-7-18(13-20)21-10-9-19(25-16-31-12-11-22(21)25)15-32-26(33)24-14-23(24)17-5-2-1-3-6-17/h1-13,16,23-24H,14-15H2,(H,32,33)/t23-,24+/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck & Co., Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human DGAT2 expressed in Sf9 cell membranes assessed as triolein formation by LC/MS/MS analysis using oleoyl-CoA as substrate |
J Med Chem 58: 9345-53 (2015)
Article DOI: 10.1021/acs.jmedchem.5b01345
BindingDB Entry DOI: 10.7270/Q28W3H9F |
More data for this
Ligand-Target Pair | |
Alpha-1,6-mannosyl-glycoprotein 2-beta-N-acetylglucosaminyltransferase
(Homo sapiens (Human)) | BDBM50499295

(CHEMBL3735591)Show SMILES COc1cccc(c1)-c1ccc(NC(=O)[C@@H]2C[C@H]2c2nc(C)no2)c2cnccc12 |r| Show InChI InChI=1S/C23H20N4O3/c1-13-25-23(30-27-13)19-11-18(19)22(28)26-21-7-6-16(17-8-9-24-12-20(17)21)14-4-3-5-15(10-14)29-2/h3-10,12,18-19H,11H2,1-2H3,(H,26,28)/t18-,19-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.28E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck & Co., Inc.
Curated by ChEMBL
| Assay Description
Inhibition of MGAT2 (unknown origin) by CPM assay |
J Med Chem 58: 9345-53 (2015)
Article DOI: 10.1021/acs.jmedchem.5b01345
BindingDB Entry DOI: 10.7270/Q28W3H9F |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50499290

(CHEMBL3734797)Show SMILES Cc1noc(n1)[C@H]1C[C@@H]1C(=O)NCc1ccc(-c2cccc(OC(F)(F)F)c2)c2CCN(Cc12)C(=O)NC(C)(C)C |r| Show InChI InChI=1S/C29H32F3N5O4/c1-16-34-26(41-36-16)23-13-22(23)25(38)33-14-18-8-9-20(17-6-5-7-19(12-17)40-29(30,31)32)21-10-11-37(15-24(18)21)27(39)35-28(2,3)4/h5-9,12,22-23H,10-11,13-15H2,1-4H3,(H,33,38)(H,35,39)/t22-,23-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.34E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck & Co., Inc.
Curated by ChEMBL
| Assay Description
Displacement of [35S]-MK-0499 from human ERG |
J Med Chem 58: 9345-53 (2015)
Article DOI: 10.1021/acs.jmedchem.5b01345
BindingDB Entry DOI: 10.7270/Q28W3H9F |
More data for this
Ligand-Target Pair | |
Diacylglycerol O-acyltransferase 2
(Homo sapiens (Human)) | BDBM50499286

(CHEMBL3735388)Show SMILES COc1cccc(c1)-c1ccc(CNC(=O)[C@@H]2C[C@H]2c2ccccc2)c2CN(CCc12)C(C)=O |r| Show InChI InChI=1S/C29H30N2O3/c1-19(32)31-14-13-25-24(21-9-6-10-23(15-21)34-2)12-11-22(28(25)18-31)17-30-29(33)27-16-26(27)20-7-4-3-5-8-20/h3-12,15,26-27H,13-14,16-18H2,1-2H3,(H,30,33)/t26-,27+/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3.46E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck & Co., Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human DGAT2 expressed in Sf9 cell membranes assessed as triolein formation by LC/MS/MS analysis using oleoyl-CoA as substrate |
J Med Chem 58: 9345-53 (2015)
Article DOI: 10.1021/acs.jmedchem.5b01345
BindingDB Entry DOI: 10.7270/Q28W3H9F |
More data for this
Ligand-Target Pair | |
Alpha-1,6-mannosyl-glycoprotein 2-beta-N-acetylglucosaminyltransferase
(Homo sapiens (Human)) | BDBM50499292

(CHEMBL3734764)Show SMILES COc1cccc(c1)-c1ccc(CNC(=O)[C@@H]2C[C@H]2c2ccccc2)c2CN(CCc12)C(=O)NC(C)(C)C |r| Show InChI InChI=1S/C32H37N3O3/c1-32(2,3)34-31(37)35-16-15-26-25(22-11-8-12-24(17-22)38-4)14-13-23(29(26)20-35)19-33-30(36)28-18-27(28)21-9-6-5-7-10-21/h5-14,17,27-28H,15-16,18-20H2,1-4H3,(H,33,36)(H,34,37)/t27-,28+/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck & Co., Inc.
Curated by ChEMBL
| Assay Description
Inhibition of MGAT2 (unknown origin) by CPM assay |
J Med Chem 58: 9345-53 (2015)
Article DOI: 10.1021/acs.jmedchem.5b01345
BindingDB Entry DOI: 10.7270/Q28W3H9F |
More data for this
Ligand-Target Pair | |
Alpha-1,6-mannosyl-glycoprotein 2-beta-N-acetylglucosaminyltransferase
(Homo sapiens (Human)) | BDBM360543

(N-((5-(3-methoxyphenyl)isoquinolin- 8-yl)methyl)sp...)Show SMILES COc1cccc(c1)-c1ccc(CNC(=O)C2CC22CCCCC2)c2cnccc12 Show InChI InChI=1S/C26H28N2O2/c1-30-20-7-5-6-18(14-20)21-9-8-19(23-17-27-13-10-22(21)23)16-28-25(29)24-15-26(24)11-3-2-4-12-26/h5-10,13-14,17,24H,2-4,11-12,15-16H2,1H3,(H,28,29) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 4.77E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck & Co., Inc.
Curated by ChEMBL
| Assay Description
Inhibition of MGAT2 (unknown origin) by CPM assay |
J Med Chem 58: 9345-53 (2015)
Article DOI: 10.1021/acs.jmedchem.5b01345
BindingDB Entry DOI: 10.7270/Q28W3H9F |
More data for this
Ligand-Target Pair | |
Alpha-1,6-mannosyl-glycoprotein 2-beta-N-acetylglucosaminyltransferase
(Homo sapiens (Human)) | BDBM50499288

(CHEMBL3736485)Show SMILES COC(=O)c1cccc(c1)-c1ccc(CNC(=O)[C@@H]2C[C@H]2c2ccccc2)c2ccccc12 |r| Show InChI InChI=1S/C29H25NO3/c1-33-29(32)21-11-7-10-20(16-21)24-15-14-22(23-12-5-6-13-25(23)24)18-30-28(31)27-17-26(27)19-8-3-2-4-9-19/h2-16,26-27H,17-18H2,1H3,(H,30,31)/t26-,27+/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 5.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck & Co., Inc.
Curated by ChEMBL
| Assay Description
Inhibition of MGAT2 (unknown origin) by CPM assay |
J Med Chem 58: 9345-53 (2015)
Article DOI: 10.1021/acs.jmedchem.5b01345
BindingDB Entry DOI: 10.7270/Q28W3H9F |
More data for this
Ligand-Target Pair | |
Alpha-1,6-mannosyl-glycoprotein 2-beta-N-acetylglucosaminyltransferase
(Homo sapiens (Human)) | BDBM50499296

(CHEMBL3736151)Show SMILES COc1cccc(c1)-c1ccc(CNC(=O)[C@@H]2C[C@H]2c2ccccc2)c2ccncc12 |r| Show InChI InChI=1S/C27H24N2O2/c1-31-21-9-5-8-19(14-21)22-11-10-20(23-12-13-28-17-26(22)23)16-29-27(30)25-15-24(25)18-6-3-2-4-7-18/h2-14,17,24-25H,15-16H2,1H3,(H,29,30)/t24-,25+/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 7.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck & Co., Inc.
Curated by ChEMBL
| Assay Description
Inhibition of MGAT2 (unknown origin) by CPM assay |
J Med Chem 58: 9345-53 (2015)
Article DOI: 10.1021/acs.jmedchem.5b01345
BindingDB Entry DOI: 10.7270/Q28W3H9F |
More data for this
Ligand-Target Pair | |
Diacylglycerol O-acyltransferase 1
(Homo sapiens (Human)) | BDBM50499290

(CHEMBL3734797)Show SMILES Cc1noc(n1)[C@H]1C[C@@H]1C(=O)NCc1ccc(-c2cccc(OC(F)(F)F)c2)c2CCN(Cc12)C(=O)NC(C)(C)C |r| Show InChI InChI=1S/C29H32F3N5O4/c1-16-34-26(41-36-16)23-13-22(23)25(38)33-14-18-8-9-20(17-6-5-7-19(12-17)40-29(30,31)32)21-10-11-37(15-24(18)21)27(39)35-28(2,3)4/h5-9,12,22-23H,10-11,13-15H2,1-4H3,(H,33,38)(H,35,39)/t22-,23-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck & Co., Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human DGAT1 by CPM assay |
J Med Chem 58: 9345-53 (2015)
Article DOI: 10.1021/acs.jmedchem.5b01345
BindingDB Entry DOI: 10.7270/Q28W3H9F |
More data for this
Ligand-Target Pair | |
Alpha-1,6-mannosyl-glycoprotein 2-beta-N-acetylglucosaminyltransferase
(Homo sapiens (Human)) | BDBM50499290

(CHEMBL3734797)Show SMILES Cc1noc(n1)[C@H]1C[C@@H]1C(=O)NCc1ccc(-c2cccc(OC(F)(F)F)c2)c2CCN(Cc12)C(=O)NC(C)(C)C |r| Show InChI InChI=1S/C29H32F3N5O4/c1-16-34-26(41-36-16)23-13-22(23)25(38)33-14-18-8-9-20(17-6-5-7-19(12-17)40-29(30,31)32)21-10-11-37(15-24(18)21)27(39)35-28(2,3)4/h5-9,12,22-23H,10-11,13-15H2,1-4H3,(H,33,38)(H,35,39)/t22-,23-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck & Co., Inc.
Curated by ChEMBL
| Assay Description
Inhibition of MGAT2 (unknown origin) by CPM assay |
J Med Chem 58: 9345-53 (2015)
Article DOI: 10.1021/acs.jmedchem.5b01345
BindingDB Entry DOI: 10.7270/Q28W3H9F |
More data for this
Ligand-Target Pair | |
Diacylglycerol O-acyltransferase 1
(Mus musculus (mouse)) | BDBM50499290

(CHEMBL3734797)Show SMILES Cc1noc(n1)[C@H]1C[C@@H]1C(=O)NCc1ccc(-c2cccc(OC(F)(F)F)c2)c2CCN(Cc12)C(=O)NC(C)(C)C |r| Show InChI InChI=1S/C29H32F3N5O4/c1-16-34-26(41-36-16)23-13-22(23)25(38)33-14-18-8-9-20(17-6-5-7-19(12-17)40-29(30,31)32)21-10-11-37(15-24(18)21)27(39)35-28(2,3)4/h5-9,12,22-23H,10-11,13-15H2,1-4H3,(H,33,38)(H,35,39)/t22-,23-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck & Co., Inc.
Curated by ChEMBL
| Assay Description
Inhibition of mouse DGAT1 by CPM assay |
J Med Chem 58: 9345-53 (2015)
Article DOI: 10.1021/acs.jmedchem.5b01345
BindingDB Entry DOI: 10.7270/Q28W3H9F |
More data for this
Ligand-Target Pair | |
Alpha-1,6-mannosyl-glycoprotein 2-beta-N-acetylglucosaminyltransferase
(Homo sapiens (Human)) | BDBM50499298

(CHEMBL3734847)Show SMILES COc1cccc(c1)-c1ccc(CNC(=O)[C@H]2C[C@@H]2c2nc(C)no2)c2CN(CCc12)C(=O)NC(C)(C)C |r| Show InChI InChI=1S/C29H35N5O4/c1-17-31-27(38-33-17)24-14-23(24)26(35)30-15-19-9-10-21(18-7-6-8-20(13-18)37-5)22-11-12-34(16-25(19)22)28(36)32-29(2,3)4/h6-10,13,23-24H,11-12,14-16H2,1-5H3,(H,30,35)(H,32,36)/t23-,24-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck & Co., Inc.
Curated by ChEMBL
| Assay Description
Inhibition of MGAT2 (unknown origin) by CPM assay |
J Med Chem 58: 9345-53 (2015)
Article DOI: 10.1021/acs.jmedchem.5b01345
BindingDB Entry DOI: 10.7270/Q28W3H9F |
More data for this
Ligand-Target Pair | |
Alpha-1,6-mannosyl-glycoprotein 2-beta-N-acetylglucosaminyltransferase
(Homo sapiens (Human)) | BDBM50499290

(CHEMBL3734797)Show SMILES Cc1noc(n1)[C@H]1C[C@@H]1C(=O)NCc1ccc(-c2cccc(OC(F)(F)F)c2)c2CCN(Cc12)C(=O)NC(C)(C)C |r| Show InChI InChI=1S/C29H32F3N5O4/c1-16-34-26(41-36-16)23-13-22(23)25(38)33-14-18-8-9-20(17-6-5-7-19(12-17)40-29(30,31)32)21-10-11-37(15-24(18)21)27(39)35-28(2,3)4/h5-9,12,22-23H,10-11,13-15H2,1-4H3,(H,33,38)(H,35,39)/t22-,23-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck & Co., Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human MGAT2 by CPM assay |
J Med Chem 58: 9345-53 (2015)
Article DOI: 10.1021/acs.jmedchem.5b01345
BindingDB Entry DOI: 10.7270/Q28W3H9F |
More data for this
Ligand-Target Pair | |
Alpha-1,6-mannosyl-glycoprotein 2-beta-N-acetylglucosaminyltransferase
(Mus musculus) | BDBM50499290

(CHEMBL3734797)Show SMILES Cc1noc(n1)[C@H]1C[C@@H]1C(=O)NCc1ccc(-c2cccc(OC(F)(F)F)c2)c2CCN(Cc12)C(=O)NC(C)(C)C |r| Show InChI InChI=1S/C29H32F3N5O4/c1-16-34-26(41-36-16)23-13-22(23)25(38)33-14-18-8-9-20(17-6-5-7-19(12-17)40-29(30,31)32)21-10-11-37(15-24(18)21)27(39)35-28(2,3)4/h5-9,12,22-23H,10-11,13-15H2,1-4H3,(H,33,38)(H,35,39)/t22-,23-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck & Co., Inc.
Curated by ChEMBL
| Assay Description
Inhibition of mouse MGAT2 by CPM assay |
J Med Chem 58: 9345-53 (2015)
Article DOI: 10.1021/acs.jmedchem.5b01345
BindingDB Entry DOI: 10.7270/Q28W3H9F |
More data for this
Ligand-Target Pair | |
Diacylglycerol O-acyltransferase 1
(Homo sapiens (Human)) | BDBM50499285

(CHEMBL3736092)Show SMILES COc1cccc(c1)-c1ccc(CNC(=O)Nc2cccc(Cl)c2Cl)c2cnccc12 Show InChI InChI=1S/C24H19Cl2N3O2/c1-31-17-5-2-4-15(12-17)18-9-8-16(20-14-27-11-10-19(18)20)13-28-24(30)29-22-7-3-6-21(25)23(22)26/h2-12,14H,13H2,1H3,(H2,28,29,30) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck & Co., Inc.
Curated by ChEMBL
| Assay Description
Inhibition of DGAT1 (unknown origin) by CPM assay |
J Med Chem 58: 9345-53 (2015)
Article DOI: 10.1021/acs.jmedchem.5b01345
BindingDB Entry DOI: 10.7270/Q28W3H9F |
More data for this
Ligand-Target Pair | |
Diacylglycerol O-acyltransferase 2
(Homo sapiens (Human)) | BDBM50499293

(CHEMBL3735532)Show InChI InChI=1S/C17H12Cl3N3O/c18-13-5-4-10(12-9-21-7-6-11(12)13)8-22-17(24)23-15-3-1-2-14(19)16(15)20/h1-7,9H,8H2,(H2,22,23,24) | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >1.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck & Co., Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human DGAT2 expressed in Sf9 cell membranes assessed as triolein formation by LC/MS/MS analysis using oleoyl-CoA as substrate |
J Med Chem 58: 9345-53 (2015)
Article DOI: 10.1021/acs.jmedchem.5b01345
BindingDB Entry DOI: 10.7270/Q28W3H9F |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50499290

(CHEMBL3734797)Show SMILES Cc1noc(n1)[C@H]1C[C@@H]1C(=O)NCc1ccc(-c2cccc(OC(F)(F)F)c2)c2CCN(Cc12)C(=O)NC(C)(C)C |r| Show InChI InChI=1S/C29H32F3N5O4/c1-16-34-26(41-36-16)23-13-22(23)25(38)33-14-18-8-9-20(17-6-5-7-19(12-17)40-29(30,31)32)21-10-11-37(15-24(18)21)27(39)35-28(2,3)4/h5-9,12,22-23H,10-11,13-15H2,1-4H3,(H,33,38)(H,35,39)/t22-,23-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck & Co., Inc.
Curated by ChEMBL
| Assay Description
Inhibition of CYP3A4 (unknown origin) |
J Med Chem 58: 9345-53 (2015)
Article DOI: 10.1021/acs.jmedchem.5b01345
BindingDB Entry DOI: 10.7270/Q28W3H9F |
More data for this
Ligand-Target Pair | |
Alpha-1,6-mannosyl-glycoprotein 2-beta-N-acetylglucosaminyltransferase
(Homo sapiens (Human)) | BDBM50499294
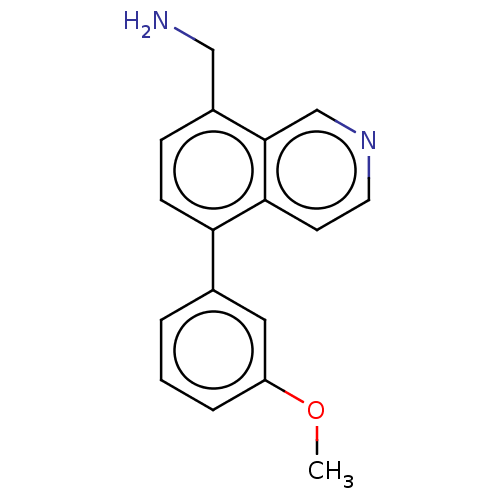
(CHEMBL3735953)Show InChI InChI=1S/C17H16N2O/c1-20-14-4-2-3-12(9-14)15-6-5-13(10-18)17-11-19-8-7-16(15)17/h2-9,11H,10,18H2,1H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.60E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck & Co., Inc.
Curated by ChEMBL
| Assay Description
Inhibition of MGAT2 (unknown origin) by CPM assay |
J Med Chem 58: 9345-53 (2015)
Article DOI: 10.1021/acs.jmedchem.5b01345
BindingDB Entry DOI: 10.7270/Q28W3H9F |
More data for this
Ligand-Target Pair | |
Alpha-1,6-mannosyl-glycoprotein 2-beta-N-acetylglucosaminyltransferase
(Homo sapiens (Human)) | BDBM50499291

(CHEMBL3735666)Show SMILES FC(F)(F)Oc1cccc(c1)-c1ccc(CNC(=O)[C@@H]2C[C@H]2c2ccccc2)c2cnccc12 |r| Show InChI InChI=1S/C27H21F3N2O2/c28-27(29,30)34-20-8-4-7-18(13-20)21-10-9-19(25-16-31-12-11-22(21)25)15-32-26(33)24-14-23(24)17-5-2-1-3-6-17/h1-13,16,23-24H,14-15H2,(H,32,33)/t23-,24+/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck & Co., Inc.
Curated by ChEMBL
| Assay Description
Inhibition of MGAT2 (unknown origin) by CPM assay |
J Med Chem 58: 9345-53 (2015)
Article DOI: 10.1021/acs.jmedchem.5b01345
BindingDB Entry DOI: 10.7270/Q28W3H9F |
More data for this
Ligand-Target Pair | |
Alpha-1,6-mannosyl-glycoprotein 2-beta-N-acetylglucosaminyltransferase
(Homo sapiens (Human)) | BDBM360598

(US9828369, Example 155 | methyl 3-(8-((3-(2,3- dic...)Show SMILES COC(=O)c1cccc(c1)-c1ccc(CNC(=O)Nc2cccc(Cl)c2Cl)c2cnccc12 Show InChI InChI=1S/C25H19Cl2N3O3/c1-33-24(31)16-5-2-4-15(12-16)18-9-8-17(20-14-28-11-10-19(18)20)13-29-25(32)30-22-7-3-6-21(26)23(22)27/h2-12,14H,13H2,1H3,(H2,29,30,32) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck & Co., Inc.
Curated by ChEMBL
| Assay Description
Inhibition of MGAT2 (unknown origin) by CPM assay |
J Med Chem 58: 9345-53 (2015)
Article DOI: 10.1021/acs.jmedchem.5b01345
BindingDB Entry DOI: 10.7270/Q28W3H9F |
More data for this
Ligand-Target Pair | |
Diacylglycerol O-acyltransferase 2
(Homo sapiens (Human)) | BDBM50499294

(CHEMBL3735953)Show InChI InChI=1S/C17H16N2O/c1-20-14-4-2-3-12(9-14)15-6-5-13(10-18)17-11-19-8-7-16(15)17/h2-9,11H,10,18H2,1H3 | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck & Co., Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human DGAT2 expressed in Sf9 cell membranes assessed as triolein formation by LC/MS/MS analysis using oleoyl-CoA as substrate |
J Med Chem 58: 9345-53 (2015)
Article DOI: 10.1021/acs.jmedchem.5b01345
BindingDB Entry DOI: 10.7270/Q28W3H9F |
More data for this
Ligand-Target Pair | |
Glycerol-3-phosphate acyltransferase 1, mitochondrial
(Homo sapiens) | BDBM50499285

(CHEMBL3736092)Show SMILES COc1cccc(c1)-c1ccc(CNC(=O)Nc2cccc(Cl)c2Cl)c2cnccc12 Show InChI InChI=1S/C24H19Cl2N3O2/c1-31-17-5-2-4-15(12-17)18-9-8-16(20-14-27-11-10-19(18)20)13-28-24(30)29-22-7-3-6-21(25)23(22)26/h2-12,14H,13H2,1H3,(H2,28,29,30) | PDB
KEGG
UniProtKB/SwissProt
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck & Co., Inc.
Curated by ChEMBL
| Assay Description
Inhibition of GPAT1 (unknown origin) by CPM assay using glycerol-3-phosphate and oleoyl-CoA as substrate |
J Med Chem 58: 9345-53 (2015)
Article DOI: 10.1021/acs.jmedchem.5b01345
BindingDB Entry DOI: 10.7270/Q28W3H9F |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data