 Found 17 hits of Enzyme Inhibition Constant Data
Found 17 hits of Enzyme Inhibition Constant Data Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50442401
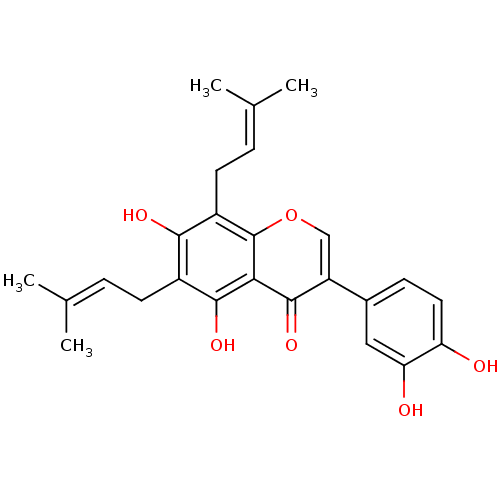
(CHEMBL2442947)Show SMILES [#6]\[#6](-[#6])=[#6]\[#6]-c1c(-[#8])c(-[#6]\[#6]=[#6](\[#6])-[#6])c2occ(-c3ccc(-[#8])c(-[#8])c3)c(=O)c2c1-[#8] Show InChI InChI=1S/C25H26O6/c1-13(2)5-8-16-22(28)17(9-6-14(3)4)25-21(23(16)29)24(30)18(12-31-25)15-7-10-19(26)20(27)11-15/h5-7,10-12,26-29H,8-9H2,1-4H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Qiqihar University
Curated by ChEMBL
| Assay Description
Non-competitive inhibition of human recombinant PTP1B using p-nitrophenyl phosphate as substrate by spectrophotometry based Lineweaver-Burk plot |
Bioorg Med Chem Lett 26: 318-21 (2016)
Article DOI: 10.1016/j.bmcl.2015.12.021
BindingDB Entry DOI: 10.7270/Q2C82C4W |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50136569
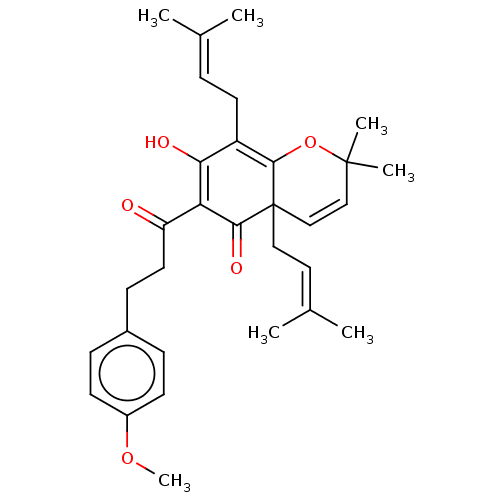
(CHEMBL3754629)Show SMILES [#6]-[#8]-c1ccc(-[#6]-[#6]-[#6](=O)-[#6]-2=[#6](-[#8])-[#6](-[#6]\[#6]=[#6](\[#6])-[#6])=[#6]3-[#8]C([#6])([#6])[#6]=[#6]C3([#6]\[#6]=[#6](\[#6])-[#6])[#6]-2=O)cc1 |c:10,24,t:18| Show InChI InChI=1S/C31H38O5/c1-20(2)8-14-24-27(33)26(25(32)15-11-22-9-12-23(35-7)13-10-22)28(34)31(17-16-21(3)4)19-18-30(5,6)36-29(24)31/h8-10,12-13,16,18-19,33H,11,14-15,17H2,1-7H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Qiqihar University
Curated by ChEMBL
| Assay Description
Competitive inhibition of human recombinant PTP1B using p-nitrophenyl phosphate as substrate by spectrophotometry based Lineweaver-Burk plot |
Bioorg Med Chem Lett 26: 318-21 (2016)
Article DOI: 10.1016/j.bmcl.2015.12.021
BindingDB Entry DOI: 10.7270/Q2C82C4W |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50442400
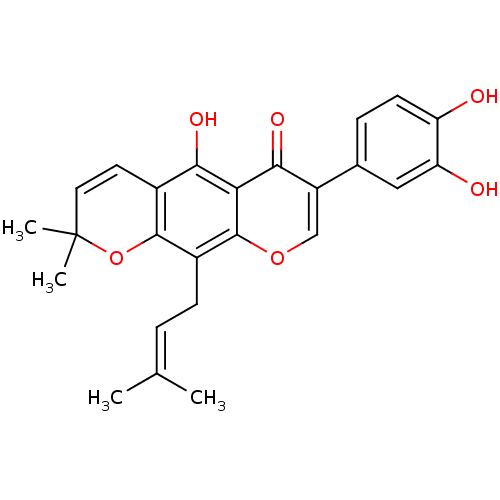
(AURICULASIN)Show SMILES [#6]\[#6](-[#6])=[#6]\[#6]-c1c2-[#8]C([#6])([#6])[#6]=[#6]-c2c(-[#8])c2c1occ(-c1ccc(-[#8])c(-[#8])c1)c2=O |c:11| Show InChI InChI=1S/C25H24O6/c1-13(2)5-7-16-23-15(9-10-25(3,4)31-23)21(28)20-22(29)17(12-30-24(16)20)14-6-8-18(26)19(27)11-14/h5-6,8-12,26-28H,7H2,1-4H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 4.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Qiqihar University
Curated by ChEMBL
| Assay Description
Non-competitive inhibition of human recombinant PTP1B using p-nitrophenyl phosphate as substrate by spectrophotometry based Lineweaver-Burk plot |
Bioorg Med Chem Lett 26: 318-21 (2016)
Article DOI: 10.1016/j.bmcl.2015.12.021
BindingDB Entry DOI: 10.7270/Q2C82C4W |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50442403
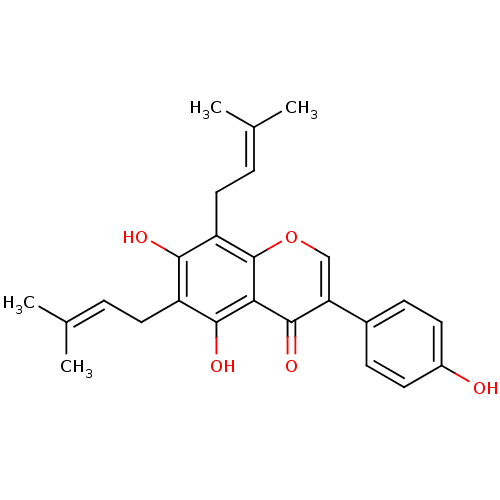
(CHEMBL494252)Show SMILES [#6]\[#6](-[#6])=[#6]\[#6]-c1c(-[#8])c(-[#6]\[#6]=[#6](\[#6])-[#6])c2occ(-c3ccc(-[#8])cc3)c(=O)c2c1-[#8] Show InChI InChI=1S/C25H26O5/c1-14(2)5-11-18-22(27)19(12-6-15(3)4)25-21(23(18)28)24(29)20(13-30-25)16-7-9-17(26)10-8-16/h5-10,13,26-28H,11-12H2,1-4H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 9.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Qiqihar University
Curated by ChEMBL
| Assay Description
Non-competitive inhibition of human recombinant PTP1B using p-nitrophenyl phosphate as substrate by spectrophotometry based Lineweaver-Burk plot |
Bioorg Med Chem Lett 26: 318-21 (2016)
Article DOI: 10.1016/j.bmcl.2015.12.021
BindingDB Entry DOI: 10.7270/Q2C82C4W |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50136568
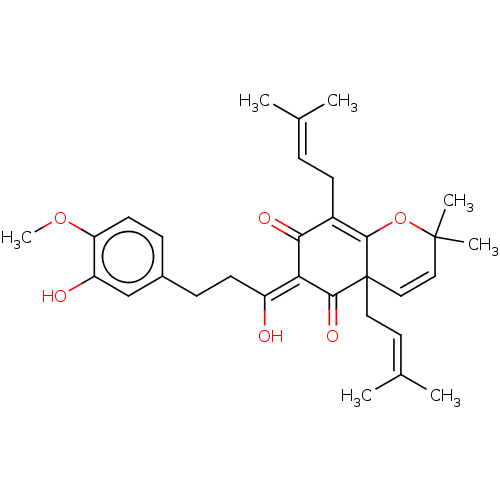
(CHEMBL3754570)Show SMILES [#6]-[#8]-c1ccc(-[#6]-[#6]\[#6](-[#8])=[#6]-2/[#6](=O)-[#6](-[#6]\[#6]=[#6](\[#6])-[#6])=[#6]3-[#8]C([#6])([#6])[#6]=[#6]C3([#6]\[#6]=[#6](\[#6])-[#6])[#6]-2=O)cc1-[#8] |c:24,t:18| Show InChI InChI=1S/C31H38O6/c1-19(2)8-11-22-27(34)26(23(32)12-9-21-10-13-25(36-7)24(33)18-21)28(35)31(15-14-20(3)4)17-16-30(5,6)37-29(22)31/h8,10,13-14,16-18,32-33H,9,11-12,15H2,1-7H3/b26-23- | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.23E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Qiqihar University
Curated by ChEMBL
| Assay Description
Competitive inhibition of human recombinant PTP1B using p-nitrophenyl phosphate as substrate by spectrophotometry based Lineweaver-Burk plot |
Bioorg Med Chem Lett 26: 318-21 (2016)
Article DOI: 10.1016/j.bmcl.2015.12.021
BindingDB Entry DOI: 10.7270/Q2C82C4W |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50136567
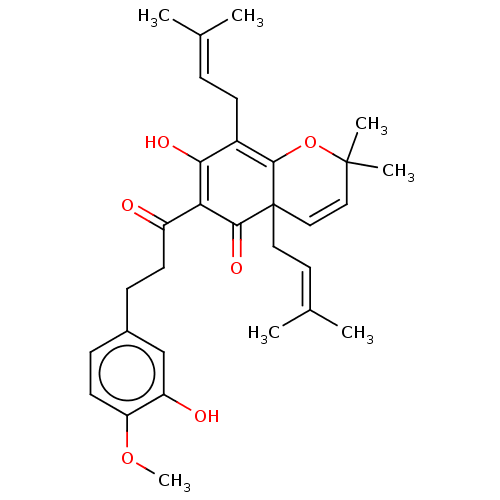
(CHEMBL3753821)Show SMILES [#6]-[#8]-c1ccc(-[#6]-[#6]-[#6](=O)-[#6]-2=[#6](-[#8])-[#6](-[#6]\[#6]=[#6](\[#6])-[#6])=[#6]3-[#8]C([#6])([#6])[#6]=[#6]C3([#6]\[#6]=[#6](\[#6])-[#6])[#6]-2=O)cc1-[#8] |c:10,24,t:18| Show InChI InChI=1S/C31H38O6/c1-19(2)8-11-22-27(34)26(23(32)12-9-21-10-13-25(36-7)24(33)18-21)28(35)31(15-14-20(3)4)17-16-30(5,6)37-29(22)31/h8,10,13-14,16-18,33-34H,9,11-12,15H2,1-7H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.76E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Qiqihar University
Curated by ChEMBL
| Assay Description
Competitive inhibition of human recombinant PTP1B using p-nitrophenyl phosphate as substrate by spectrophotometry based Lineweaver-Burk plot |
Bioorg Med Chem Lett 26: 318-21 (2016)
Article DOI: 10.1016/j.bmcl.2015.12.021
BindingDB Entry DOI: 10.7270/Q2C82C4W |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50442402
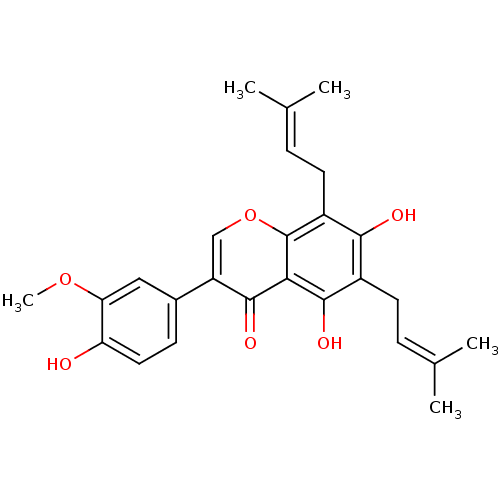
(FLEMINGSIN)Show SMILES [#6]-[#8]-c1cc(ccc1-[#8])-c1coc2c(-[#6]\[#6]=[#6](\[#6])-[#6])c(-[#8])c(-[#6]\[#6]=[#6](\[#6])-[#6])c(-[#8])c2c1=O Show InChI InChI=1S/C26H28O6/c1-14(2)6-9-17-23(28)18(10-7-15(3)4)26-22(24(17)29)25(30)19(13-32-26)16-8-11-20(27)21(12-16)31-5/h6-8,11-13,27-29H,9-10H2,1-5H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.92E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Qiqihar University
Curated by ChEMBL
| Assay Description
Non-competitive inhibition of human recombinant PTP1B using p-nitrophenyl phosphate as substrate by spectrophotometry based Lineweaver-Burk plot |
Bioorg Med Chem Lett 26: 318-21 (2016)
Article DOI: 10.1016/j.bmcl.2015.12.021
BindingDB Entry DOI: 10.7270/Q2C82C4W |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50442397
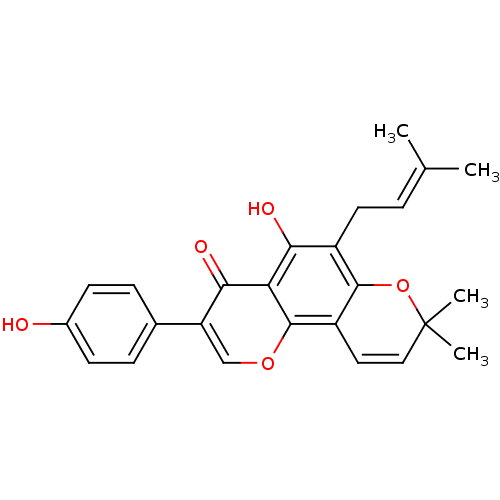
(OSAJIN)Show SMILES [#6]\[#6](-[#6])=[#6]\[#6]-c1c(-[#8])c2c(occ(-c3ccc(-[#8])cc3)c2=O)c2-[#6]=[#6]C([#6])([#6])[#8]-c12 |c:25| Show InChI InChI=1S/C25H24O5/c1-14(2)5-10-17-21(27)20-22(28)19(15-6-8-16(26)9-7-15)13-29-24(20)18-11-12-25(3,4)30-23(17)18/h5-9,11-13,26-27H,10H2,1-4H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.99E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Qiqihar University
Curated by ChEMBL
| Assay Description
Non-competitive inhibition of human recombinant PTP1B using p-nitrophenyl phosphate as substrate by spectrophotometry based Lineweaver-Burk plot |
Bioorg Med Chem Lett 26: 318-21 (2016)
Article DOI: 10.1016/j.bmcl.2015.12.021
BindingDB Entry DOI: 10.7270/Q2C82C4W |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50442401

(CHEMBL2442947)Show SMILES [#6]\[#6](-[#6])=[#6]\[#6]-c1c(-[#8])c(-[#6]\[#6]=[#6](\[#6])-[#6])c2occ(-c3ccc(-[#8])c(-[#8])c3)c(=O)c2c1-[#8] Show InChI InChI=1S/C25H26O6/c1-13(2)5-8-16-22(28)17(9-6-14(3)4)25-21(23(16)29)24(30)18(12-31-25)15-7-10-19(26)20(27)11-15/h5-7,10-12,26-29H,8-9H2,1-4H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Qiqihar University
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant PTP1B using p-nitrophenyl phosphate as substrate by spectrophotometric analysis |
Bioorg Med Chem Lett 26: 318-21 (2016)
Article DOI: 10.1016/j.bmcl.2015.12.021
BindingDB Entry DOI: 10.7270/Q2C82C4W |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50442400

(AURICULASIN)Show SMILES [#6]\[#6](-[#6])=[#6]\[#6]-c1c2-[#8]C([#6])([#6])[#6]=[#6]-c2c(-[#8])c2c1occ(-c1ccc(-[#8])c(-[#8])c1)c2=O |c:11| Show InChI InChI=1S/C25H24O6/c1-13(2)5-7-16-23-15(9-10-25(3,4)31-23)21(28)20-22(29)17(12-30-24(16)20)14-6-8-18(26)19(27)11-14/h5-6,8-12,26-28H,7H2,1-4H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Qiqihar University
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant PTP1B using p-nitrophenyl phosphate as substrate by spectrophotometric analysis |
Bioorg Med Chem Lett 26: 318-21 (2016)
Article DOI: 10.1016/j.bmcl.2015.12.021
BindingDB Entry DOI: 10.7270/Q2C82C4W |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50136569

(CHEMBL3754629)Show SMILES [#6]-[#8]-c1ccc(-[#6]-[#6]-[#6](=O)-[#6]-2=[#6](-[#8])-[#6](-[#6]\[#6]=[#6](\[#6])-[#6])=[#6]3-[#8]C([#6])([#6])[#6]=[#6]C3([#6]\[#6]=[#6](\[#6])-[#6])[#6]-2=O)cc1 |c:10,24,t:18| Show InChI InChI=1S/C31H38O5/c1-20(2)8-14-24-27(33)26(25(32)15-11-22-9-12-23(35-7)13-10-22)28(34)31(17-16-21(3)4)19-18-30(5,6)36-29(24)31/h8-10,12-13,16,18-19,33H,11,14-15,17H2,1-7H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Qiqihar University
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant PTP1B using p-nitrophenyl phosphate as substrate by spectrophotometric analysis |
Bioorg Med Chem Lett 26: 318-21 (2016)
Article DOI: 10.1016/j.bmcl.2015.12.021
BindingDB Entry DOI: 10.7270/Q2C82C4W |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50442403

(CHEMBL494252)Show SMILES [#6]\[#6](-[#6])=[#6]\[#6]-c1c(-[#8])c(-[#6]\[#6]=[#6](\[#6])-[#6])c2occ(-c3ccc(-[#8])cc3)c(=O)c2c1-[#8] Show InChI InChI=1S/C25H26O5/c1-14(2)5-11-18-22(27)19(12-6-15(3)4)25-21(23(18)28)24(29)20(13-30-25)16-7-9-17(26)10-8-16/h5-10,13,26-28H,11-12H2,1-4H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 9.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Qiqihar University
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant PTP1B using p-nitrophenyl phosphate as substrate by spectrophotometric analysis |
Bioorg Med Chem Lett 26: 318-21 (2016)
Article DOI: 10.1016/j.bmcl.2015.12.021
BindingDB Entry DOI: 10.7270/Q2C82C4W |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50148911

((3beta)-3-hydroxyurs-12-en-28-oic acid | 3beta-hyd...)Show SMILES C[C@@H]1CC[C@@]2(CC[C@]3(C)C(=CC[C@@H]4[C@@]5(C)CC[C@H](O)C(C)(C)[C@@H]5CC[C@@]34C)[C@@H]2[C@H]1C)C(O)=O |r,c:9| Show InChI InChI=1S/C30H48O3/c1-18-10-15-30(25(32)33)17-16-28(6)20(24(30)19(18)2)8-9-22-27(5)13-12-23(31)26(3,4)21(27)11-14-29(22,28)7/h8,18-19,21-24,31H,9-17H2,1-7H3,(H,32,33)/t18-,19+,21+,22-,23+,24+,27+,28-,29-,30+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.55E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Qiqihar University
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant PTP1B using p-nitrophenyl phosphate as substrate by spectrophotometric analysis |
Bioorg Med Chem Lett 26: 318-21 (2016)
Article DOI: 10.1016/j.bmcl.2015.12.021
BindingDB Entry DOI: 10.7270/Q2C82C4W |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50442402

(FLEMINGSIN)Show SMILES [#6]-[#8]-c1cc(ccc1-[#8])-c1coc2c(-[#6]\[#6]=[#6](\[#6])-[#6])c(-[#8])c(-[#6]\[#6]=[#6](\[#6])-[#6])c(-[#8])c2c1=O Show InChI InChI=1S/C26H28O6/c1-14(2)6-9-17-23(28)18(10-7-15(3)4)26-22(24(17)29)25(30)19(13-32-26)16-8-11-20(27)21(12-16)31-5/h6-8,11-13,27-29H,9-10H2,1-5H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.74E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Qiqihar University
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant PTP1B using p-nitrophenyl phosphate as substrate by spectrophotometric analysis |
Bioorg Med Chem Lett 26: 318-21 (2016)
Article DOI: 10.1016/j.bmcl.2015.12.021
BindingDB Entry DOI: 10.7270/Q2C82C4W |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50136568

(CHEMBL3754570)Show SMILES [#6]-[#8]-c1ccc(-[#6]-[#6]\[#6](-[#8])=[#6]-2/[#6](=O)-[#6](-[#6]\[#6]=[#6](\[#6])-[#6])=[#6]3-[#8]C([#6])([#6])[#6]=[#6]C3([#6]\[#6]=[#6](\[#6])-[#6])[#6]-2=O)cc1-[#8] |c:24,t:18| Show InChI InChI=1S/C31H38O6/c1-19(2)8-11-22-27(34)26(23(32)12-9-21-10-13-25(36-7)24(33)18-21)28(35)31(15-14-20(3)4)17-16-30(5,6)37-29(22)31/h8,10,13-14,16-18,32-33H,9,11-12,15H2,1-7H3/b26-23- | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.02E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Qiqihar University
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant PTP1B using p-nitrophenyl phosphate as substrate by spectrophotometric analysis |
Bioorg Med Chem Lett 26: 318-21 (2016)
Article DOI: 10.1016/j.bmcl.2015.12.021
BindingDB Entry DOI: 10.7270/Q2C82C4W |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50442397

(OSAJIN)Show SMILES [#6]\[#6](-[#6])=[#6]\[#6]-c1c(-[#8])c2c(occ(-c3ccc(-[#8])cc3)c2=O)c2-[#6]=[#6]C([#6])([#6])[#8]-c12 |c:25| Show InChI InChI=1S/C25H24O5/c1-14(2)5-10-17-21(27)20-22(28)19(15-6-8-16(26)9-7-15)13-29-24(20)18-11-12-25(3,4)30-23(17)18/h5-9,11-13,26-27H,10H2,1-4H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.36E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Qiqihar University
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant PTP1B using p-nitrophenyl phosphate as substrate by spectrophotometric analysis |
Bioorg Med Chem Lett 26: 318-21 (2016)
Article DOI: 10.1016/j.bmcl.2015.12.021
BindingDB Entry DOI: 10.7270/Q2C82C4W |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50136567

(CHEMBL3753821)Show SMILES [#6]-[#8]-c1ccc(-[#6]-[#6]-[#6](=O)-[#6]-2=[#6](-[#8])-[#6](-[#6]\[#6]=[#6](\[#6])-[#6])=[#6]3-[#8]C([#6])([#6])[#6]=[#6]C3([#6]\[#6]=[#6](\[#6])-[#6])[#6]-2=O)cc1-[#8] |c:10,24,t:18| Show InChI InChI=1S/C31H38O6/c1-19(2)8-11-22-27(34)26(23(32)12-9-21-10-13-25(36-7)24(33)18-21)28(35)31(15-14-20(3)4)17-16-30(5,6)37-29(22)31/h8,10,13-14,16-18,33-34H,9,11-12,15H2,1-7H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.94E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Qiqihar University
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant PTP1B using p-nitrophenyl phosphate as substrate by spectrophotometric analysis |
Bioorg Med Chem Lett 26: 318-21 (2016)
Article DOI: 10.1016/j.bmcl.2015.12.021
BindingDB Entry DOI: 10.7270/Q2C82C4W |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data