

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Vitamin D3 receptor (Homo sapiens (Human)) | BDBM50139645 (CHEBI:47828 | CHEMBL3544529) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL KEGG PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | 2.29E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Wisconsin Curated by ChEMBL | Assay Description Antagonist activity against VDR-LBD (unknown origin) expressed in Escherichia coli assessed as inhibition of VDR agonist LG190178-induced SRC2-3 coac... | Eur J Med Chem 109: 238-46 (2016) Article DOI: 10.1016/j.ejmech.2016.01.002 BindingDB Entry DOI: 10.7270/Q21N82ZW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vitamin D3 receptor (Homo sapiens (Human)) | BDBM50139647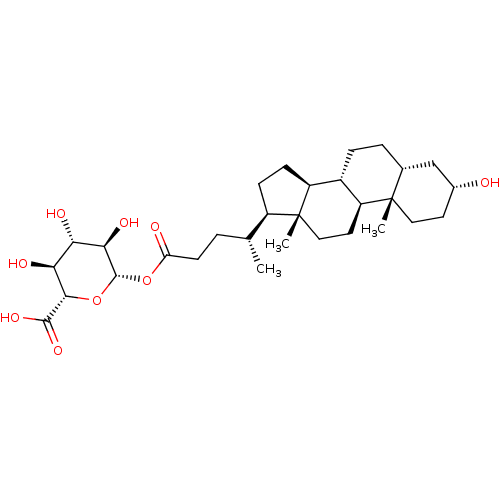 (CHEMBL3764957) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.86E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Wisconsin Curated by ChEMBL | Assay Description Antagonist activity against VDR-LBD (unknown origin) expressed in Escherichia coli assessed as inhibition of VDR agonist LG190178-induced SRC2-3 coac... | Eur J Med Chem 109: 238-46 (2016) Article DOI: 10.1016/j.ejmech.2016.01.002 BindingDB Entry DOI: 10.7270/Q21N82ZW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vitamin D3 receptor (Homo sapiens (Human)) | BDBM50375582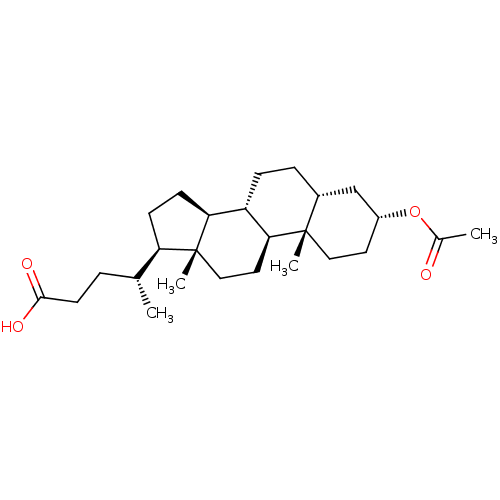 (CHEMBL272041) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | 1.10E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Wisconsin Curated by ChEMBL | Assay Description Agonist activity at VDR-LBD (unknown origin) expressed in Escherichia coli assessed as SRC2-3 coactivator peptide recruitment after 30 mins by fluore... | Eur J Med Chem 109: 238-46 (2016) Article DOI: 10.1016/j.ejmech.2016.01.002 BindingDB Entry DOI: 10.7270/Q21N82ZW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vitamin D3 receptor (Homo sapiens (Human)) | BDBM50236238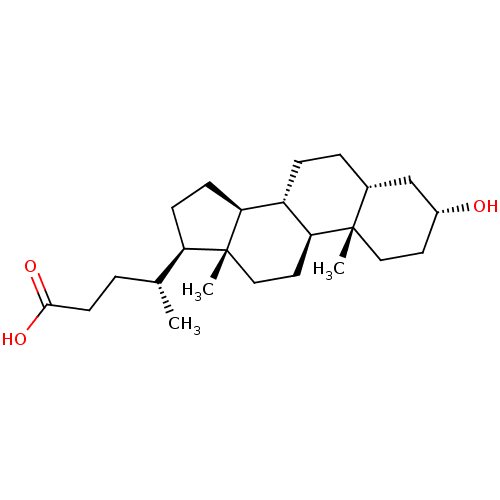 ((3alpha,5beta)-3-hydroxycholan-24-oic acid | 3alph...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 1.36E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Wisconsin Curated by ChEMBL | Assay Description Agonist activity at VDR-LBD (unknown origin) expressed in Escherichia coli assessed as SRC2-3 coactivator peptide recruitment after 30 mins by fluore... | Eur J Med Chem 109: 238-46 (2016) Article DOI: 10.1016/j.ejmech.2016.01.002 BindingDB Entry DOI: 10.7270/Q21N82ZW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vitamin D3 receptor (Homo sapiens (Human)) | BDBM50375593 (CHEMBL408701) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.45E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Wisconsin Curated by ChEMBL | Assay Description Antagonist activity against VDR-LBD (unknown origin) expressed in Escherichia coli assessed as inhibition of VDR agonist LG190178-induced SRC2-3 coac... | Eur J Med Chem 109: 238-46 (2016) Article DOI: 10.1016/j.ejmech.2016.01.002 BindingDB Entry DOI: 10.7270/Q21N82ZW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vitamin D3 receptor (Homo sapiens (Human)) | BDBM50375564 (CHEMBL260735 | LCAME) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.51E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Wisconsin Curated by ChEMBL | Assay Description Agonist activity at VP16 tagged-VDR-LBD (unknown origin) expressed in HEK293T cells assessed as SRC1 coactivator peptide recruitment after 16 hrs by ... | Eur J Med Chem 109: 238-46 (2016) Article DOI: 10.1016/j.ejmech.2016.01.002 BindingDB Entry DOI: 10.7270/Q21N82ZW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vitamin D3 receptor (Homo sapiens (Human)) | BDBM50375583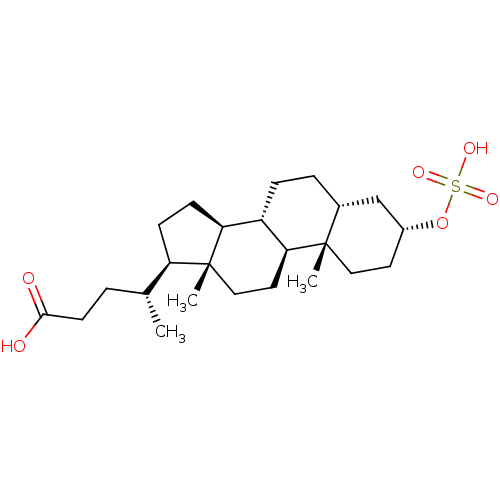 (CHEMBL260315) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.33E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Wisconsin Curated by ChEMBL | Assay Description Antagonist activity against VDR-LBD (unknown origin) expressed in Escherichia coli assessed as inhibition of VDR agonist LG190178-induced SRC2-3 coac... | Eur J Med Chem 109: 238-46 (2016) Article DOI: 10.1016/j.ejmech.2016.01.002 BindingDB Entry DOI: 10.7270/Q21N82ZW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vitamin D3 receptor (Homo sapiens (Human)) | BDBM50139646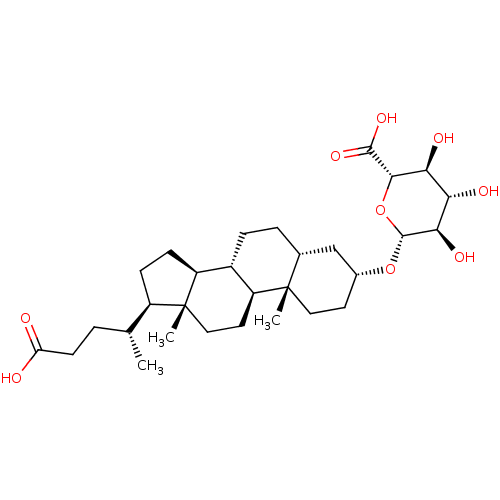 (CHEMBL3763659) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.15E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Wisconsin Curated by ChEMBL | Assay Description Antagonist activity against VDR-LBD (unknown origin) expressed in Escherichia coli assessed as inhibition of VDR agonist LG190178-induced SRC2-3 coac... | Eur J Med Chem 109: 238-46 (2016) Article DOI: 10.1016/j.ejmech.2016.01.002 BindingDB Entry DOI: 10.7270/Q21N82ZW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vitamin D3 receptor (Homo sapiens (Human)) | BDBM50375588 (CHEMBL258818 | LCAGLY) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.01E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Wisconsin Curated by ChEMBL | Assay Description Antagonist activity against VDR-LBD (unknown origin) expressed in Escherichia coli assessed as inhibition of VDR agonist LG190178-induced SRC2-3 coac... | Eur J Med Chem 109: 238-46 (2016) Article DOI: 10.1016/j.ejmech.2016.01.002 BindingDB Entry DOI: 10.7270/Q21N82ZW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vitamin D3 receptor (Homo sapiens (Human)) | BDBM50236238 ((3alpha,5beta)-3-hydroxycholan-24-oic acid | 3alph...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 4.67E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Wisconsin Curated by ChEMBL | Assay Description Antagonist activity against VDR-LBD (unknown origin) expressed in Escherichia coli assessed as inhibition of VDR agonist LG190178-induced SRC2-3 coac... | Eur J Med Chem 109: 238-46 (2016) Article DOI: 10.1016/j.ejmech.2016.01.002 BindingDB Entry DOI: 10.7270/Q21N82ZW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vitamin D3 receptor (Homo sapiens (Human)) | BDBM50375596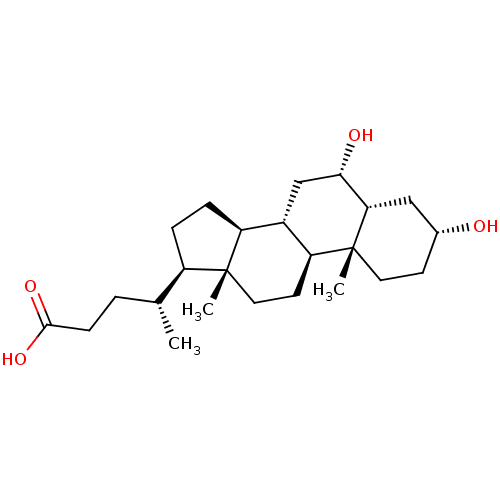 (HDCA | hyodeoxycholic acid) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Wisconsin Curated by ChEMBL | Assay Description Agonist activity at human VP16 tagged-VDR-LBD after 16 hrs by luciferase reporter gene based transcription assay | Eur J Med Chem 109: 238-46 (2016) Article DOI: 10.1016/j.ejmech.2016.01.002 BindingDB Entry DOI: 10.7270/Q21N82ZW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vitamin D3 receptor (Homo sapiens (Human)) | BDBM50375583 (CHEMBL260315) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Wisconsin Curated by ChEMBL | Assay Description Antagonist activity against VP16 tagged-VDR-LBD (unknown origin) expressed in HEK293T cells assessed as inhibition of 1,25-dihydroxyvitamin D3-induce... | Eur J Med Chem 109: 238-46 (2016) Article DOI: 10.1016/j.ejmech.2016.01.002 BindingDB Entry DOI: 10.7270/Q21N82ZW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vitamin D3 receptor (Homo sapiens (Human)) | BDBM50375588 (CHEMBL258818 | LCAGLY) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Wisconsin Curated by ChEMBL | Assay Description Antagonist activity against VP16 tagged-VDR-LBD (unknown origin) expressed in HEK293T cells assessed as inhibition of 1,25-dihydroxyvitamin D3-induce... | Eur J Med Chem 109: 238-46 (2016) Article DOI: 10.1016/j.ejmech.2016.01.002 BindingDB Entry DOI: 10.7270/Q21N82ZW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vitamin D3 receptor (Homo sapiens (Human)) | BDBM50375582 (CHEMBL272041) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Wisconsin Curated by ChEMBL | Assay Description Agonist activity at VP16 tagged-VDR-LBD (unknown origin) expressed in HEK293T cells assessed as SRC1 coactivator peptide recruitment after 16 hrs by ... | Eur J Med Chem 109: 238-46 (2016) Article DOI: 10.1016/j.ejmech.2016.01.002 BindingDB Entry DOI: 10.7270/Q21N82ZW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vitamin D3 receptor (Homo sapiens (Human)) | BDBM21674 ((4R)-4-[(1S,2S,5R,7S,9R,10R,11S,14R,15R)-5,9-dihyd...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Wisconsin Curated by ChEMBL | Assay Description Antagonist activity against VP16 tagged-VDR-LBD (unknown origin) expressed in HEK293T cells assessed as inhibition of 1,25-dihydroxyvitamin D3-induce... | Eur J Med Chem 109: 238-46 (2016) Article DOI: 10.1016/j.ejmech.2016.01.002 BindingDB Entry DOI: 10.7270/Q21N82ZW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vitamin D3 receptor (Homo sapiens (Human)) | BDBM50375599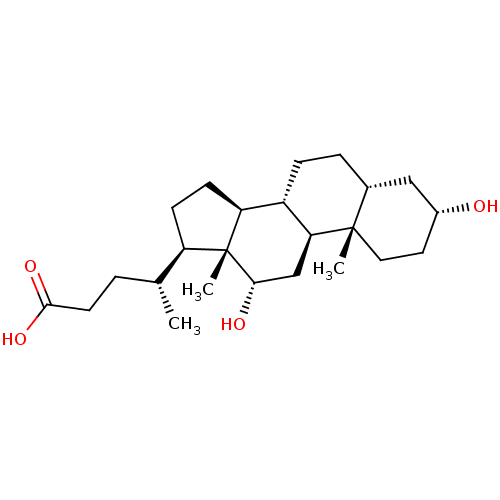 (DEOXYCHOLATE | Deoxycholic Acid | KYBELLA) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase DrugBank MCE KEGG MMDB PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Wisconsin Curated by ChEMBL | Assay Description Antagonist activity against VP16 tagged-VDR-LBD (unknown origin) expressed in HEK293T cells assessed as inhibition of 1,25-dihydroxyvitamin D3-induce... | Eur J Med Chem 109: 238-46 (2016) Article DOI: 10.1016/j.ejmech.2016.01.002 BindingDB Entry DOI: 10.7270/Q21N82ZW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vitamin D3 receptor (Homo sapiens (Human)) | BDBM53721 ((4R)-4-[(3R,5S,7S,8R,9S,10S,13R,14S,17R)-10,13-dim...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Wisconsin Curated by ChEMBL | Assay Description Antagonist activity against VP16 tagged-VDR-LBD (unknown origin) expressed in HEK293T cells assessed as inhibition of 1,25-dihydroxyvitamin D3-induce... | Eur J Med Chem 109: 238-46 (2016) Article DOI: 10.1016/j.ejmech.2016.01.002 BindingDB Entry DOI: 10.7270/Q21N82ZW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vitamin D3 receptor (Homo sapiens (Human)) | BDBM50375593 (CHEMBL408701) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Wisconsin Curated by ChEMBL | Assay Description Agonist activity at VP16 tagged-VDR-LBD (unknown origin) expressed in HEK293T cells assessed as SRC1 coactivator peptide recruitment after 16 hrs by ... | Eur J Med Chem 109: 238-46 (2016) Article DOI: 10.1016/j.ejmech.2016.01.002 BindingDB Entry DOI: 10.7270/Q21N82ZW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vitamin D3 receptor (Homo sapiens (Human)) | BDBM50139645 (CHEBI:47828 | CHEMBL3544529) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL KEGG PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Wisconsin Curated by ChEMBL | Assay Description Antagonist activity against VDR-LBD (unknown origin) expressed in Escherichia coli assessed as inhibition of VDR agonist LG190178-induced SRC2-3 coac... | Eur J Med Chem 109: 238-46 (2016) Article DOI: 10.1016/j.ejmech.2016.01.002 BindingDB Entry DOI: 10.7270/Q21N82ZW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vitamin D3 receptor (Homo sapiens (Human)) | BDBM21674 ((4R)-4-[(1S,2S,5R,7S,9R,10R,11S,14R,15R)-5,9-dihyd...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | >1.50E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Wisconsin Curated by ChEMBL | Assay Description Antagonist activity against VP16 tagged-VDR-LBD (unknown origin) expressed in HEK293T cells assessed as inhibition of 1,25-dihydroxyvitamin D3-induce... | Eur J Med Chem 109: 238-46 (2016) Article DOI: 10.1016/j.ejmech.2016.01.002 BindingDB Entry DOI: 10.7270/Q21N82ZW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vitamin D3 receptor (Homo sapiens (Human)) | BDBM21680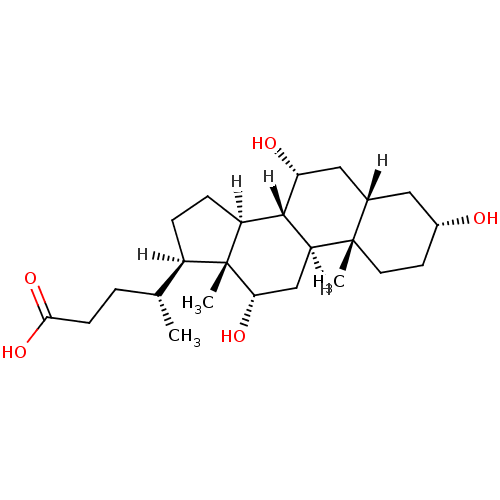 ((4R)-4-[(1S,2S,5R,7S,9R,10R,11S,14R,15R,16S)-5,9,1...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | >1.50E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Wisconsin Curated by ChEMBL | Assay Description Agonist activity at VDR-LBD (unknown origin) expressed in Escherichia coli assessed as SRC2-3 coactivator peptide recruitment after 30 mins by fluore... | Eur J Med Chem 109: 238-46 (2016) Article DOI: 10.1016/j.ejmech.2016.01.002 BindingDB Entry DOI: 10.7270/Q21N82ZW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vitamin D3 receptor (Homo sapiens (Human)) | BDBM50139647 (CHEMBL3764957) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.50E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Wisconsin Curated by ChEMBL | Assay Description Antagonist activity against VP16 tagged-VDR-LBD (unknown origin) expressed in HEK293T cells assessed as inhibition of 1,25-dihydroxyvitamin D3-induce... | Eur J Med Chem 109: 238-46 (2016) Article DOI: 10.1016/j.ejmech.2016.01.002 BindingDB Entry DOI: 10.7270/Q21N82ZW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vitamin D3 receptor (Homo sapiens (Human)) | BDBM21680 ((4R)-4-[(1S,2S,5R,7S,9R,10R,11S,14R,15R,16S)-5,9,1...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | >1.50E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Wisconsin Curated by ChEMBL | Assay Description Agonist activity at VDR-LBD (unknown origin) expressed in Escherichia coli assessed as SRC2-3 coactivator peptide recruitment after 30 mins by fluore... | Eur J Med Chem 109: 238-46 (2016) Article DOI: 10.1016/j.ejmech.2016.01.002 BindingDB Entry DOI: 10.7270/Q21N82ZW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vitamin D3 receptor (Homo sapiens (Human)) | BDBM50375599 (DEOXYCHOLATE | Deoxycholic Acid | KYBELLA) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase DrugBank MCE KEGG MMDB PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.50E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Wisconsin Curated by ChEMBL | Assay Description Agonist activity at human VP16 tagged-VDR-LBD after 16 hrs by luciferase reporter gene based transcription assay | Eur J Med Chem 109: 238-46 (2016) Article DOI: 10.1016/j.ejmech.2016.01.002 BindingDB Entry DOI: 10.7270/Q21N82ZW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vitamin D3 receptor (Homo sapiens (Human)) | BDBM50375596 (HDCA | hyodeoxycholic acid) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.50E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Wisconsin Curated by ChEMBL | Assay Description Antagonist activity against VP16 tagged-VDR-LBD (unknown origin) expressed in HEK293T cells assessed as inhibition of 1,25-dihydroxyvitamin D3-induce... | Eur J Med Chem 109: 238-46 (2016) Article DOI: 10.1016/j.ejmech.2016.01.002 BindingDB Entry DOI: 10.7270/Q21N82ZW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vitamin D3 receptor (Homo sapiens (Human)) | BDBM50375564 (CHEMBL260735 | LCAME) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.50E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Wisconsin Curated by ChEMBL | Assay Description Agonist activity at VP16 tagged-VDR-LBD (unknown origin) expressed in HEK293T cells assessed as SRC1 coactivator peptide recruitment after 16 hrs by ... | Eur J Med Chem 109: 238-46 (2016) Article DOI: 10.1016/j.ejmech.2016.01.002 BindingDB Entry DOI: 10.7270/Q21N82ZW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vitamin D3 receptor (Homo sapiens (Human)) | BDBM50139646 (CHEMBL3763659) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.50E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Wisconsin Curated by ChEMBL | Assay Description Antagonist activity against VP16 tagged-VDR-LBD (unknown origin) expressed in HEK293T cells assessed as inhibition of 1,25-dihydroxyvitamin D3-induce... | Eur J Med Chem 109: 238-46 (2016) Article DOI: 10.1016/j.ejmech.2016.01.002 BindingDB Entry DOI: 10.7270/Q21N82ZW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vitamin D3 receptor (Homo sapiens (Human)) | BDBM53721 ((4R)-4-[(3R,5S,7S,8R,9S,10S,13R,14S,17R)-10,13-dim...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | >1.50E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Wisconsin Curated by ChEMBL | Assay Description Agonist activity at VDR-LBD (unknown origin) expressed in Escherichia coli assessed as SRC2-3 coactivator peptide recruitment after 30 mins by fluore... | Eur J Med Chem 109: 238-46 (2016) Article DOI: 10.1016/j.ejmech.2016.01.002 BindingDB Entry DOI: 10.7270/Q21N82ZW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vitamin D3 receptor (Homo sapiens (Human)) | BDBM50139646 (CHEMBL3763659) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a |
University of Wisconsin Curated by ChEMBL | Assay Description Antagonist activity against VDR-LBD (unknown origin) expressed in Escherichia coli assessed as inhibition of VDR agonist LG190178-induced SRC2-3 coac... | Eur J Med Chem 109: 238-46 (2016) Article DOI: 10.1016/j.ejmech.2016.01.002 BindingDB Entry DOI: 10.7270/Q21N82ZW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vitamin D3 receptor (Homo sapiens (Human)) | BDBM50375564 (CHEMBL260735 | LCAME) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | >1.50E+5 | n/a | n/a | n/a | n/a |
University of Wisconsin Curated by ChEMBL | Assay Description Agonist activity at VP16 tagged-VDR-LBD (unknown origin) expressed in HEK293T cells assessed as SRC1 coactivator peptide recruitment after 16 hrs by ... | Eur J Med Chem 109: 238-46 (2016) Article DOI: 10.1016/j.ejmech.2016.01.002 BindingDB Entry DOI: 10.7270/Q21N82ZW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vitamin D3 receptor (Homo sapiens (Human)) | BDBM50139647 (CHEMBL3764957) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 2.13E+4 | n/a | n/a | n/a | n/a |
University of Wisconsin Curated by ChEMBL | Assay Description Antagonist activity against VP16 tagged-VDR-LBD (unknown origin) expressed in HEK293T cells assessed as inhibition of 1,25-dihydroxyvitamin D3-induce... | Eur J Med Chem 109: 238-46 (2016) Article DOI: 10.1016/j.ejmech.2016.01.002 BindingDB Entry DOI: 10.7270/Q21N82ZW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vitamin D3 receptor (Homo sapiens (Human)) | BDBM50375588 (CHEMBL258818 | LCAGLY) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | >1.50E+5 | n/a | n/a | n/a | n/a |
University of Wisconsin Curated by ChEMBL | Assay Description Agonist activity at VP16 tagged-VDR-LBD (unknown origin) expressed in HEK293T cells assessed as SRC1 coactivator peptide recruitment after 16 hrs by ... | Eur J Med Chem 109: 238-46 (2016) Article DOI: 10.1016/j.ejmech.2016.01.002 BindingDB Entry DOI: 10.7270/Q21N82ZW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vitamin D3 receptor (Homo sapiens (Human)) | BDBM50375599 (DEOXYCHOLATE | Deoxycholic Acid | KYBELLA) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase DrugBank MCE KEGG MMDB PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | >1.50E+5 | n/a | n/a | n/a | n/a |
University of Wisconsin Curated by ChEMBL | Assay Description Antagonist activity against VP16 tagged-VDR-LBD (unknown origin) expressed in HEK293T cells assessed as inhibition of 1,25-dihydroxyvitamin D3-induce... | Eur J Med Chem 109: 238-46 (2016) Article DOI: 10.1016/j.ejmech.2016.01.002 BindingDB Entry DOI: 10.7270/Q21N82ZW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vitamin D3 receptor (Homo sapiens (Human)) | BDBM50375588 (CHEMBL258818 | LCAGLY) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | >1.50E+5 | n/a | n/a | n/a | n/a |
University of Wisconsin Curated by ChEMBL | Assay Description Antagonist activity against VDR-LBD (unknown origin) expressed in Escherichia coli assessed as inhibition of VDR agonist LG190178-induced SRC2-3 coac... | Eur J Med Chem 109: 238-46 (2016) Article DOI: 10.1016/j.ejmech.2016.01.002 BindingDB Entry DOI: 10.7270/Q21N82ZW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vitamin D3 receptor (Homo sapiens (Human)) | BDBM50139646 (CHEMBL3763659) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 2.05E+4 | n/a | n/a | n/a | n/a |
University of Wisconsin Curated by ChEMBL | Assay Description Antagonist activity against VDR-LBD (unknown origin) expressed in Escherichia coli assessed as inhibition of VDR agonist LG190178-induced SRC2-3 coac... | Eur J Med Chem 109: 238-46 (2016) Article DOI: 10.1016/j.ejmech.2016.01.002 BindingDB Entry DOI: 10.7270/Q21N82ZW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vitamin D3 receptor (Homo sapiens (Human)) | BDBM21680 ((4R)-4-[(1S,2S,5R,7S,9R,10R,11S,14R,15R,16S)-5,9,1...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | >1.50E+5 | n/a | n/a | n/a | n/a |
University of Wisconsin Curated by ChEMBL | Assay Description Agonist activity at VP16 tagged-VDR-LBD (unknown origin) expressed in HEK293T cells assessed as SRC1 coactivator peptide recruitment after 16 hrs by ... | Eur J Med Chem 109: 238-46 (2016) Article DOI: 10.1016/j.ejmech.2016.01.002 BindingDB Entry DOI: 10.7270/Q21N82ZW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vitamin D3 receptor (Homo sapiens (Human)) | BDBM50375596 (HDCA | hyodeoxycholic acid) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | >1.50E+5 | n/a | n/a | n/a | n/a |
University of Wisconsin Curated by ChEMBL | Assay Description Agonist activity at VDR-LBD (unknown origin) expressed in Escherichia coli assessed as SRC2-3 coactivator peptide recruitment after 30 mins by fluore... | Eur J Med Chem 109: 238-46 (2016) Article DOI: 10.1016/j.ejmech.2016.01.002 BindingDB Entry DOI: 10.7270/Q21N82ZW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vitamin D3 receptor (Homo sapiens (Human)) | BDBM53721 ((4R)-4-[(3R,5S,7S,8R,9S,10S,13R,14S,17R)-10,13-dim...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | >1.50E+5 | n/a | n/a | n/a | n/a |
University of Wisconsin Curated by ChEMBL | Assay Description Agonist activity at VDR-LBD (unknown origin) expressed in Escherichia coli assessed as SRC2-3 coactivator peptide recruitment after 30 mins by fluore... | Eur J Med Chem 109: 238-46 (2016) Article DOI: 10.1016/j.ejmech.2016.01.002 BindingDB Entry DOI: 10.7270/Q21N82ZW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vitamin D3 receptor (Homo sapiens (Human)) | BDBM50375593 (CHEMBL408701) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | >1.50E+5 | n/a | n/a | n/a | n/a |
University of Wisconsin Curated by ChEMBL | Assay Description Agonist activity at VDR-LBD (unknown origin) expressed in Escherichia coli assessed as SRC2-3 coactivator peptide recruitment after 30 mins by fluore... | Eur J Med Chem 109: 238-46 (2016) Article DOI: 10.1016/j.ejmech.2016.01.002 BindingDB Entry DOI: 10.7270/Q21N82ZW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vitamin D3 receptor (Homo sapiens (Human)) | BDBM50139647 (CHEMBL3764957) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | >1.50E+5 | n/a | n/a | n/a | n/a |
University of Wisconsin Curated by ChEMBL | Assay Description Antagonist activity against VDR-LBD (unknown origin) expressed in Escherichia coli assessed as inhibition of VDR agonist LG190178-induced SRC2-3 coac... | Eur J Med Chem 109: 238-46 (2016) Article DOI: 10.1016/j.ejmech.2016.01.002 BindingDB Entry DOI: 10.7270/Q21N82ZW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vitamin D3 receptor (Homo sapiens (Human)) | BDBM21680 ((4R)-4-[(1S,2S,5R,7S,9R,10R,11S,14R,15R,16S)-5,9,1...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | >1.50E+5 | n/a | n/a | n/a | n/a |
University of Wisconsin Curated by ChEMBL | Assay Description Agonist activity at VDR-LBD (unknown origin) expressed in Escherichia coli assessed as SRC2-3 coactivator peptide recruitment after 30 mins by fluore... | Eur J Med Chem 109: 238-46 (2016) Article DOI: 10.1016/j.ejmech.2016.01.002 BindingDB Entry DOI: 10.7270/Q21N82ZW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vitamin D3 receptor (Homo sapiens (Human)) | BDBM50375583 (CHEMBL260315) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | >1.50E+5 | n/a | n/a | n/a | n/a |
University of Wisconsin Curated by ChEMBL | Assay Description Antagonist activity against VDR-LBD (unknown origin) expressed in Escherichia coli assessed as inhibition of VDR agonist LG190178-induced SRC2-3 coac... | Eur J Med Chem 109: 238-46 (2016) Article DOI: 10.1016/j.ejmech.2016.01.002 BindingDB Entry DOI: 10.7270/Q21N82ZW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vitamin D3 receptor (Homo sapiens (Human)) | BDBM21674 ((4R)-4-[(1S,2S,5R,7S,9R,10R,11S,14R,15R)-5,9-dihyd...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | >1.50E+5 | n/a | n/a | n/a | n/a |
University of Wisconsin Curated by ChEMBL | Assay Description Antagonist activity against VP16 tagged-VDR-LBD (unknown origin) expressed in HEK293T cells assessed as inhibition of 1,25-dihydroxyvitamin D3-induce... | Eur J Med Chem 109: 238-46 (2016) Article DOI: 10.1016/j.ejmech.2016.01.002 BindingDB Entry DOI: 10.7270/Q21N82ZW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vitamin D3 receptor (Homo sapiens (Human)) | BDBM21674 ((4R)-4-[(1S,2S,5R,7S,9R,10R,11S,14R,15R)-5,9-dihyd...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | >1.50E+5 | n/a | n/a | n/a | n/a |
University of Wisconsin Curated by ChEMBL | Assay Description Agonist activity at VP16 tagged-VDR-LBD (unknown origin) expressed in HEK293T cells assessed as SRC1 coactivator peptide recruitment after 16 hrs by ... | Eur J Med Chem 109: 238-46 (2016) Article DOI: 10.1016/j.ejmech.2016.01.002 BindingDB Entry DOI: 10.7270/Q21N82ZW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vitamin D3 receptor (Homo sapiens (Human)) | BDBM50236238 ((3alpha,5beta)-3-hydroxycholan-24-oic acid | 3alph...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 2.14E+3 | n/a | n/a | n/a | n/a |
University of Wisconsin Curated by ChEMBL | Assay Description Agonist activity at VDR-LBD (unknown origin) expressed in Escherichia coli assessed as SRC2-3 coactivator peptide recruitment after 30 mins by fluore... | Eur J Med Chem 109: 238-46 (2016) Article DOI: 10.1016/j.ejmech.2016.01.002 BindingDB Entry DOI: 10.7270/Q21N82ZW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vitamin D3 receptor (Homo sapiens (Human)) | BDBM50139647 (CHEMBL3764957) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 6.52E+3 | n/a | n/a | n/a | n/a |
University of Wisconsin Curated by ChEMBL | Assay Description Agonist activity at VP16 tagged-VDR-LBD (unknown origin) expressed in HEK293T cells assessed as SRC1 coactivator peptide recruitment after 16 hrs by ... | Eur J Med Chem 109: 238-46 (2016) Article DOI: 10.1016/j.ejmech.2016.01.002 BindingDB Entry DOI: 10.7270/Q21N82ZW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vitamin D3 receptor (Homo sapiens (Human)) | BDBM50375582 (CHEMBL272041) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | >1.50E+5 | n/a | n/a | n/a | n/a |
University of Wisconsin Curated by ChEMBL | Assay Description Agonist activity at VP16 tagged-VDR-LBD (unknown origin) expressed in HEK293T cells assessed as SRC1 coactivator peptide recruitment after 16 hrs by ... | Eur J Med Chem 109: 238-46 (2016) Article DOI: 10.1016/j.ejmech.2016.01.002 BindingDB Entry DOI: 10.7270/Q21N82ZW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vitamin D3 receptor (Homo sapiens (Human)) | BDBM50375564 (CHEMBL260735 | LCAME) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | >1.50E+5 | n/a | n/a | n/a | n/a |
University of Wisconsin Curated by ChEMBL | Assay Description Agonist activity at VP16 tagged-VDR-LBD (unknown origin) expressed in HEK293T cells assessed as SRC1 coactivator peptide recruitment after 16 hrs by ... | Eur J Med Chem 109: 238-46 (2016) Article DOI: 10.1016/j.ejmech.2016.01.002 BindingDB Entry DOI: 10.7270/Q21N82ZW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vitamin D3 receptor (Homo sapiens (Human)) | BDBM50139645 (CHEBI:47828 | CHEMBL3544529) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL KEGG PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 870 | n/a | n/a | n/a | n/a |
University of Wisconsin Curated by ChEMBL | Assay Description Antagonist activity against VP16 tagged-VDR-LBD (unknown origin) expressed in HEK293T cells assessed as inhibition of 1,25-dihydroxyvitamin D3-induce... | Eur J Med Chem 109: 238-46 (2016) Article DOI: 10.1016/j.ejmech.2016.01.002 BindingDB Entry DOI: 10.7270/Q21N82ZW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vitamin D3 receptor (Homo sapiens (Human)) | BDBM50139646 (CHEMBL3763659) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 1.23E+4 | n/a | n/a | n/a | n/a |
University of Wisconsin Curated by ChEMBL | Assay Description Antagonist activity against VP16 tagged-VDR-LBD (unknown origin) expressed in HEK293T cells assessed as inhibition of 1,25-dihydroxyvitamin D3-induce... | Eur J Med Chem 109: 238-46 (2016) Article DOI: 10.1016/j.ejmech.2016.01.002 BindingDB Entry DOI: 10.7270/Q21N82ZW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vitamin D3 receptor (Homo sapiens (Human)) | BDBM50375596 (HDCA | hyodeoxycholic acid) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | >1.50E+5 | n/a | n/a | n/a | n/a |
University of Wisconsin Curated by ChEMBL | Assay Description Agonist activity at VP16 tagged-VDR-LBD (unknown origin) expressed in HEK293T cells assessed as SRC1 coactivator peptide recruitment after 16 hrs by ... | Eur J Med Chem 109: 238-46 (2016) Article DOI: 10.1016/j.ejmech.2016.01.002 BindingDB Entry DOI: 10.7270/Q21N82ZW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vitamin D3 receptor (Homo sapiens (Human)) | BDBM50375582 (CHEMBL272041) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 1.49E+3 | n/a | n/a | n/a | n/a |
University of Wisconsin Curated by ChEMBL | Assay Description Agonist activity at VP16 tagged-VDR-LBD (unknown origin) expressed in HEK293T cells assessed as SRC1 coactivator peptide recruitment after 16 hrs by ... | Eur J Med Chem 109: 238-46 (2016) Article DOI: 10.1016/j.ejmech.2016.01.002 BindingDB Entry DOI: 10.7270/Q21N82ZW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vitamin D3 receptor (Homo sapiens (Human)) | BDBM50139645 (CHEBI:47828 | CHEMBL3544529) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL KEGG PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a |
University of Wisconsin Curated by ChEMBL | Assay Description Agonist activity at VDR-LBD (unknown origin) expressed in Escherichia coli assessed as SRC2-3 coactivator peptide recruitment after 30 mins by fluore... | Eur J Med Chem 109: 238-46 (2016) Article DOI: 10.1016/j.ejmech.2016.01.002 BindingDB Entry DOI: 10.7270/Q21N82ZW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vitamin D3 receptor (Homo sapiens (Human)) | BDBM50375593 (CHEMBL408701) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | >1.50E+5 | n/a | n/a | n/a | n/a |
University of Wisconsin Curated by ChEMBL | Assay Description Agonist activity at VDR-LBD (unknown origin) expressed in Escherichia coli assessed as SRC2-3 coactivator peptide recruitment after 30 mins by fluore... | Eur J Med Chem 109: 238-46 (2016) Article DOI: 10.1016/j.ejmech.2016.01.002 BindingDB Entry DOI: 10.7270/Q21N82ZW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vitamin D3 receptor (Homo sapiens (Human)) | BDBM53721 ((4R)-4-[(3R,5S,7S,8R,9S,10S,13R,14S,17R)-10,13-dim...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | >1.50E+5 | n/a | n/a | n/a | n/a |
University of Wisconsin Curated by ChEMBL | Assay Description Agonist activity at VDR-LBD (unknown origin) expressed in Escherichia coli assessed as SRC2-3 coactivator peptide recruitment after 30 mins by fluore... | Eur J Med Chem 109: 238-46 (2016) Article DOI: 10.1016/j.ejmech.2016.01.002 BindingDB Entry DOI: 10.7270/Q21N82ZW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vitamin D3 receptor (Homo sapiens (Human)) | BDBM50236238 ((3alpha,5beta)-3-hydroxycholan-24-oic acid | 3alph...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a |
University of Wisconsin Curated by ChEMBL | Assay Description Agonist activity at VDR-LBD (unknown origin) expressed in Escherichia coli assessed as SRC2-3 coactivator peptide recruitment after 30 mins by fluore... | Eur J Med Chem 109: 238-46 (2016) Article DOI: 10.1016/j.ejmech.2016.01.002 BindingDB Entry DOI: 10.7270/Q21N82ZW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vitamin D3 receptor (Homo sapiens (Human)) | BDBM50375599 (DEOXYCHOLATE | Deoxycholic Acid | KYBELLA) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase DrugBank MCE KEGG MMDB PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | >1.50E+5 | n/a | n/a | n/a | n/a |
University of Wisconsin Curated by ChEMBL | Assay Description Agonist activity at VP16 tagged-VDR-LBD (unknown origin) expressed in HEK293T cells assessed as SRC1 coactivator peptide recruitment after 16 hrs by ... | Eur J Med Chem 109: 238-46 (2016) Article DOI: 10.1016/j.ejmech.2016.01.002 BindingDB Entry DOI: 10.7270/Q21N82ZW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vitamin D3 receptor (Homo sapiens (Human)) | BDBM50375583 (CHEMBL260315) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | >1.50E+5 | n/a | n/a | n/a | n/a |
University of Wisconsin Curated by ChEMBL | Assay Description Antagonist activity against VDR-LBD (unknown origin) expressed in Escherichia coli assessed as inhibition of VDR agonist LG190178-induced SRC2-3 coac... | Eur J Med Chem 109: 238-46 (2016) Article DOI: 10.1016/j.ejmech.2016.01.002 BindingDB Entry DOI: 10.7270/Q21N82ZW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||