

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50500691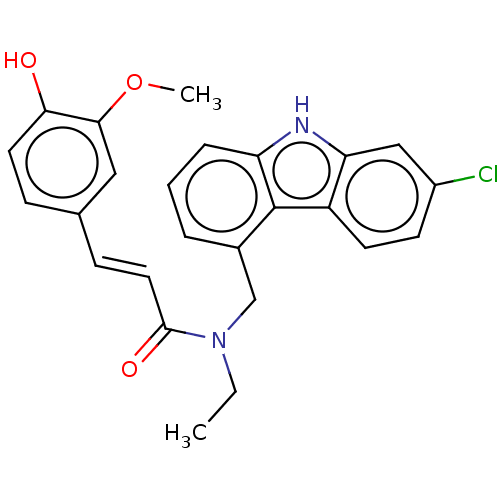 (CHEMBL3754448) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Southeast University Curated by ChEMBL | Assay Description Inhibition of electric eel AChE using ATC as substrate preincubated for 5 mins followed by substrate addition measured after 2 mins by Ellman's assay | Bioorg Med Chem 24: 886-93 (2016) Article DOI: 10.1016/j.bmc.2016.01.010 BindingDB Entry DOI: 10.7270/Q23R0WWJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50500686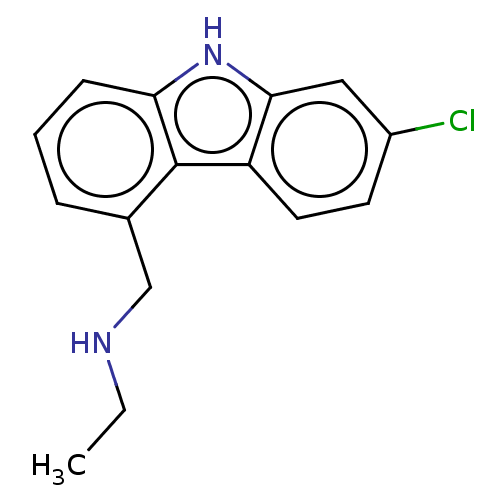 (CHEMBL3752232) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Southeast University Curated by ChEMBL | Assay Description Inhibition of equine BChE using BTC as substrate preincubated for 5 mins followed by substrate addition measured after 2 mins by Ellman's assay | Bioorg Med Chem 24: 886-93 (2016) Article DOI: 10.1016/j.bmc.2016.01.010 BindingDB Entry DOI: 10.7270/Q23R0WWJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50500686 (CHEMBL3752232) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Southeast University Curated by ChEMBL | Assay Description Inhibition of electric eel AChE using ATC as substrate preincubated for 5 mins followed by substrate addition measured after 2 mins by Ellman's assay | Bioorg Med Chem 24: 886-93 (2016) Article DOI: 10.1016/j.bmc.2016.01.010 BindingDB Entry DOI: 10.7270/Q23R0WWJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM50500691 (CHEMBL3754448) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Southeast University Curated by ChEMBL | Assay Description Inhibition of human BChE using BTC as substrate preincubated for 5 mins followed by substrate addition measured after 2 mins by Ellman's assay | Bioorg Med Chem 24: 886-93 (2016) Article DOI: 10.1016/j.bmc.2016.01.010 BindingDB Entry DOI: 10.7270/Q23R0WWJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50500691 (CHEMBL3754448) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Southeast University Curated by ChEMBL | Assay Description Inhibition of equine BChE using BTC as substrate preincubated for 5 mins followed by substrate addition measured after 2 mins by Ellman's assay | Bioorg Med Chem 24: 886-93 (2016) Article DOI: 10.1016/j.bmc.2016.01.010 BindingDB Entry DOI: 10.7270/Q23R0WWJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50500686 (CHEMBL3752232) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 5.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Southeast University Curated by ChEMBL | Assay Description Inhibition of human AChE using ATC as substrate preincubated for 5 mins followed by substrate addition measured after 2 mins by Ellman's assay | Bioorg Med Chem 24: 886-93 (2016) Article DOI: 10.1016/j.bmc.2016.01.010 BindingDB Entry DOI: 10.7270/Q23R0WWJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50500691 (CHEMBL3754448) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 6.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Southeast University Curated by ChEMBL | Assay Description Inhibition of human AChE using ATC as substrate preincubated for 5 mins followed by substrate addition measured after 2 mins by Ellman's assay | Bioorg Med Chem 24: 886-93 (2016) Article DOI: 10.1016/j.bmc.2016.01.010 BindingDB Entry DOI: 10.7270/Q23R0WWJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM50500686 (CHEMBL3752232) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 7.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Southeast University Curated by ChEMBL | Assay Description Inhibition of human BChE using BTC as substrate preincubated for 5 mins followed by substrate addition measured after 2 mins by Ellman's assay | Bioorg Med Chem 24: 886-93 (2016) Article DOI: 10.1016/j.bmc.2016.01.010 BindingDB Entry DOI: 10.7270/Q23R0WWJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM50500690 (CHEMBL3752974) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 8.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Southeast University Curated by ChEMBL | Assay Description Inhibition of human BChE using BTC as substrate preincubated for 5 mins followed by substrate addition measured after 2 mins by Ellman's assay | Bioorg Med Chem 24: 886-93 (2016) Article DOI: 10.1016/j.bmc.2016.01.010 BindingDB Entry DOI: 10.7270/Q23R0WWJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM10404 ((1S,12S,14R)-9-methoxy-4-methyl-11-oxa-4-azatetrac...) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 8.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Southeast University Curated by ChEMBL | Assay Description Inhibition of electric eel AChE using ATC as substrate preincubated for 5 mins followed by substrate addition measured after 2 mins by Ellman's assay | Bioorg Med Chem 24: 886-93 (2016) Article DOI: 10.1016/j.bmc.2016.01.010 BindingDB Entry DOI: 10.7270/Q23R0WWJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50500690 (CHEMBL3752974) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 8.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Southeast University Curated by ChEMBL | Assay Description Inhibition of equine BChE using BTC as substrate preincubated for 5 mins followed by substrate addition measured after 2 mins by Ellman's assay | Bioorg Med Chem 24: 886-93 (2016) Article DOI: 10.1016/j.bmc.2016.01.010 BindingDB Entry DOI: 10.7270/Q23R0WWJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM50500688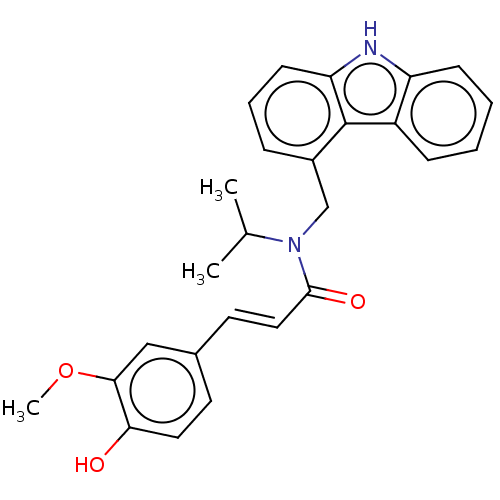 (CHEMBL3754631) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 9.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Southeast University Curated by ChEMBL | Assay Description Inhibition of human BChE using BTC as substrate preincubated for 5 mins followed by substrate addition measured after 2 mins by Ellman's assay | Bioorg Med Chem 24: 886-93 (2016) Article DOI: 10.1016/j.bmc.2016.01.010 BindingDB Entry DOI: 10.7270/Q23R0WWJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50500688 (CHEMBL3754631) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.11E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Southeast University Curated by ChEMBL | Assay Description Inhibition of electric eel AChE using ATC as substrate preincubated for 5 mins followed by substrate addition measured after 2 mins by Ellman's assay | Bioorg Med Chem 24: 886-93 (2016) Article DOI: 10.1016/j.bmc.2016.01.010 BindingDB Entry DOI: 10.7270/Q23R0WWJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50500688 (CHEMBL3754631) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.27E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Southeast University Curated by ChEMBL | Assay Description Inhibition of equine BChE using BTC as substrate preincubated for 5 mins followed by substrate addition measured after 2 mins by Ellman's assay | Bioorg Med Chem 24: 886-93 (2016) Article DOI: 10.1016/j.bmc.2016.01.010 BindingDB Entry DOI: 10.7270/Q23R0WWJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50500685 (CHEMBL3754419) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.38E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Southeast University Curated by ChEMBL | Assay Description Inhibition of equine BChE using BTC as substrate preincubated for 5 mins followed by substrate addition measured after 2 mins by Ellman's assay | Bioorg Med Chem 24: 886-93 (2016) Article DOI: 10.1016/j.bmc.2016.01.010 BindingDB Entry DOI: 10.7270/Q23R0WWJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50500695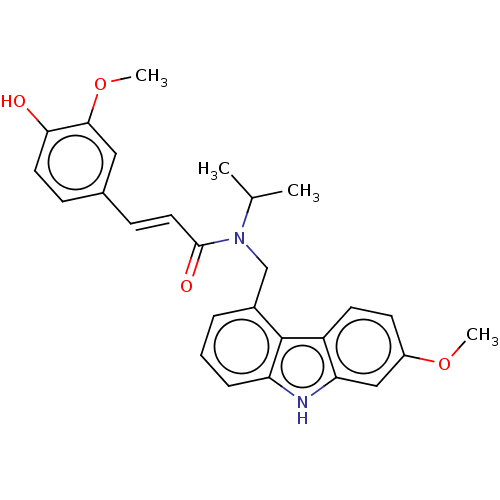 (CHEMBL3752518) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.39E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Southeast University Curated by ChEMBL | Assay Description Inhibition of equine BChE using BTC as substrate preincubated for 5 mins followed by substrate addition measured after 2 mins by Ellman's assay | Bioorg Med Chem 24: 886-93 (2016) Article DOI: 10.1016/j.bmc.2016.01.010 BindingDB Entry DOI: 10.7270/Q23R0WWJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50500685 (CHEMBL3754419) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.80E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Southeast University Curated by ChEMBL | Assay Description Inhibition of electric eel AChE using ATC as substrate preincubated for 5 mins followed by substrate addition measured after 2 mins by Ellman's assay | Bioorg Med Chem 24: 886-93 (2016) Article DOI: 10.1016/j.bmc.2016.01.010 BindingDB Entry DOI: 10.7270/Q23R0WWJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50500690 (CHEMBL3752974) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.80E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Southeast University Curated by ChEMBL | Assay Description Inhibition of human AChE using ATC as substrate preincubated for 5 mins followed by substrate addition measured after 2 mins by Ellman's assay | Bioorg Med Chem 24: 886-93 (2016) Article DOI: 10.1016/j.bmc.2016.01.010 BindingDB Entry DOI: 10.7270/Q23R0WWJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50500687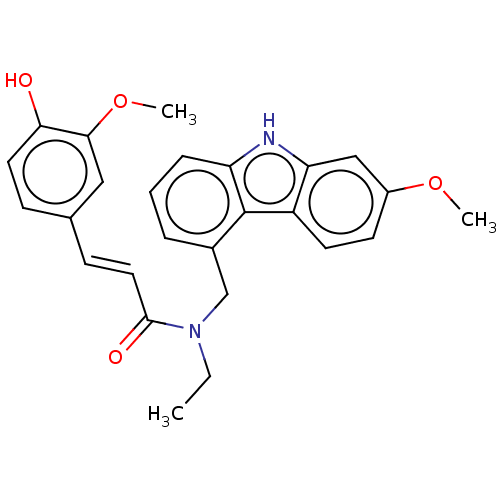 (CHEMBL3754289) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.98E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Southeast University Curated by ChEMBL | Assay Description Inhibition of equine BChE using BTC as substrate preincubated for 5 mins followed by substrate addition measured after 2 mins by Ellman's assay | Bioorg Med Chem 24: 886-93 (2016) Article DOI: 10.1016/j.bmc.2016.01.010 BindingDB Entry DOI: 10.7270/Q23R0WWJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50500697 (CHEMBL3752521) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.05E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Southeast University Curated by ChEMBL | Assay Description Inhibition of equine BChE using BTC as substrate preincubated for 5 mins followed by substrate addition measured after 2 mins by Ellman's assay | Bioorg Med Chem 24: 886-93 (2016) Article DOI: 10.1016/j.bmc.2016.01.010 BindingDB Entry DOI: 10.7270/Q23R0WWJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50500690 (CHEMBL3752974) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.16E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Southeast University Curated by ChEMBL | Assay Description Inhibition of electric eel AChE using ATC as substrate preincubated for 5 mins followed by substrate addition measured after 2 mins by Ellman's assay | Bioorg Med Chem 24: 886-93 (2016) Article DOI: 10.1016/j.bmc.2016.01.010 BindingDB Entry DOI: 10.7270/Q23R0WWJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50500692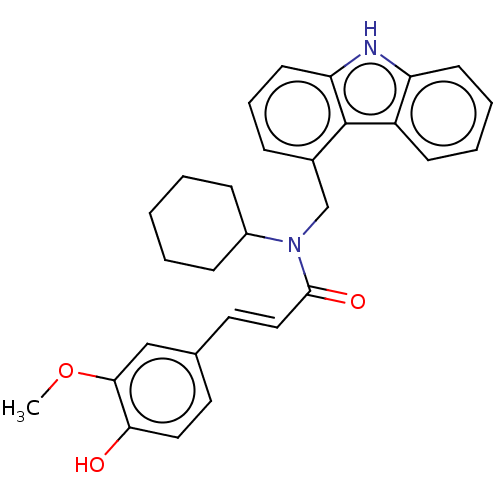 (CHEMBL3754652) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Southeast University Curated by ChEMBL | Assay Description Inhibition of equine BChE using BTC as substrate preincubated for 5 mins followed by substrate addition measured after 2 mins by Ellman's assay | Bioorg Med Chem 24: 886-93 (2016) Article DOI: 10.1016/j.bmc.2016.01.010 BindingDB Entry DOI: 10.7270/Q23R0WWJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50500688 (CHEMBL3754631) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Southeast University Curated by ChEMBL | Assay Description Inhibition of human AChE using ATC as substrate preincubated for 5 mins followed by substrate addition measured after 2 mins by Ellman's assay | Bioorg Med Chem 24: 886-93 (2016) Article DOI: 10.1016/j.bmc.2016.01.010 BindingDB Entry DOI: 10.7270/Q23R0WWJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50500693 (CHEMBL3753231) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.59E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Southeast University Curated by ChEMBL | Assay Description Inhibition of equine BChE using BTC as substrate preincubated for 5 mins followed by substrate addition measured after 2 mins by Ellman's assay | Bioorg Med Chem 24: 886-93 (2016) Article DOI: 10.1016/j.bmc.2016.01.010 BindingDB Entry DOI: 10.7270/Q23R0WWJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM10404 ((1S,12S,14R)-9-methoxy-4-methyl-11-oxa-4-azatetrac...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 2.81E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Southeast University Curated by ChEMBL | Assay Description Inhibition of equine BChE using BTC as substrate preincubated for 5 mins followed by substrate addition measured after 2 mins by Ellman's assay | Bioorg Med Chem 24: 886-93 (2016) Article DOI: 10.1016/j.bmc.2016.01.010 BindingDB Entry DOI: 10.7270/Q23R0WWJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50500689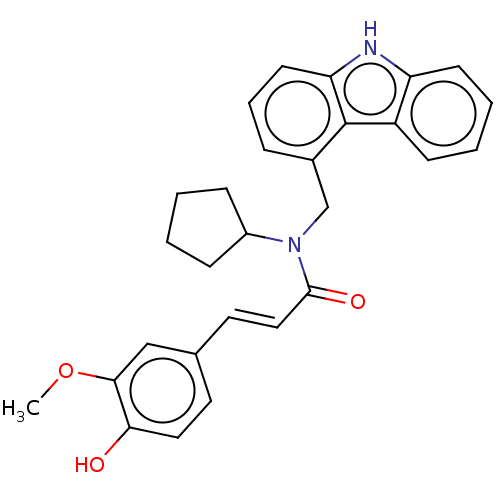 (CHEMBL3752517) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3.16E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Southeast University Curated by ChEMBL | Assay Description Inhibition of equine BChE using BTC as substrate preincubated for 5 mins followed by substrate addition measured after 2 mins by Ellman's assay | Bioorg Med Chem 24: 886-93 (2016) Article DOI: 10.1016/j.bmc.2016.01.010 BindingDB Entry DOI: 10.7270/Q23R0WWJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50500695 (CHEMBL3752518) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 5.82E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Southeast University Curated by ChEMBL | Assay Description Inhibition of electric eel AChE using ATC as substrate preincubated for 5 mins followed by substrate addition measured after 2 mins by Ellman's assay | Bioorg Med Chem 24: 886-93 (2016) Article DOI: 10.1016/j.bmc.2016.01.010 BindingDB Entry DOI: 10.7270/Q23R0WWJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50500687 (CHEMBL3754289) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 6.16E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Southeast University Curated by ChEMBL | Assay Description Inhibition of electric eel AChE using ATC as substrate preincubated for 5 mins followed by substrate addition measured after 2 mins by Ellman's assay | Bioorg Med Chem 24: 886-93 (2016) Article DOI: 10.1016/j.bmc.2016.01.010 BindingDB Entry DOI: 10.7270/Q23R0WWJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50500693 (CHEMBL3753231) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 8.82E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Southeast University Curated by ChEMBL | Assay Description Inhibition of electric eel AChE using ATC as substrate preincubated for 5 mins followed by substrate addition measured after 2 mins by Ellman's assay | Bioorg Med Chem 24: 886-93 (2016) Article DOI: 10.1016/j.bmc.2016.01.010 BindingDB Entry DOI: 10.7270/Q23R0WWJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50500689 (CHEMBL3752517) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Southeast University Curated by ChEMBL | Assay Description Inhibition of electric eel AChE using ATC as substrate preincubated for 5 mins followed by substrate addition measured after 2 mins by Ellman's assay | Bioorg Med Chem 24: 886-93 (2016) Article DOI: 10.1016/j.bmc.2016.01.010 BindingDB Entry DOI: 10.7270/Q23R0WWJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50500692 (CHEMBL3754652) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Southeast University Curated by ChEMBL | Assay Description Inhibition of electric eel AChE using ATC as substrate preincubated for 5 mins followed by substrate addition measured after 2 mins by Ellman's assay | Bioorg Med Chem 24: 886-93 (2016) Article DOI: 10.1016/j.bmc.2016.01.010 BindingDB Entry DOI: 10.7270/Q23R0WWJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50500697 (CHEMBL3752521) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Southeast University Curated by ChEMBL | Assay Description Inhibition of electric eel AChE using ATC as substrate preincubated for 5 mins followed by substrate addition measured after 2 mins by Ellman's assay | Bioorg Med Chem 24: 886-93 (2016) Article DOI: 10.1016/j.bmc.2016.01.010 BindingDB Entry DOI: 10.7270/Q23R0WWJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50500694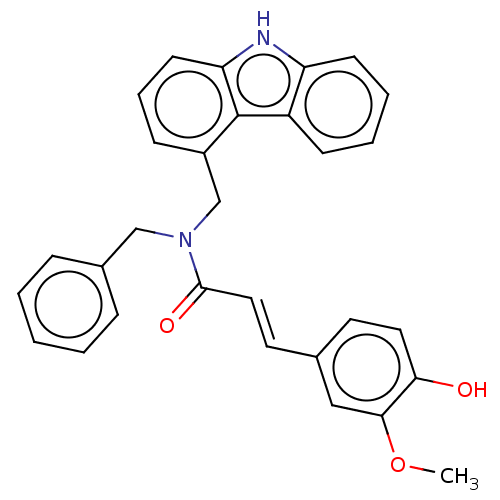 (CHEMBL3753827) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Southeast University Curated by ChEMBL | Assay Description Inhibition of electric eel AChE using ATC as substrate preincubated for 5 mins followed by substrate addition measured after 2 mins by Ellman's assay | Bioorg Med Chem 24: 886-93 (2016) Article DOI: 10.1016/j.bmc.2016.01.010 BindingDB Entry DOI: 10.7270/Q23R0WWJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50500696 (CHEMBL3752703) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Southeast University Curated by ChEMBL | Assay Description Inhibition of electric eel AChE using ATC as substrate preincubated for 5 mins followed by substrate addition measured after 2 mins by Ellman's assay | Bioorg Med Chem 24: 886-93 (2016) Article DOI: 10.1016/j.bmc.2016.01.010 BindingDB Entry DOI: 10.7270/Q23R0WWJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||