

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cholinesterase (Equus caballus (Horse)) | BDBM50500766 (CHEMBL3754409) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 270 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Physiologically Active Compounds Russian Academy of Sciences Curated by ChEMBL | Assay Description Competitive inhibition of equine serum BChE using butyrylthiocholine iodide as substrate assessed as steady state inhibition constant preincubated fo... | Bioorg Med Chem 24: 1050-62 (2016) Article DOI: 10.1016/j.bmc.2016.01.031 BindingDB Entry DOI: 10.7270/Q2B85C4Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Liver carboxylesterase (Sus scrofa) | BDBM50500766 (CHEMBL3754409) | PDB KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Physiologically Active Compounds Russian Academy of Sciences Curated by ChEMBL | Assay Description Noncompetitive inhibition of porcine liver carboxylesterase using 4-nitrophenol acetate as substrate assessed as steady state inhibition constant pre... | Bioorg Med Chem 24: 1050-62 (2016) Article DOI: 10.1016/j.bmc.2016.01.031 BindingDB Entry DOI: 10.7270/Q2B85C4Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Liver carboxylesterase (Sus scrofa) | BDBM50500752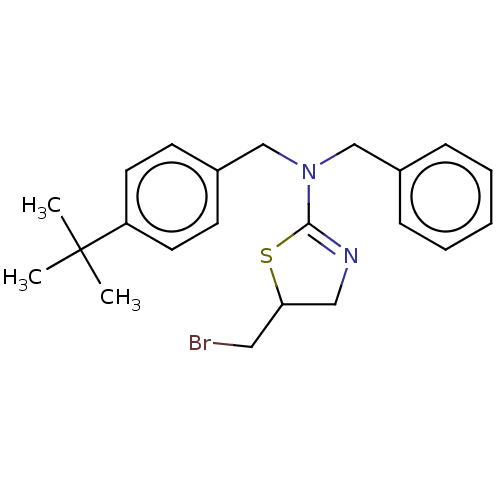 (CHEMBL3754327) | PDB KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 440 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Physiologically Active Compounds Russian Academy of Sciences Curated by ChEMBL | Assay Description Noncompetitive inhibition of porcine liver carboxylesterase using 4-nitrophenol acetate as substrate assessed as steady state inhibition constant pre... | Bioorg Med Chem 24: 1050-62 (2016) Article DOI: 10.1016/j.bmc.2016.01.031 BindingDB Entry DOI: 10.7270/Q2B85C4Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50500752 (CHEMBL3754327) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 620 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Physiologically Active Compounds Russian Academy of Sciences Curated by ChEMBL | Assay Description Competitive inhibition of equine serum BChE using butyrylthiocholine iodide as substrate assessed as steady state inhibition constant preincubated fo... | Bioorg Med Chem 24: 1050-62 (2016) Article DOI: 10.1016/j.bmc.2016.01.031 BindingDB Entry DOI: 10.7270/Q2B85C4Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50500760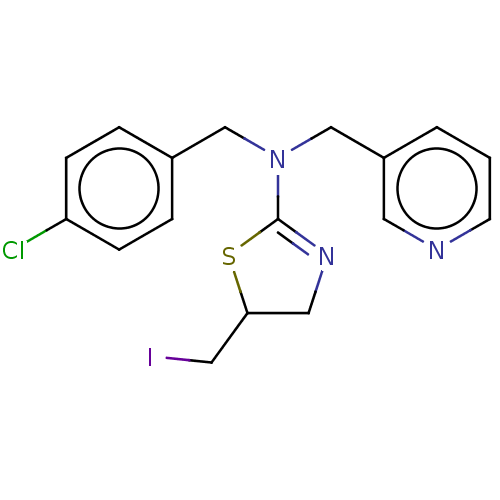 (CHEMBL3754622) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 670 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Physiologically Active Compounds Russian Academy of Sciences Curated by ChEMBL | Assay Description Competitive inhibition of equine serum BChE using butyrylthiocholine iodide as substrate assessed as steady state inhibition constant preincubated fo... | Bioorg Med Chem 24: 1050-62 (2016) Article DOI: 10.1016/j.bmc.2016.01.031 BindingDB Entry DOI: 10.7270/Q2B85C4Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50500766 (CHEMBL3754409) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Physiologically Active Compounds Russian Academy of Sciences Curated by ChEMBL | Assay Description Noncompetitive inhibition of equine serum BChE using butyrylthiocholine iodide as substrate assessed as steady state inhibition constant preincubated... | Bioorg Med Chem 24: 1050-62 (2016) Article DOI: 10.1016/j.bmc.2016.01.031 BindingDB Entry DOI: 10.7270/Q2B85C4Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50500755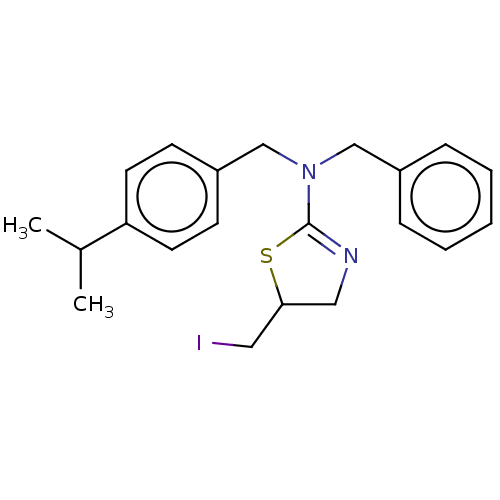 (CHEMBL3752466) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.34E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Physiologically Active Compounds Russian Academy of Sciences Curated by ChEMBL | Assay Description Competitive inhibition of equine serum BChE using butyrylthiocholine iodide as substrate assessed as steady state inhibition constant preincubated fo... | Bioorg Med Chem 24: 1050-62 (2016) Article DOI: 10.1016/j.bmc.2016.01.031 BindingDB Entry DOI: 10.7270/Q2B85C4Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50500756 (CHEMBL3752908) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.53E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Physiologically Active Compounds Russian Academy of Sciences Curated by ChEMBL | Assay Description Noncompetitive inhibition of equine serum BChE using butyrylthiocholine iodide as substrate assessed as steady state inhibition constant preincubated... | Bioorg Med Chem 24: 1050-62 (2016) Article DOI: 10.1016/j.bmc.2016.01.031 BindingDB Entry DOI: 10.7270/Q2B85C4Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Liver carboxylesterase (Sus scrofa) | BDBM50500755 (CHEMBL3752466) | PDB KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 2.09E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Physiologically Active Compounds Russian Academy of Sciences Curated by ChEMBL | Assay Description Noncompetitive inhibition of porcine liver carboxylesterase using 4-nitrophenol acetate as substrate assessed as steady state inhibition constant pre... | Bioorg Med Chem 24: 1050-62 (2016) Article DOI: 10.1016/j.bmc.2016.01.031 BindingDB Entry DOI: 10.7270/Q2B85C4Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Liver carboxylesterase (Sus scrofa) | BDBM50500757 (CHEMBL3752682) | PDB KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 3.54E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Physiologically Active Compounds Russian Academy of Sciences Curated by ChEMBL | Assay Description Noncompetitive inhibition of porcine liver carboxylesterase using 4-nitrophenol acetate as substrate assessed as steady state inhibition constant pre... | Bioorg Med Chem 24: 1050-62 (2016) Article DOI: 10.1016/j.bmc.2016.01.031 BindingDB Entry DOI: 10.7270/Q2B85C4Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50500760 (CHEMBL3754622) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 3.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Physiologically Active Compounds Russian Academy of Sciences Curated by ChEMBL | Assay Description Noncompetitive inhibition of equine serum BChE using butyrylthiocholine iodide as substrate assessed as steady state inhibition constant preincubated... | Bioorg Med Chem 24: 1050-62 (2016) Article DOI: 10.1016/j.bmc.2016.01.031 BindingDB Entry DOI: 10.7270/Q2B85C4Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Liver carboxylesterase (Sus scrofa) | BDBM50500758 (CHEMBL3753216) | PDB KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 7.37E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Physiologically Active Compounds Russian Academy of Sciences Curated by ChEMBL | Assay Description Noncompetitive inhibition of porcine liver carboxylesterase using 4-nitrophenol acetate as substrate assessed as steady state inhibition constant pre... | Bioorg Med Chem 24: 1050-62 (2016) Article DOI: 10.1016/j.bmc.2016.01.031 BindingDB Entry DOI: 10.7270/Q2B85C4Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50500755 (CHEMBL3752466) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 7.56E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Physiologically Active Compounds Russian Academy of Sciences Curated by ChEMBL | Assay Description Noncompetitive inhibition of equine serum BChE using butyrylthiocholine iodide as substrate assessed as steady state inhibition constant preincubated... | Bioorg Med Chem 24: 1050-62 (2016) Article DOI: 10.1016/j.bmc.2016.01.031 BindingDB Entry DOI: 10.7270/Q2B85C4Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Liver carboxylesterase (Sus scrofa) | BDBM50500748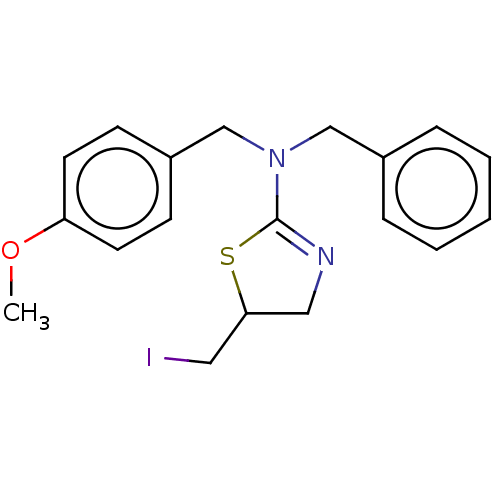 (CHEMBL3753156) | PDB KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | Article PubMed | 7.81E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Physiologically Active Compounds Russian Academy of Sciences Curated by ChEMBL | Assay Description Noncompetitive inhibition of porcine liver carboxylesterase using 4-nitrophenol acetate as substrate assessed as steady state inhibition constant pre... | Bioorg Med Chem 24: 1050-62 (2016) Article DOI: 10.1016/j.bmc.2016.01.031 BindingDB Entry DOI: 10.7270/Q2B85C4Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50500746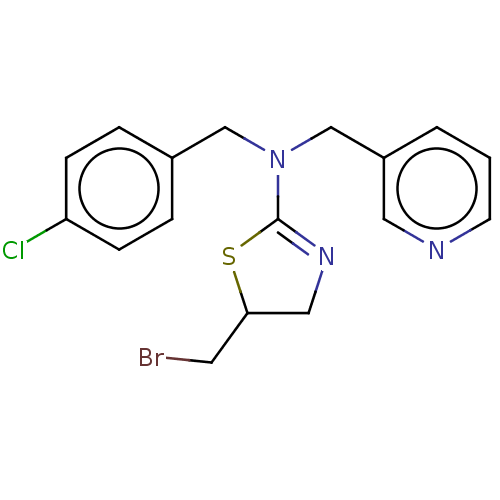 (CHEMBL3753531) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 9.51E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Physiologically Active Compounds Russian Academy of Sciences Curated by ChEMBL | Assay Description Competitive inhibition of equine serum BChE using butyrylthiocholine iodide as substrate assessed as steady state inhibition constant preincubated fo... | Bioorg Med Chem 24: 1050-62 (2016) Article DOI: 10.1016/j.bmc.2016.01.031 BindingDB Entry DOI: 10.7270/Q2B85C4Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50500746 (CHEMBL3753531) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.99E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Physiologically Active Compounds Russian Academy of Sciences Curated by ChEMBL | Assay Description Noncompetitive inhibition of equine serum BChE using butyrylthiocholine iodide as substrate assessed as steady state inhibition constant preincubated... | Bioorg Med Chem 24: 1050-62 (2016) Article DOI: 10.1016/j.bmc.2016.01.031 BindingDB Entry DOI: 10.7270/Q2B85C4Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM8960 ((+/-)-2-[(1-benzylpiperidin-4-yl)methyl]-5,6-dimet...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE PC cid PC sid PDB UniChem Patents Similars | DrugBank PDB Article PubMed | n/a | n/a | 22 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Physiologically Active Compounds Russian Academy of Sciences Curated by ChEMBL | Assay Description Inhibition of human erythrocyte AChE using acetylthiocholine iodide as substrate preincubated for 10 mins followed by substrate addition by Ellman's ... | Bioorg Med Chem 24: 1050-62 (2016) Article DOI: 10.1016/j.bmc.2016.01.031 BindingDB Entry DOI: 10.7270/Q2B85C4Q | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM8961 (1,2,3,4-tetrahydro-9-acridinamine | 1,2,3,4-tetrah...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 34 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Physiologically Active Compounds Russian Academy of Sciences Curated by ChEMBL | Assay Description Inhibition of human BChE using butyrylthiocholine iodide as substrate preincubated followed by substrate addition by Ellman's method | Bioorg Med Chem 24: 1050-62 (2016) Article DOI: 10.1016/j.bmc.2016.01.031 BindingDB Entry DOI: 10.7270/Q2B85C4Q | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM8961 (1,2,3,4-tetrahydro-9-acridinamine | 1,2,3,4-tetrah...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 79 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Physiologically Active Compounds Russian Academy of Sciences Curated by ChEMBL | Assay Description Inhibition of human erythrocyte AChE using acetylthiocholine iodide as substrate preincubated for 10 mins followed by substrate addition by Ellman's ... | Bioorg Med Chem 24: 1050-62 (2016) Article DOI: 10.1016/j.bmc.2016.01.031 BindingDB Entry DOI: 10.7270/Q2B85C4Q | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Liver carboxylesterase (Sus scrofa) | BDBM50500766 (CHEMBL3754409) | PDB KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 220 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Physiologically Active Compounds Russian Academy of Sciences Curated by ChEMBL | Assay Description Inhibition of porcine liver carboxylesterase using 4-nitrophenol acetate as substrate by spectrophotometric analysis | Bioorg Med Chem 24: 1050-62 (2016) Article DOI: 10.1016/j.bmc.2016.01.031 BindingDB Entry DOI: 10.7270/Q2B85C4Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Liver carboxylesterase (Sus scrofa) | BDBM50500752 (CHEMBL3754327) | PDB KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 320 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Physiologically Active Compounds Russian Academy of Sciences Curated by ChEMBL | Assay Description Inhibition of porcine liver carboxylesterase using 4-nitrophenol acetate as substrate by spectrophotometric analysis | Bioorg Med Chem 24: 1050-62 (2016) Article DOI: 10.1016/j.bmc.2016.01.031 BindingDB Entry DOI: 10.7270/Q2B85C4Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Liver carboxylesterase 1 (Homo sapiens (Human)) | BDBM22722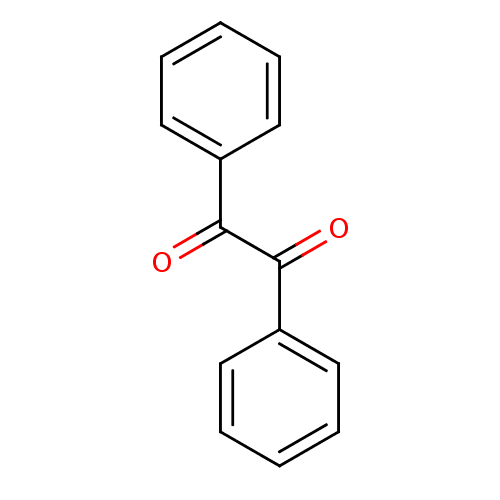 (1,2-diphenylethane-1,2-dione | Benzil | CHEMBL1898...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 370 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Physiologically Active Compounds Russian Academy of Sciences Curated by ChEMBL | Assay Description Inhibition of human carboxylesterase 1 using o-nitrophenylacetate as substrate by spectrophotometry analysis | Bioorg Med Chem 24: 1050-62 (2016) Article DOI: 10.1016/j.bmc.2016.01.031 BindingDB Entry DOI: 10.7270/Q2B85C4Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50500766 (CHEMBL3754409) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 770 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Physiologically Active Compounds Russian Academy of Sciences Curated by ChEMBL | Assay Description Inhibition of equine serum BChE using butyrylthiocholine iodide as substrate preincubated for 10 mins followed by substrate addition by Ellman's meth... | Bioorg Med Chem 24: 1050-62 (2016) Article DOI: 10.1016/j.bmc.2016.01.031 BindingDB Entry DOI: 10.7270/Q2B85C4Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Liver carboxylesterase (Sus scrofa) | BDBM50500755 (CHEMBL3752466) | PDB KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.13E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Physiologically Active Compounds Russian Academy of Sciences Curated by ChEMBL | Assay Description Inhibition of porcine liver carboxylesterase using 4-nitrophenol acetate as substrate by spectrophotometric analysis | Bioorg Med Chem 24: 1050-62 (2016) Article DOI: 10.1016/j.bmc.2016.01.031 BindingDB Entry DOI: 10.7270/Q2B85C4Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50500752 (CHEMBL3754327) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.34E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Physiologically Active Compounds Russian Academy of Sciences Curated by ChEMBL | Assay Description Inhibition of equine serum BChE using butyrylthiocholine iodide as substrate preincubated for 10 mins followed by substrate addition by Ellman's meth... | Bioorg Med Chem 24: 1050-62 (2016) Article DOI: 10.1016/j.bmc.2016.01.031 BindingDB Entry DOI: 10.7270/Q2B85C4Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50500760 (CHEMBL3754622) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.47E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Physiologically Active Compounds Russian Academy of Sciences Curated by ChEMBL | Assay Description Inhibition of equine serum BChE using butyrylthiocholine iodide as substrate preincubated for 10 mins followed by substrate addition by Ellman's meth... | Bioorg Med Chem 24: 1050-62 (2016) Article DOI: 10.1016/j.bmc.2016.01.031 BindingDB Entry DOI: 10.7270/Q2B85C4Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Liver carboxylesterase (Sus scrofa) | BDBM50500757 (CHEMBL3752682) | PDB KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.55E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Physiologically Active Compounds Russian Academy of Sciences Curated by ChEMBL | Assay Description Inhibition of porcine liver carboxylesterase using 4-nitrophenol acetate as substrate by spectrophotometric analysis | Bioorg Med Chem 24: 1050-62 (2016) Article DOI: 10.1016/j.bmc.2016.01.031 BindingDB Entry DOI: 10.7270/Q2B85C4Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50500756 (CHEMBL3752908) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.74E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Physiologically Active Compounds Russian Academy of Sciences Curated by ChEMBL | Assay Description Inhibition of equine serum BChE using butyrylthiocholine iodide as substrate preincubated for 10 mins followed by substrate addition by Ellman's meth... | Bioorg Med Chem 24: 1050-62 (2016) Article DOI: 10.1016/j.bmc.2016.01.031 BindingDB Entry DOI: 10.7270/Q2B85C4Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50500755 (CHEMBL3752466) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 4.14E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Physiologically Active Compounds Russian Academy of Sciences Curated by ChEMBL | Assay Description Inhibition of equine serum BChE using butyrylthiocholine iodide as substrate preincubated for 10 mins followed by substrate addition by Ellman's meth... | Bioorg Med Chem 24: 1050-62 (2016) Article DOI: 10.1016/j.bmc.2016.01.031 BindingDB Entry DOI: 10.7270/Q2B85C4Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Liver carboxylesterase (Sus scrofa) | BDBM50500748 (CHEMBL3753156) | PDB KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | Article PubMed | n/a | n/a | 4.66E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Physiologically Active Compounds Russian Academy of Sciences Curated by ChEMBL | Assay Description Inhibition of porcine liver carboxylesterase using 4-nitrophenol acetate as substrate by spectrophotometric analysis | Bioorg Med Chem 24: 1050-62 (2016) Article DOI: 10.1016/j.bmc.2016.01.031 BindingDB Entry DOI: 10.7270/Q2B85C4Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Liver carboxylesterase (Sus scrofa) | BDBM50500758 (CHEMBL3753216) | PDB KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 4.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Physiologically Active Compounds Russian Academy of Sciences Curated by ChEMBL | Assay Description Inhibition of porcine liver carboxylesterase using 4-nitrophenol acetate as substrate by spectrophotometric analysis | Bioorg Med Chem 24: 1050-62 (2016) Article DOI: 10.1016/j.bmc.2016.01.031 BindingDB Entry DOI: 10.7270/Q2B85C4Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM8960 ((+/-)-2-[(1-benzylpiperidin-4-yl)methyl]-5,6-dimet...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 7.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Physiologically Active Compounds Russian Academy of Sciences Curated by ChEMBL | Assay Description Inhibition of human BChE using butyrylthiocholine iodide as substrate preincubated followed by substrate addition by Ellman's method | Bioorg Med Chem 24: 1050-62 (2016) Article DOI: 10.1016/j.bmc.2016.01.031 BindingDB Entry DOI: 10.7270/Q2B85C4Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50500757 (CHEMBL3752682) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.10E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Physiologically Active Compounds Russian Academy of Sciences Curated by ChEMBL | Assay Description Inhibition of equine serum BChE using butyrylthiocholine iodide as substrate preincubated for 10 mins followed by substrate addition by Ellman's meth... | Bioorg Med Chem 24: 1050-62 (2016) Article DOI: 10.1016/j.bmc.2016.01.031 BindingDB Entry DOI: 10.7270/Q2B85C4Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50500770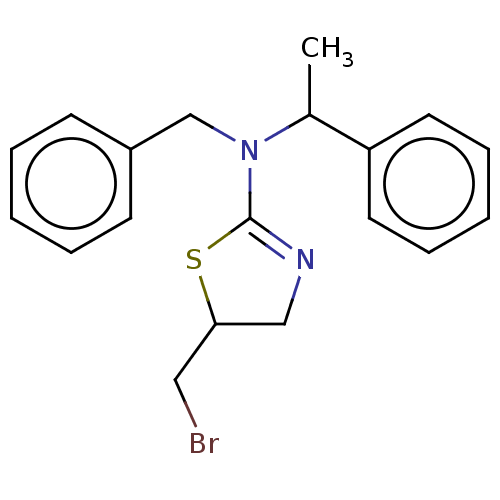 (CHEMBL3752043) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.40E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Physiologically Active Compounds Russian Academy of Sciences Curated by ChEMBL | Assay Description Inhibition of equine serum BChE using butyrylthiocholine iodide as substrate preincubated for 10 mins followed by substrate addition by Ellman's meth... | Bioorg Med Chem 24: 1050-62 (2016) Article DOI: 10.1016/j.bmc.2016.01.031 BindingDB Entry DOI: 10.7270/Q2B85C4Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Liver carboxylesterase (Sus scrofa) | BDBM50500749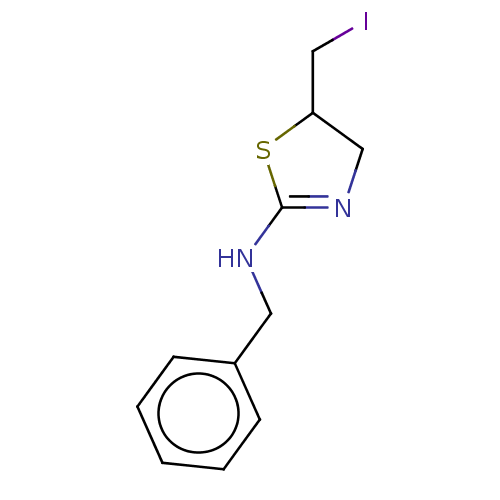 (CHEMBL3754187) | PDB KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.70E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Physiologically Active Compounds Russian Academy of Sciences Curated by ChEMBL | Assay Description Inhibition of porcine liver carboxylesterase using 4-nitrophenol acetate as substrate by spectrophotometric analysis | Bioorg Med Chem 24: 1050-62 (2016) Article DOI: 10.1016/j.bmc.2016.01.031 BindingDB Entry DOI: 10.7270/Q2B85C4Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50500751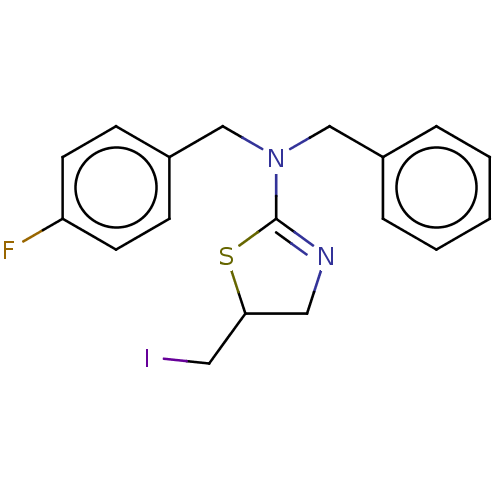 (CHEMBL3754088) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.70E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Physiologically Active Compounds Russian Academy of Sciences Curated by ChEMBL | Assay Description Inhibition of equine serum BChE using butyrylthiocholine iodide as substrate preincubated for 10 mins followed by substrate addition by Ellman's meth... | Bioorg Med Chem 24: 1050-62 (2016) Article DOI: 10.1016/j.bmc.2016.01.031 BindingDB Entry DOI: 10.7270/Q2B85C4Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Liver carboxylesterase (Sus scrofa) | BDBM50500760 (CHEMBL3754622) | PDB KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.80E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Physiologically Active Compounds Russian Academy of Sciences Curated by ChEMBL | Assay Description Inhibition of porcine liver carboxylesterase using 4-nitrophenol acetate as substrate by spectrophotometric analysis | Bioorg Med Chem 24: 1050-62 (2016) Article DOI: 10.1016/j.bmc.2016.01.031 BindingDB Entry DOI: 10.7270/Q2B85C4Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Liver carboxylesterase (Sus scrofa) | BDBM50500759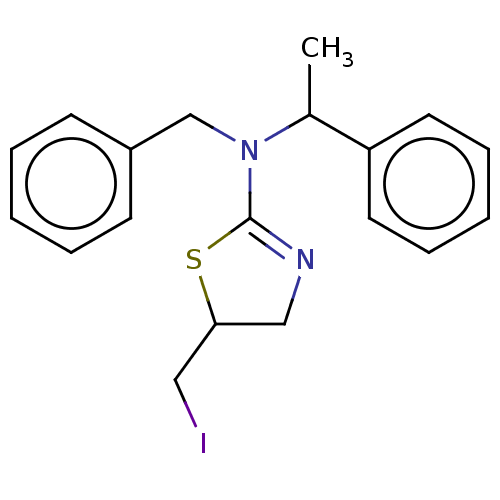 (CHEMBL3754616) | PDB KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.90E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Physiologically Active Compounds Russian Academy of Sciences Curated by ChEMBL | Assay Description Inhibition of porcine liver carboxylesterase using 4-nitrophenol acetate as substrate by spectrophotometric analysis | Bioorg Med Chem 24: 1050-62 (2016) Article DOI: 10.1016/j.bmc.2016.01.031 BindingDB Entry DOI: 10.7270/Q2B85C4Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50500762 (CHEMBL3752710) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Physiologically Active Compounds Russian Academy of Sciences Curated by ChEMBL | Assay Description Inhibition of equine serum BChE using butyrylthiocholine iodide as substrate preincubated for 10 mins followed by substrate addition by Ellman's meth... | Bioorg Med Chem 24: 1050-62 (2016) Article DOI: 10.1016/j.bmc.2016.01.031 BindingDB Entry DOI: 10.7270/Q2B85C4Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50500767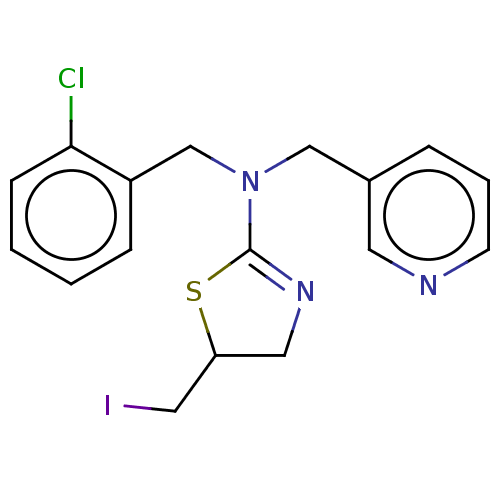 (CHEMBL3752953) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.10E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Physiologically Active Compounds Russian Academy of Sciences Curated by ChEMBL | Assay Description Inhibition of equine serum BChE using butyrylthiocholine iodide as substrate preincubated for 10 mins followed by substrate addition by Ellman's meth... | Bioorg Med Chem 24: 1050-62 (2016) Article DOI: 10.1016/j.bmc.2016.01.031 BindingDB Entry DOI: 10.7270/Q2B85C4Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50500772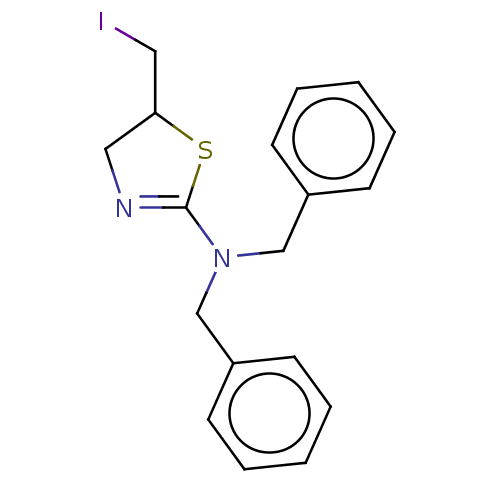 (CHEMBL3751974) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Physiologically Active Compounds Russian Academy of Sciences Curated by ChEMBL | Assay Description Inhibition of equine serum BChE using butyrylthiocholine iodide as substrate preincubated for 10 mins followed by substrate addition by Ellman's meth... | Bioorg Med Chem 24: 1050-62 (2016) Article DOI: 10.1016/j.bmc.2016.01.031 BindingDB Entry DOI: 10.7270/Q2B85C4Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50500750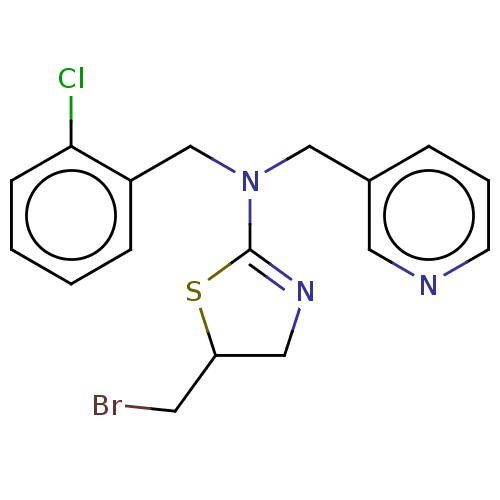 (CHEMBL3752399) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Physiologically Active Compounds Russian Academy of Sciences Curated by ChEMBL | Assay Description Inhibition of equine serum BChE using butyrylthiocholine iodide as substrate preincubated for 10 mins followed by substrate addition by Ellman's meth... | Bioorg Med Chem 24: 1050-62 (2016) Article DOI: 10.1016/j.bmc.2016.01.031 BindingDB Entry DOI: 10.7270/Q2B85C4Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50500748 (CHEMBL3753156) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Physiologically Active Compounds Russian Academy of Sciences Curated by ChEMBL | Assay Description Inhibition of equine serum BChE using butyrylthiocholine iodide as substrate preincubated for 10 mins followed by substrate addition by Ellman's meth... | Bioorg Med Chem 24: 1050-62 (2016) Article DOI: 10.1016/j.bmc.2016.01.031 BindingDB Entry DOI: 10.7270/Q2B85C4Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50500753 (CHEMBL3752331) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.60E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Physiologically Active Compounds Russian Academy of Sciences Curated by ChEMBL | Assay Description Inhibition of equine serum BChE using butyrylthiocholine iodide as substrate preincubated for 10 mins followed by substrate addition by Ellman's meth... | Bioorg Med Chem 24: 1050-62 (2016) Article DOI: 10.1016/j.bmc.2016.01.031 BindingDB Entry DOI: 10.7270/Q2B85C4Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50500754 (CHEMBL3753110) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.90E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Physiologically Active Compounds Russian Academy of Sciences Curated by ChEMBL | Assay Description Inhibition of equine serum BChE using butyrylthiocholine iodide as substrate preincubated for 10 mins followed by substrate addition by Ellman's meth... | Bioorg Med Chem 24: 1050-62 (2016) Article DOI: 10.1016/j.bmc.2016.01.031 BindingDB Entry DOI: 10.7270/Q2B85C4Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50500746 (CHEMBL3753531) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Physiologically Active Compounds Russian Academy of Sciences Curated by ChEMBL | Assay Description Inhibition of equine serum BChE using butyrylthiocholine iodide as substrate preincubated for 10 mins followed by substrate addition by Ellman's meth... | Bioorg Med Chem 24: 1050-62 (2016) Article DOI: 10.1016/j.bmc.2016.01.031 BindingDB Entry DOI: 10.7270/Q2B85C4Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Liver carboxylesterase (Sus scrofa) | BDBM50500753 (CHEMBL3752331) | PDB KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Physiologically Active Compounds Russian Academy of Sciences Curated by ChEMBL | Assay Description Inhibition of porcine liver carboxylesterase using 4-nitrophenol acetate as substrate by spectrophotometric analysis | Bioorg Med Chem 24: 1050-62 (2016) Article DOI: 10.1016/j.bmc.2016.01.031 BindingDB Entry DOI: 10.7270/Q2B85C4Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50500758 (CHEMBL3753216) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3.40E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Physiologically Active Compounds Russian Academy of Sciences Curated by ChEMBL | Assay Description Inhibition of equine serum BChE using butyrylthiocholine iodide as substrate preincubated for 10 mins followed by substrate addition by Ellman's meth... | Bioorg Med Chem 24: 1050-62 (2016) Article DOI: 10.1016/j.bmc.2016.01.031 BindingDB Entry DOI: 10.7270/Q2B85C4Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50500769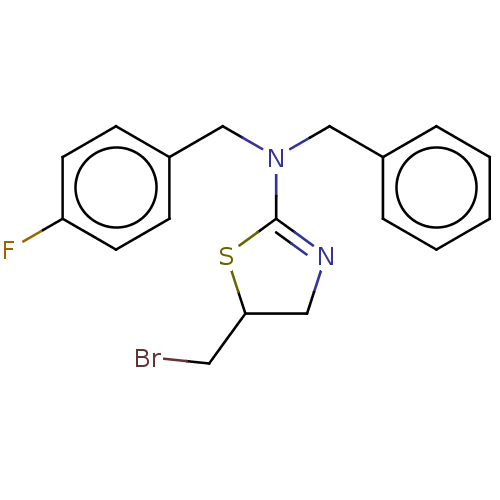 (CHEMBL3754339) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3.80E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Physiologically Active Compounds Russian Academy of Sciences Curated by ChEMBL | Assay Description Inhibition of equine serum BChE using butyrylthiocholine iodide as substrate preincubated for 10 mins followed by substrate addition by Ellman's meth... | Bioorg Med Chem 24: 1050-62 (2016) Article DOI: 10.1016/j.bmc.2016.01.031 BindingDB Entry DOI: 10.7270/Q2B85C4Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Liver carboxylesterase (Sus scrofa) | BDBM50500769 (CHEMBL3754339) | PDB KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 4.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Physiologically Active Compounds Russian Academy of Sciences Curated by ChEMBL | Assay Description Inhibition of porcine liver carboxylesterase using 4-nitrophenol acetate as substrate by spectrophotometric analysis | Bioorg Med Chem 24: 1050-62 (2016) Article DOI: 10.1016/j.bmc.2016.01.031 BindingDB Entry DOI: 10.7270/Q2B85C4Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50500763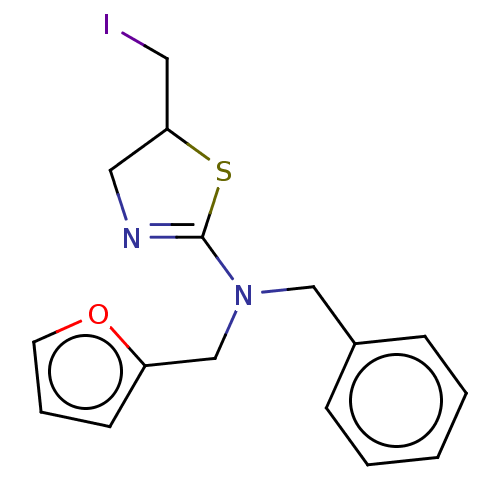 (CHEMBL3753018) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | Article PubMed | n/a | n/a | 4.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Physiologically Active Compounds Russian Academy of Sciences Curated by ChEMBL | Assay Description Inhibition of equine serum BChE using butyrylthiocholine iodide as substrate preincubated for 10 mins followed by substrate addition by Ellman's meth... | Bioorg Med Chem 24: 1050-62 (2016) Article DOI: 10.1016/j.bmc.2016.01.031 BindingDB Entry DOI: 10.7270/Q2B85C4Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50500761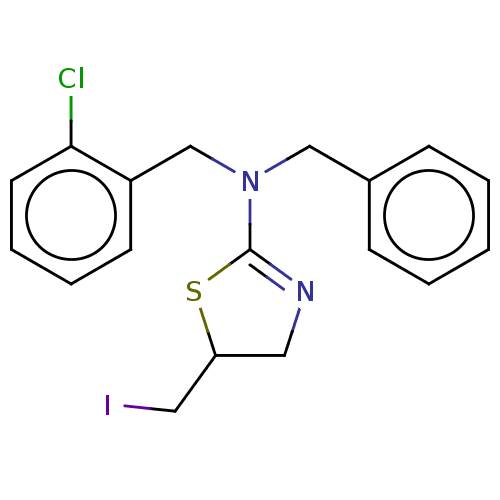 (CHEMBL3752873) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 4.40E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Physiologically Active Compounds Russian Academy of Sciences Curated by ChEMBL | Assay Description Inhibition of equine serum BChE using butyrylthiocholine iodide as substrate preincubated for 10 mins followed by substrate addition by Ellman's meth... | Bioorg Med Chem 24: 1050-62 (2016) Article DOI: 10.1016/j.bmc.2016.01.031 BindingDB Entry DOI: 10.7270/Q2B85C4Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Liver carboxylesterase (Sus scrofa) | BDBM50500772 (CHEMBL3751974) | PDB KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | Article PubMed | n/a | n/a | 4.60E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Physiologically Active Compounds Russian Academy of Sciences Curated by ChEMBL | Assay Description Inhibition of porcine liver carboxylesterase using 4-nitrophenol acetate as substrate by spectrophotometric analysis | Bioorg Med Chem 24: 1050-62 (2016) Article DOI: 10.1016/j.bmc.2016.01.031 BindingDB Entry DOI: 10.7270/Q2B85C4Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50500765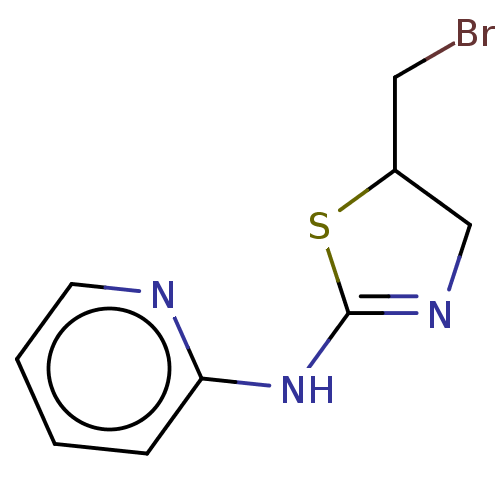 (CHEMBL3754585) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | Article PubMed | n/a | n/a | 6.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Physiologically Active Compounds Russian Academy of Sciences Curated by ChEMBL | Assay Description Inhibition of equine serum BChE using butyrylthiocholine iodide as substrate preincubated for 10 mins followed by substrate addition by Ellman's meth... | Bioorg Med Chem 24: 1050-62 (2016) Article DOI: 10.1016/j.bmc.2016.01.031 BindingDB Entry DOI: 10.7270/Q2B85C4Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50500756 (CHEMBL3752908) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 7.10E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Physiologically Active Compounds Russian Academy of Sciences Curated by ChEMBL | Assay Description Inhibition of human erythrocyte AChE using acetylthiocholine iodide as substrate preincubated for 10 mins followed by substrate addition by Ellman's ... | Bioorg Med Chem 24: 1050-62 (2016) Article DOI: 10.1016/j.bmc.2016.01.031 BindingDB Entry DOI: 10.7270/Q2B85C4Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Liver carboxylesterase (Sus scrofa) | BDBM50500751 (CHEMBL3754088) | PDB KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | Article PubMed | n/a | n/a | 8.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Physiologically Active Compounds Russian Academy of Sciences Curated by ChEMBL | Assay Description Inhibition of porcine liver carboxylesterase using 4-nitrophenol acetate as substrate by spectrophotometric analysis | Bioorg Med Chem 24: 1050-62 (2016) Article DOI: 10.1016/j.bmc.2016.01.031 BindingDB Entry DOI: 10.7270/Q2B85C4Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Liver carboxylesterase (Sus scrofa) | BDBM50500765 (CHEMBL3754585) | PDB KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | Article PubMed | n/a | n/a | 9.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Physiologically Active Compounds Russian Academy of Sciences Curated by ChEMBL | Assay Description Inhibition of porcine liver carboxylesterase using 4-nitrophenol acetate as substrate by spectrophotometric analysis | Bioorg Med Chem 24: 1050-62 (2016) Article DOI: 10.1016/j.bmc.2016.01.031 BindingDB Entry DOI: 10.7270/Q2B85C4Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50500747 (CHEMBL3753661) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | Article PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Physiologically Active Compounds Russian Academy of Sciences Curated by ChEMBL | Assay Description Inhibition of equine serum BChE using butyrylthiocholine iodide as substrate preincubated for 10 mins followed by substrate addition by Ellman's meth... | Bioorg Med Chem 24: 1050-62 (2016) Article DOI: 10.1016/j.bmc.2016.01.031 BindingDB Entry DOI: 10.7270/Q2B85C4Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Liver carboxylesterase (Sus scrofa) | BDBM50500764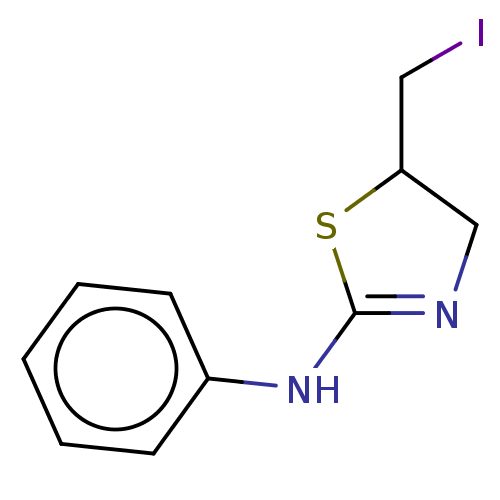 (CHEMBL3754511) | PDB KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Physiologically Active Compounds Russian Academy of Sciences Curated by ChEMBL | Assay Description Inhibition of porcine liver carboxylesterase using 4-nitrophenol acetate as substrate by spectrophotometric analysis | Bioorg Med Chem 24: 1050-62 (2016) Article DOI: 10.1016/j.bmc.2016.01.031 BindingDB Entry DOI: 10.7270/Q2B85C4Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Liver carboxylesterase (Sus scrofa) | BDBM50500767 (CHEMBL3752953) | PDB KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Physiologically Active Compounds Russian Academy of Sciences Curated by ChEMBL | Assay Description Inhibition of porcine liver carboxylesterase using 4-nitrophenol acetate as substrate by spectrophotometric analysis | Bioorg Med Chem 24: 1050-62 (2016) Article DOI: 10.1016/j.bmc.2016.01.031 BindingDB Entry DOI: 10.7270/Q2B85C4Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Liver carboxylesterase (Sus scrofa) | BDBM50500762 (CHEMBL3752710) | PDB KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Physiologically Active Compounds Russian Academy of Sciences Curated by ChEMBL | Assay Description Inhibition of porcine liver carboxylesterase using 4-nitrophenol acetate as substrate by spectrophotometric analysis | Bioorg Med Chem 24: 1050-62 (2016) Article DOI: 10.1016/j.bmc.2016.01.031 BindingDB Entry DOI: 10.7270/Q2B85C4Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Liver carboxylesterase (Sus scrofa) | BDBM50500747 (CHEMBL3753661) | PDB KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | Article PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Physiologically Active Compounds Russian Academy of Sciences Curated by ChEMBL | Assay Description Inhibition of porcine liver carboxylesterase using 4-nitrophenol acetate as substrate by spectrophotometric analysis | Bioorg Med Chem 24: 1050-62 (2016) Article DOI: 10.1016/j.bmc.2016.01.031 BindingDB Entry DOI: 10.7270/Q2B85C4Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Liver carboxylesterase (Sus scrofa) | BDBM50500770 (CHEMBL3752043) | PDB KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Physiologically Active Compounds Russian Academy of Sciences Curated by ChEMBL | Assay Description Inhibition of porcine liver carboxylesterase using 4-nitrophenol acetate as substrate by spectrophotometric analysis | Bioorg Med Chem 24: 1050-62 (2016) Article DOI: 10.1016/j.bmc.2016.01.031 BindingDB Entry DOI: 10.7270/Q2B85C4Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Liver carboxylesterase (Sus scrofa) | BDBM50500768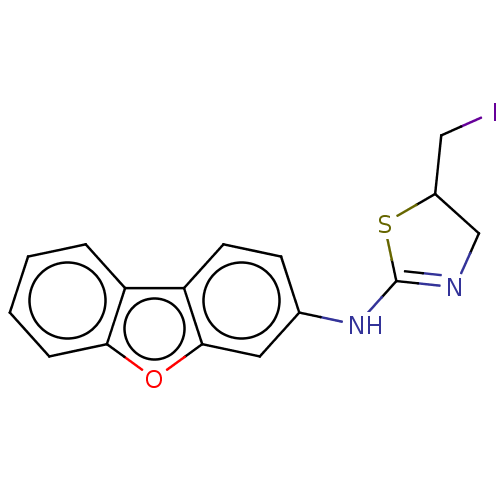 (CHEMBL3751906) | PDB KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | Article PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Physiologically Active Compounds Russian Academy of Sciences Curated by ChEMBL | Assay Description Inhibition of porcine liver carboxylesterase using 4-nitrophenol acetate as substrate by spectrophotometric analysis | Bioorg Med Chem 24: 1050-62 (2016) Article DOI: 10.1016/j.bmc.2016.01.031 BindingDB Entry DOI: 10.7270/Q2B85C4Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50500768 (CHEMBL3751906) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | Article PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Physiologically Active Compounds Russian Academy of Sciences Curated by ChEMBL | Assay Description Inhibition of equine serum BChE using butyrylthiocholine iodide as substrate preincubated for 10 mins followed by substrate addition by Ellman's meth... | Bioorg Med Chem 24: 1050-62 (2016) Article DOI: 10.1016/j.bmc.2016.01.031 BindingDB Entry DOI: 10.7270/Q2B85C4Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM22722 (1,2-diphenylethane-1,2-dione | Benzil | CHEMBL1898...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Physiologically Active Compounds Russian Academy of Sciences Curated by ChEMBL | Assay Description Inhibition of human BChE using butyrylthiocholine iodide as substrate preincubated followed by substrate addition by Ellman's method | Bioorg Med Chem 24: 1050-62 (2016) Article DOI: 10.1016/j.bmc.2016.01.031 BindingDB Entry DOI: 10.7270/Q2B85C4Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Liver carboxylesterase 1 (Homo sapiens (Human)) | BDBM8961 (1,2,3,4-tetrahydro-9-acridinamine | 1,2,3,4-tetrah...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Physiologically Active Compounds Russian Academy of Sciences Curated by ChEMBL | Assay Description Inhibition of human carboxylesterase 1 using o-nitrophenylacetate as substrate by spectrophotometry analysis | Bioorg Med Chem 24: 1050-62 (2016) Article DOI: 10.1016/j.bmc.2016.01.031 BindingDB Entry DOI: 10.7270/Q2B85C4Q | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Liver carboxylesterase (Sus scrofa) | BDBM50500746 (CHEMBL3753531) | PDB KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Physiologically Active Compounds Russian Academy of Sciences Curated by ChEMBL | Assay Description Inhibition of porcine liver carboxylesterase using 4-nitrophenol acetate as substrate by spectrophotometric analysis | Bioorg Med Chem 24: 1050-62 (2016) Article DOI: 10.1016/j.bmc.2016.01.031 BindingDB Entry DOI: 10.7270/Q2B85C4Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Liver carboxylesterase (Sus scrofa) | BDBM50500771 (CHEMBL3753197) | PDB KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | Article PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Physiologically Active Compounds Russian Academy of Sciences Curated by ChEMBL | Assay Description Inhibition of porcine liver carboxylesterase using 4-nitrophenol acetate as substrate by spectrophotometric analysis | Bioorg Med Chem 24: 1050-62 (2016) Article DOI: 10.1016/j.bmc.2016.01.031 BindingDB Entry DOI: 10.7270/Q2B85C4Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50500749 (CHEMBL3754187) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Physiologically Active Compounds Russian Academy of Sciences Curated by ChEMBL | Assay Description Inhibition of equine serum BChE using butyrylthiocholine iodide as substrate preincubated for 10 mins followed by substrate addition by Ellman's meth... | Bioorg Med Chem 24: 1050-62 (2016) Article DOI: 10.1016/j.bmc.2016.01.031 BindingDB Entry DOI: 10.7270/Q2B85C4Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Liver carboxylesterase (Sus scrofa) | BDBM50500750 (CHEMBL3752399) | PDB KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Physiologically Active Compounds Russian Academy of Sciences Curated by ChEMBL | Assay Description Inhibition of porcine liver carboxylesterase using 4-nitrophenol acetate as substrate by spectrophotometric analysis | Bioorg Med Chem 24: 1050-62 (2016) Article DOI: 10.1016/j.bmc.2016.01.031 BindingDB Entry DOI: 10.7270/Q2B85C4Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50500759 (CHEMBL3754616) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Physiologically Active Compounds Russian Academy of Sciences Curated by ChEMBL | Assay Description Inhibition of equine serum BChE using butyrylthiocholine iodide as substrate preincubated for 10 mins followed by substrate addition by Ellman's meth... | Bioorg Med Chem 24: 1050-62 (2016) Article DOI: 10.1016/j.bmc.2016.01.031 BindingDB Entry DOI: 10.7270/Q2B85C4Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50500760 (CHEMBL3754622) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Physiologically Active Compounds Russian Academy of Sciences Curated by ChEMBL | Assay Description Inhibition of human erythrocyte AChE using acetylthiocholine iodide as substrate preincubated for 10 mins followed by substrate addition by Ellman's ... | Bioorg Med Chem 24: 1050-62 (2016) Article DOI: 10.1016/j.bmc.2016.01.031 BindingDB Entry DOI: 10.7270/Q2B85C4Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50500762 (CHEMBL3752710) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Physiologically Active Compounds Russian Academy of Sciences Curated by ChEMBL | Assay Description Inhibition of human erythrocyte AChE using acetylthiocholine iodide as substrate preincubated for 10 mins followed by substrate addition by Ellman's ... | Bioorg Med Chem 24: 1050-62 (2016) Article DOI: 10.1016/j.bmc.2016.01.031 BindingDB Entry DOI: 10.7270/Q2B85C4Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Liver carboxylesterase 1 (Homo sapiens (Human)) | BDBM8960 ((+/-)-2-[(1-benzylpiperidin-4-yl)methyl]-5,6-dimet...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Physiologically Active Compounds Russian Academy of Sciences Curated by ChEMBL | Assay Description Inhibition of human carboxylesterase 1 using o-nitrophenylacetate as substrate by spectrophotometry analysis | Bioorg Med Chem 24: 1050-62 (2016) Article DOI: 10.1016/j.bmc.2016.01.031 BindingDB Entry DOI: 10.7270/Q2B85C4Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM22722 (1,2-diphenylethane-1,2-dione | Benzil | CHEMBL1898...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Physiologically Active Compounds Russian Academy of Sciences Curated by ChEMBL | Assay Description Inhibition of human erythrocyte AChE using acetylthiocholine iodide as substrate preincubated for 10 mins followed by substrate addition by Ellman's ... | Bioorg Med Chem 24: 1050-62 (2016) Article DOI: 10.1016/j.bmc.2016.01.031 BindingDB Entry DOI: 10.7270/Q2B85C4Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50500773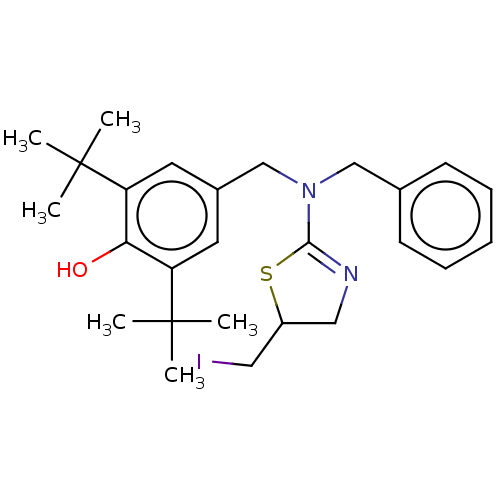 (CHEMBL3752567) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Physiologically Active Compounds Russian Academy of Sciences Curated by ChEMBL | Assay Description Inhibition of equine serum BChE using butyrylthiocholine iodide as substrate preincubated for 10 mins followed by substrate addition by Ellman's meth... | Bioorg Med Chem 24: 1050-62 (2016) Article DOI: 10.1016/j.bmc.2016.01.031 BindingDB Entry DOI: 10.7270/Q2B85C4Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Liver carboxylesterase (Sus scrofa) | BDBM50500754 (CHEMBL3753110) | PDB KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Physiologically Active Compounds Russian Academy of Sciences Curated by ChEMBL | Assay Description Inhibition of porcine liver carboxylesterase using 4-nitrophenol acetate as substrate by spectrophotometric analysis | Bioorg Med Chem 24: 1050-62 (2016) Article DOI: 10.1016/j.bmc.2016.01.031 BindingDB Entry DOI: 10.7270/Q2B85C4Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Liver carboxylesterase (Sus scrofa) | BDBM50500774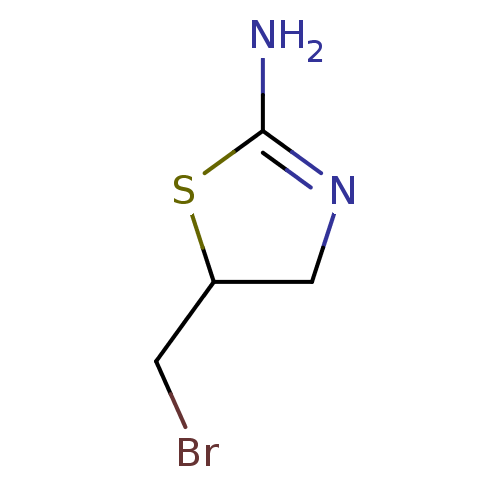 (CHEMBL3754756) | PDB KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | Article PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Physiologically Active Compounds Russian Academy of Sciences Curated by ChEMBL | Assay Description Inhibition of porcine liver carboxylesterase using 4-nitrophenol acetate as substrate by spectrophotometric analysis | Bioorg Med Chem 24: 1050-62 (2016) Article DOI: 10.1016/j.bmc.2016.01.031 BindingDB Entry DOI: 10.7270/Q2B85C4Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50500765 (CHEMBL3754585) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.10E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Physiologically Active Compounds Russian Academy of Sciences Curated by ChEMBL | Assay Description Inhibition of human erythrocyte AChE using acetylthiocholine iodide as substrate preincubated for 10 mins followed by substrate addition by Ellman's ... | Bioorg Med Chem 24: 1050-62 (2016) Article DOI: 10.1016/j.bmc.2016.01.031 BindingDB Entry DOI: 10.7270/Q2B85C4Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Liver carboxylesterase (Sus scrofa) | BDBM50500761 (CHEMBL3752873) | PDB KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.17E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Physiologically Active Compounds Russian Academy of Sciences Curated by ChEMBL | Assay Description Inhibition of porcine liver carboxylesterase using 4-nitrophenol acetate as substrate by spectrophotometric analysis | Bioorg Med Chem 24: 1050-62 (2016) Article DOI: 10.1016/j.bmc.2016.01.031 BindingDB Entry DOI: 10.7270/Q2B85C4Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Liver carboxylesterase (Sus scrofa) | BDBM50500763 (CHEMBL3753018) | PDB KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.31E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Physiologically Active Compounds Russian Academy of Sciences Curated by ChEMBL | Assay Description Inhibition of porcine liver carboxylesterase using 4-nitrophenol acetate as substrate by spectrophotometric analysis | Bioorg Med Chem 24: 1050-62 (2016) Article DOI: 10.1016/j.bmc.2016.01.031 BindingDB Entry DOI: 10.7270/Q2B85C4Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Liver carboxylesterase (Sus scrofa) | BDBM50500756 (CHEMBL3752908) | PDB KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 6.10E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Physiologically Active Compounds Russian Academy of Sciences Curated by ChEMBL | Assay Description Inhibition of porcine liver carboxylesterase using 4-nitrophenol acetate as substrate by spectrophotometric analysis | Bioorg Med Chem 24: 1050-62 (2016) Article DOI: 10.1016/j.bmc.2016.01.031 BindingDB Entry DOI: 10.7270/Q2B85C4Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||