

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM8961 (1,2,3,4-tetrahydro-9-acridinamine | 1,2,3,4-tetrah...) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
University of British Columbia Curated by ChEMBL | Assay Description Inhibition of electric eel AChE using acetylthiocholine iodide as substrate by Ellman's method | Bioorg Med Chem Lett 26: 1624-8 (2016) Article DOI: 10.1016/j.bmcl.2016.01.080 BindingDB Entry DOI: 10.7270/Q2W66NND | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM11682 (2,3-dihydroxybutanedioic acid; 3-[(1S)-1-(dimethyl...) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase DrugBank MCE KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of British Columbia Curated by ChEMBL | Assay Description Inhibition of electric eel AChE using acetylthiocholine iodide as substrate by Ellman's method | Bioorg Med Chem Lett 26: 1624-8 (2016) Article DOI: 10.1016/j.bmcl.2016.01.080 BindingDB Entry DOI: 10.7270/Q2W66NND | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50158402 (CHEMBL3781198) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.88E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of British Columbia Curated by ChEMBL | Assay Description Inhibition of electric eel AChE preincubated for 5 mins followed by addition of acetylthiocholine iodide measured after 2 mins by Ellman's method | Bioorg Med Chem Lett 26: 1624-8 (2016) Article DOI: 10.1016/j.bmcl.2016.01.080 BindingDB Entry DOI: 10.7270/Q2W66NND | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50158534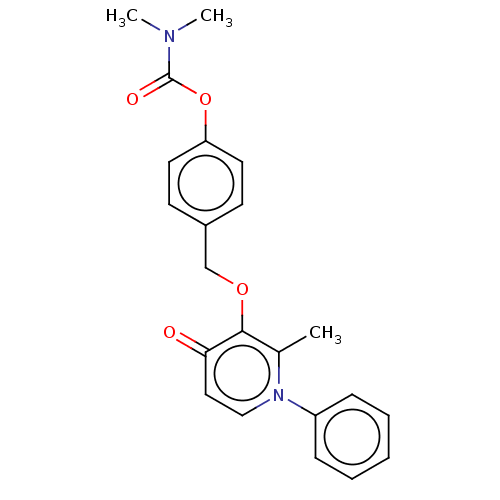 (CHEMBL3781763) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.17E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of British Columbia Curated by ChEMBL | Assay Description Inhibition of electric eel AChE using acetylthiocholine iodide as substrate by Ellman's method | Bioorg Med Chem Lett 26: 1624-8 (2016) Article DOI: 10.1016/j.bmcl.2016.01.080 BindingDB Entry DOI: 10.7270/Q2W66NND | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50086229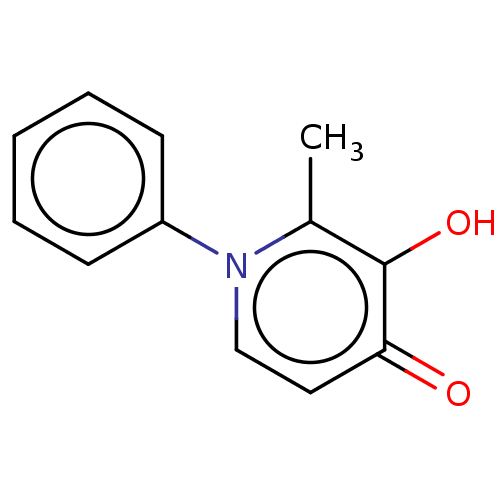 (CHEMBL326815) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.20E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of British Columbia Curated by ChEMBL | Assay Description Inhibition of electric eel AChE using acetylthiocholine iodide as substrate by Ellman's method | Bioorg Med Chem Lett 26: 1624-8 (2016) Article DOI: 10.1016/j.bmcl.2016.01.080 BindingDB Entry DOI: 10.7270/Q2W66NND | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50158537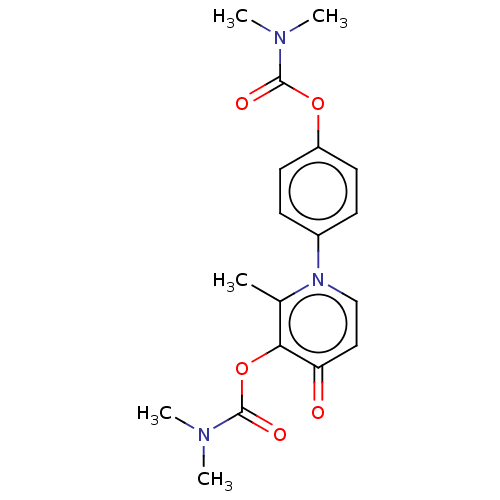 (CHEMBL3781438) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.52E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of British Columbia Curated by ChEMBL | Assay Description Inhibition of electric eel AChE using acetylthiocholine iodide as substrate by Ellman's method | Bioorg Med Chem Lett 26: 1624-8 (2016) Article DOI: 10.1016/j.bmcl.2016.01.080 BindingDB Entry DOI: 10.7270/Q2W66NND | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50158538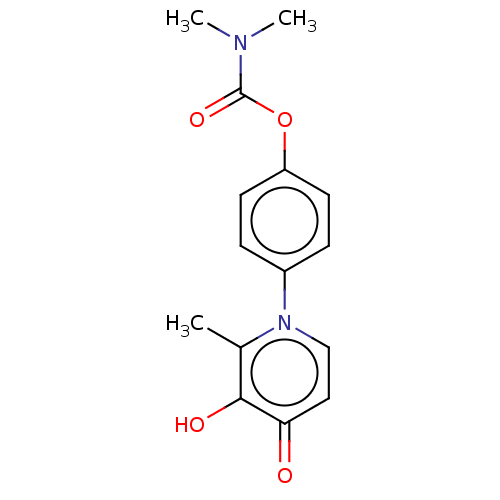 (CHEMBL3780941) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.48E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of British Columbia Curated by ChEMBL | Assay Description Inhibition of electric eel AChE using acetylthiocholine iodide as substrate by Ellman's method | Bioorg Med Chem Lett 26: 1624-8 (2016) Article DOI: 10.1016/j.bmcl.2016.01.080 BindingDB Entry DOI: 10.7270/Q2W66NND | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50158403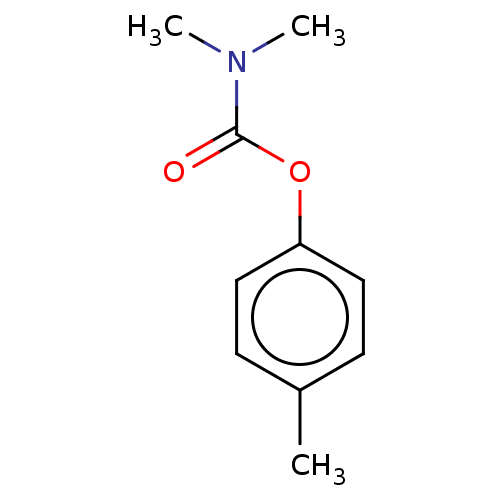 (P-TOLYL DIMETHYLCARBAMATE) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.28E+6 | n/a | n/a | n/a | n/a | n/a | n/a |
University of British Columbia Curated by ChEMBL | Assay Description Inhibition of electric eel AChE using acetylthiocholine iodide as substrate by Ellman's method | Bioorg Med Chem Lett 26: 1624-8 (2016) Article DOI: 10.1016/j.bmcl.2016.01.080 BindingDB Entry DOI: 10.7270/Q2W66NND | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||