 Found 75 hits of Enzyme Inhibition Constant Data
Found 75 hits of Enzyme Inhibition Constant Data Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit beta isoform
(Homo sapiens (Human)) | BDBM50500920
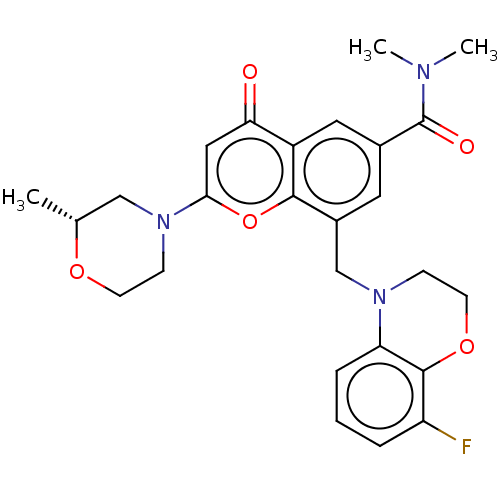
(CHEMBL3798914)Show SMILES C[C@@H]1CN(CCO1)c1cc(=O)c2cc(cc(CN3CCOc4c(F)cccc34)c2o1)C(=O)N(C)C |r| Show InChI InChI=1S/C26H28FN3O5/c1-16-14-30(8-9-33-16)23-13-22(31)19-12-17(26(32)28(2)3)11-18(24(19)35-23)15-29-7-10-34-25-20(27)5-4-6-21(25)29/h4-6,11-13,16H,7-10,14-15H2,1-3H3/t16-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kbeta in PTEN deficient human MDA-MB-468 cells assessed as inhibition of AKT phosphorylation at Ser-473 residue after 2 hrs by plate... |
Bioorg Med Chem Lett 26: 2318-23 (2016)
Article DOI: 10.1016/j.bmcl.2016.03.034
BindingDB Entry DOI: 10.7270/Q2W098ZZ |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit beta isoform
(Homo sapiens (Human)) | BDBM50500917
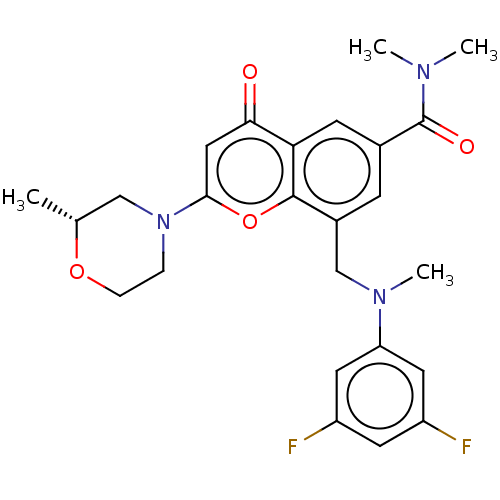
(CHEMBL3797698)Show SMILES C[C@@H]1CN(CCO1)c1cc(=O)c2cc(cc(CN(C)c3cc(F)cc(F)c3)c2o1)C(=O)N(C)C |r| Show InChI InChI=1S/C25H27F2N3O4/c1-15-13-30(5-6-33-15)23-12-22(31)21-8-16(25(32)28(2)3)7-17(24(21)34-23)14-29(4)20-10-18(26)9-19(27)11-20/h7-12,15H,5-6,13-14H2,1-4H3/t15-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kbeta in PTEN deficient human MDA-MB-468 cells assessed as inhibition of AKT phosphorylation at Ser-473 residue after 2 hrs by plate... |
Bioorg Med Chem Lett 26: 2318-23 (2016)
Article DOI: 10.1016/j.bmcl.2016.03.034
BindingDB Entry DOI: 10.7270/Q2W098ZZ |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit beta isoform
(Homo sapiens (Human)) | BDBM50500922
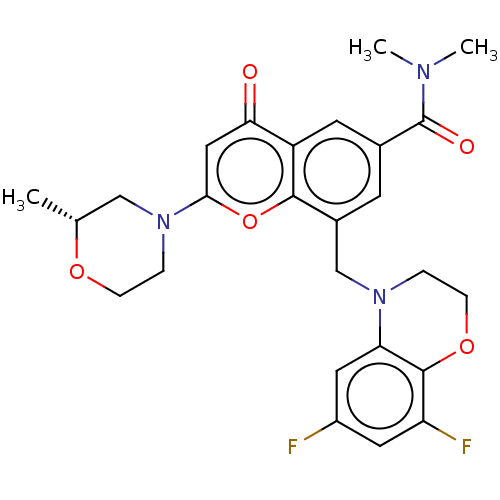
(CHEMBL3797812)Show SMILES C[C@@H]1CN(CCO1)c1cc(=O)c2cc(cc(CN3CCOc4c(F)cc(F)cc34)c2o1)C(=O)N(C)C |r| Show InChI InChI=1S/C26H27F2N3O5/c1-15-13-31(5-6-34-15)23-12-22(32)19-9-16(26(33)29(2)3)8-17(24(19)36-23)14-30-4-7-35-25-20(28)10-18(27)11-21(25)30/h8-12,15H,4-7,13-14H2,1-3H3/t15-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kbeta in PTEN deficient human MDA-MB-468 cells assessed as inhibition of AKT phosphorylation at Ser-473 residue after 2 hrs by plate... |
Bioorg Med Chem Lett 26: 2318-23 (2016)
Article DOI: 10.1016/j.bmcl.2016.03.034
BindingDB Entry DOI: 10.7270/Q2W098ZZ |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit beta isoform
(Homo sapiens (Human)) | BDBM50500918
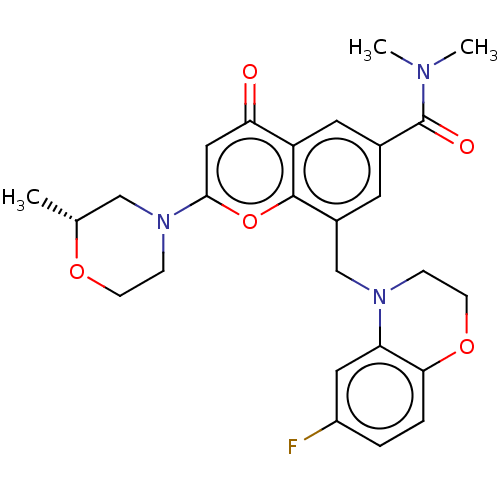
(CHEMBL3799511)Show SMILES C[C@@H]1CN(CCO1)c1cc(=O)c2cc(cc(CN3CCOc4ccc(F)cc34)c2o1)C(=O)N(C)C |r| Show InChI InChI=1S/C26H28FN3O5/c1-16-14-30(7-8-33-16)24-13-22(31)20-11-17(26(32)28(2)3)10-18(25(20)35-24)15-29-6-9-34-23-5-4-19(27)12-21(23)29/h4-5,10-13,16H,6-9,14-15H2,1-3H3/t16-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kbeta in PTEN deficient human MDA-MB-468 cells assessed as inhibition of AKT phosphorylation at Ser-473 residue after 2 hrs by plate... |
Bioorg Med Chem Lett 26: 2318-23 (2016)
Article DOI: 10.1016/j.bmcl.2016.03.034
BindingDB Entry DOI: 10.7270/Q2W098ZZ |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit beta isoform
(Homo sapiens (Human)) | BDBM50500924
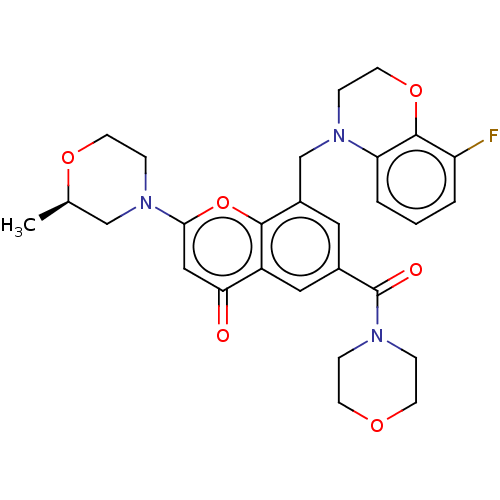
(CHEMBL3799158)Show SMILES C[C@@H]1CN(CCO1)c1cc(=O)c2cc(cc(CN3CCOc4c(F)cccc34)c2o1)C(=O)N1CCOCC1 |r| Show InChI InChI=1S/C28H30FN3O6/c1-18-16-32(8-11-36-18)25-15-24(33)21-14-19(28(34)30-5-9-35-10-6-30)13-20(26(21)38-25)17-31-7-12-37-27-22(29)3-2-4-23(27)31/h2-4,13-15,18H,5-12,16-17H2,1H3/t18-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kbeta in PTEN deficient human MDA-MB-468 cells assessed as inhibition of AKT phosphorylation at Ser-473 residue after 2 hrs by plate... |
Bioorg Med Chem Lett 26: 2318-23 (2016)
Article DOI: 10.1016/j.bmcl.2016.03.034
BindingDB Entry DOI: 10.7270/Q2W098ZZ |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit beta isoform
(Homo sapiens (Human)) | BDBM50500929
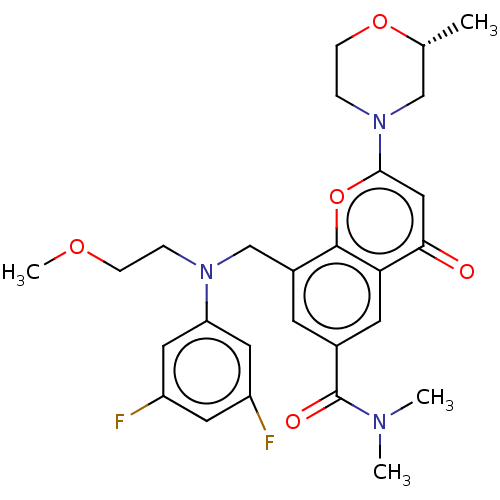
(CHEMBL3800231)Show SMILES COCCN(Cc1cc(cc2c1oc(cc2=O)N1CCO[C@H](C)C1)C(=O)N(C)C)c1cc(F)cc(F)c1 |r| Show InChI InChI=1S/C27H31F2N3O5/c1-17-15-32(6-8-36-17)25-14-24(33)23-10-18(27(34)30(2)3)9-19(26(23)37-25)16-31(5-7-35-4)22-12-20(28)11-21(29)13-22/h9-14,17H,5-8,15-16H2,1-4H3/t17-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kbeta in PTEN deficient human MDA-MB-468 cells assessed as inhibition of AKT phosphorylation at Ser-473 residue after 2 hrs by plate... |
Bioorg Med Chem Lett 26: 2318-23 (2016)
Article DOI: 10.1016/j.bmcl.2016.03.034
BindingDB Entry DOI: 10.7270/Q2W098ZZ |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50500917

(CHEMBL3797698)Show SMILES C[C@@H]1CN(CCO1)c1cc(=O)c2cc(cc(CN(C)c3cc(F)cc(F)c3)c2o1)C(=O)N(C)C |r| Show InChI InChI=1S/C25H27F2N3O4/c1-15-13-30(5-6-33-15)23-12-22(31)21-8-16(25(32)28(2)3)7-17(24(21)34-23)14-29(4)20-10-18(26)9-19(27)11-20/h7-12,15H,5-6,13-14H2,1-4H3/t15-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant PI3Kalpha using PIP2 as substrate preincubated for 20 mins followed by substrate addition measured after 80 mins by l... |
Bioorg Med Chem Lett 26: 2318-23 (2016)
Article DOI: 10.1016/j.bmcl.2016.03.034
BindingDB Entry DOI: 10.7270/Q2W098ZZ |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit beta isoform
(Homo sapiens (Human)) | BDBM50500922

(CHEMBL3797812)Show SMILES C[C@@H]1CN(CCO1)c1cc(=O)c2cc(cc(CN3CCOc4c(F)cc(F)cc34)c2o1)C(=O)N(C)C |r| Show InChI InChI=1S/C26H27F2N3O5/c1-15-13-31(5-6-34-15)23-12-22(32)19-9-16(26(33)29(2)3)8-17(24(19)36-23)14-30-4-7-35-25-20(28)10-18(27)11-21(25)30/h8-12,15H,4-7,13-14H2,1-3H3/t15-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant PI3Kbeta using PIP2 as substrate preincubated for 20 mins followed by substrate addition measured after 80 mins by lu... |
Bioorg Med Chem Lett 26: 2318-23 (2016)
Article DOI: 10.1016/j.bmcl.2016.03.034
BindingDB Entry DOI: 10.7270/Q2W098ZZ |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit beta isoform
(Homo sapiens (Human)) | BDBM50500925
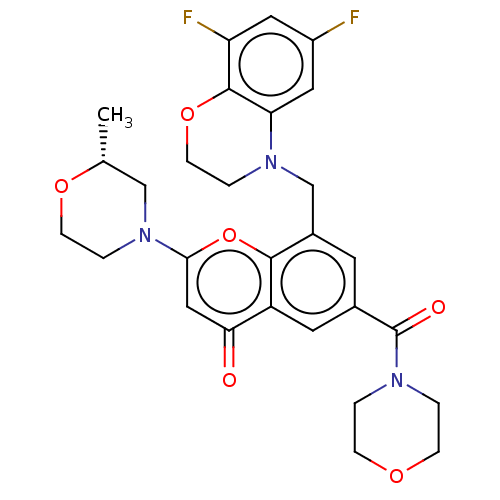
(CHEMBL3799673)Show SMILES C[C@@H]1CN(CCO1)c1cc(=O)c2cc(cc(CN3CCOc4c(F)cc(F)cc34)c2o1)C(=O)N1CCOCC1 |r| Show InChI InChI=1S/C28H29F2N3O6/c1-17-15-33(5-8-37-17)25-14-24(34)21-11-18(28(35)31-2-6-36-7-3-31)10-19(26(21)39-25)16-32-4-9-38-27-22(30)12-20(29)13-23(27)32/h10-14,17H,2-9,15-16H2,1H3/t17-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kbeta in PTEN deficient human MDA-MB-468 cells assessed as inhibition of AKT phosphorylation at Ser-473 residue after 2 hrs by plate... |
Bioorg Med Chem Lett 26: 2318-23 (2016)
Article DOI: 10.1016/j.bmcl.2016.03.034
BindingDB Entry DOI: 10.7270/Q2W098ZZ |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit beta isoform
(Homo sapiens (Human)) | BDBM50500915
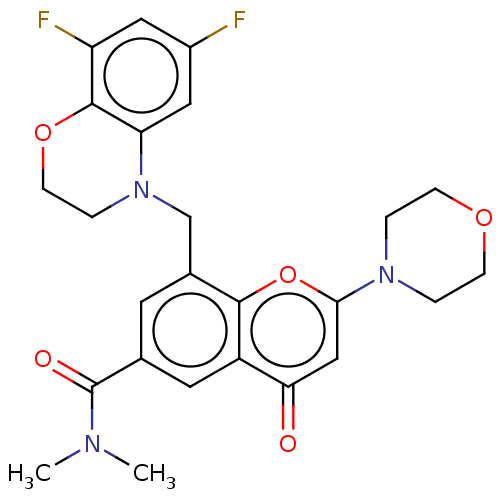
(CHEMBL3797750)Show SMILES CN(C)C(=O)c1cc(CN2CCOc3c(F)cc(F)cc23)c2oc(cc(=O)c2c1)N1CCOCC1 Show InChI InChI=1S/C25H25F2N3O5/c1-28(2)25(32)15-9-16(14-30-5-8-34-24-19(27)11-17(26)12-20(24)30)23-18(10-15)21(31)13-22(35-23)29-3-6-33-7-4-29/h9-13H,3-8,14H2,1-2H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant PI3Kbeta using PIP2 as substrate preincubated for 20 mins followed by substrate addition measured after 80 mins by lu... |
Bioorg Med Chem Lett 26: 2318-23 (2016)
Article DOI: 10.1016/j.bmcl.2016.03.034
BindingDB Entry DOI: 10.7270/Q2W098ZZ |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50500920

(CHEMBL3798914)Show SMILES C[C@@H]1CN(CCO1)c1cc(=O)c2cc(cc(CN3CCOc4c(F)cccc34)c2o1)C(=O)N(C)C |r| Show InChI InChI=1S/C26H28FN3O5/c1-16-14-30(8-9-33-16)23-13-22(31)19-12-17(26(32)28(2)3)11-18(24(19)35-23)15-29-7-10-34-25-20(27)5-4-6-21(25)29/h4-6,11-13,16H,7-10,14-15H2,1-3H3/t16-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant PI3Kalpha using PIP2 as substrate preincubated for 20 mins followed by substrate addition measured after 80 mins by l... |
Bioorg Med Chem Lett 26: 2318-23 (2016)
Article DOI: 10.1016/j.bmcl.2016.03.034
BindingDB Entry DOI: 10.7270/Q2W098ZZ |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit beta isoform
(Homo sapiens (Human)) | BDBM50500918

(CHEMBL3799511)Show SMILES C[C@@H]1CN(CCO1)c1cc(=O)c2cc(cc(CN3CCOc4ccc(F)cc34)c2o1)C(=O)N(C)C |r| Show InChI InChI=1S/C26H28FN3O5/c1-16-14-30(7-8-33-16)24-13-22(31)20-11-17(26(32)28(2)3)10-18(25(20)35-24)15-29-6-9-34-23-5-4-19(27)12-21(23)29/h4-5,10-13,16H,6-9,14-15H2,1-3H3/t16-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant PI3Kbeta using PIP2 as substrate preincubated for 20 mins followed by substrate addition measured after 80 mins by lu... |
Bioorg Med Chem Lett 26: 2318-23 (2016)
Article DOI: 10.1016/j.bmcl.2016.03.034
BindingDB Entry DOI: 10.7270/Q2W098ZZ |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit beta isoform
(Homo sapiens (Human)) | BDBM50500917

(CHEMBL3797698)Show SMILES C[C@@H]1CN(CCO1)c1cc(=O)c2cc(cc(CN(C)c3cc(F)cc(F)c3)c2o1)C(=O)N(C)C |r| Show InChI InChI=1S/C25H27F2N3O4/c1-15-13-30(5-6-33-15)23-12-22(31)21-8-16(25(32)28(2)3)7-17(24(21)34-23)14-29(4)20-10-18(26)9-19(27)11-20/h7-12,15H,5-6,13-14H2,1-4H3/t15-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant PI3Kbeta using PIP2 as substrate preincubated for 20 mins followed by substrate addition measured after 80 mins by lu... |
Bioorg Med Chem Lett 26: 2318-23 (2016)
Article DOI: 10.1016/j.bmcl.2016.03.034
BindingDB Entry DOI: 10.7270/Q2W098ZZ |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50500922

(CHEMBL3797812)Show SMILES C[C@@H]1CN(CCO1)c1cc(=O)c2cc(cc(CN3CCOc4c(F)cc(F)cc34)c2o1)C(=O)N(C)C |r| Show InChI InChI=1S/C26H27F2N3O5/c1-15-13-31(5-6-34-15)23-12-22(32)19-9-16(26(33)29(2)3)8-17(24(19)36-23)14-30-4-7-35-25-20(28)10-18(27)11-21(25)30/h8-12,15H,4-7,13-14H2,1-3H3/t15-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant PI3Kalpha using PIP2 as substrate preincubated for 20 mins followed by substrate addition measured after 80 mins by l... |
Bioorg Med Chem Lett 26: 2318-23 (2016)
Article DOI: 10.1016/j.bmcl.2016.03.034
BindingDB Entry DOI: 10.7270/Q2W098ZZ |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM50500917

(CHEMBL3797698)Show SMILES C[C@@H]1CN(CCO1)c1cc(=O)c2cc(cc(CN(C)c3cc(F)cc(F)c3)c2o1)C(=O)N(C)C |r| Show InChI InChI=1S/C25H27F2N3O4/c1-15-13-30(5-6-33-15)23-12-22(31)21-8-16(25(32)28(2)3)7-17(24(21)34-23)14-29(4)20-10-18(26)9-19(27)11-20/h7-12,15H,5-6,13-14H2,1-4H3/t15-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant PI3Kdelta using PIP2 as substrate preincubated for 20 mins followed by substrate addition measured after 80 mins by l... |
Bioorg Med Chem Lett 26: 2318-23 (2016)
Article DOI: 10.1016/j.bmcl.2016.03.034
BindingDB Entry DOI: 10.7270/Q2W098ZZ |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit beta isoform
(Homo sapiens (Human)) | BDBM50070301
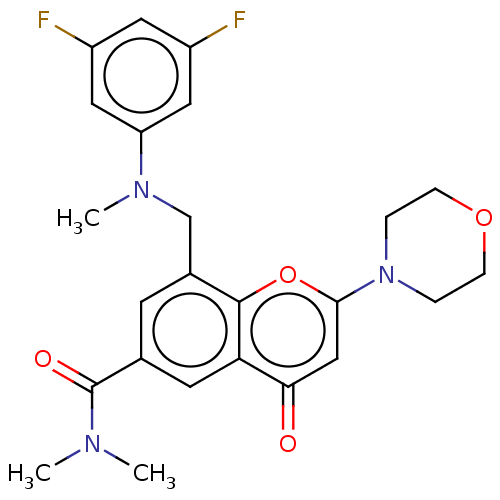
(CHEMBL3408273)Show SMILES CN(C)C(=O)c1cc(CN(C)c2cc(F)cc(F)c2)c2oc(cc(=O)c2c1)N1CCOCC1 Show InChI InChI=1S/C24H25F2N3O4/c1-27(2)24(31)15-8-16(14-28(3)19-11-17(25)10-18(26)12-19)23-20(9-15)21(30)13-22(33-23)29-4-6-32-7-5-29/h8-13H,4-7,14H2,1-3H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant PI3Kbeta using PIP2 as substrate preincubated for 20 mins followed by substrate addition measured after 80 mins by lu... |
Bioorg Med Chem Lett 26: 2318-23 (2016)
Article DOI: 10.1016/j.bmcl.2016.03.034
BindingDB Entry DOI: 10.7270/Q2W098ZZ |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit beta isoform
(Homo sapiens (Human)) | BDBM50500920

(CHEMBL3798914)Show SMILES C[C@@H]1CN(CCO1)c1cc(=O)c2cc(cc(CN3CCOc4c(F)cccc34)c2o1)C(=O)N(C)C |r| Show InChI InChI=1S/C26H28FN3O5/c1-16-14-30(8-9-33-16)23-13-22(31)19-12-17(26(32)28(2)3)11-18(24(19)35-23)15-29-7-10-34-25-20(27)5-4-6-21(25)29/h4-6,11-13,16H,7-10,14-15H2,1-3H3/t16-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant PI3Kbeta using PIP2 as substrate preincubated for 20 mins followed by substrate addition measured after 80 mins by lu... |
Bioorg Med Chem Lett 26: 2318-23 (2016)
Article DOI: 10.1016/j.bmcl.2016.03.034
BindingDB Entry DOI: 10.7270/Q2W098ZZ |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit beta isoform
(Homo sapiens (Human)) | BDBM50500914
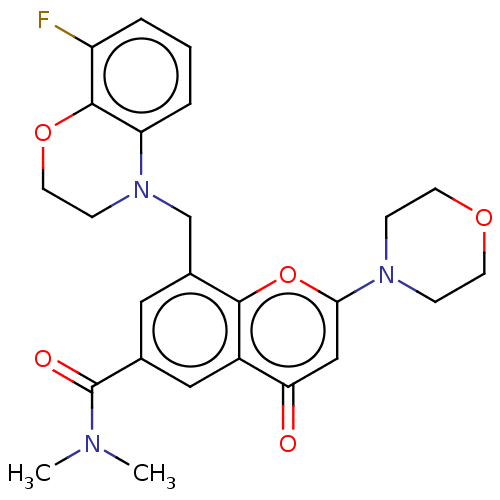
(CHEMBL3799831)Show SMILES CN(C)C(=O)c1cc(CN2CCOc3c(F)cccc23)c2oc(cc(=O)c2c1)N1CCOCC1 Show InChI InChI=1S/C25H26FN3O5/c1-27(2)25(31)16-12-17(15-29-8-11-33-24-19(26)4-3-5-20(24)29)23-18(13-16)21(30)14-22(34-23)28-6-9-32-10-7-28/h3-5,12-14H,6-11,15H2,1-2H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant PI3Kbeta using PIP2 as substrate preincubated for 20 mins followed by substrate addition measured after 80 mins by lu... |
Bioorg Med Chem Lett 26: 2318-23 (2016)
Article DOI: 10.1016/j.bmcl.2016.03.034
BindingDB Entry DOI: 10.7270/Q2W098ZZ |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM50500922

(CHEMBL3797812)Show SMILES C[C@@H]1CN(CCO1)c1cc(=O)c2cc(cc(CN3CCOc4c(F)cc(F)cc34)c2o1)C(=O)N(C)C |r| Show InChI InChI=1S/C26H27F2N3O5/c1-15-13-31(5-6-34-15)23-12-22(32)19-9-16(26(33)29(2)3)8-17(24(19)36-23)14-30-4-7-35-25-20(28)10-18(27)11-21(25)30/h8-12,15H,4-7,13-14H2,1-3H3/t15-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant PI3Kdelta using PIP2 as substrate preincubated for 20 mins followed by substrate addition measured after 80 mins by l... |
Bioorg Med Chem Lett 26: 2318-23 (2016)
Article DOI: 10.1016/j.bmcl.2016.03.034
BindingDB Entry DOI: 10.7270/Q2W098ZZ |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50500918

(CHEMBL3799511)Show SMILES C[C@@H]1CN(CCO1)c1cc(=O)c2cc(cc(CN3CCOc4ccc(F)cc34)c2o1)C(=O)N(C)C |r| Show InChI InChI=1S/C26H28FN3O5/c1-16-14-30(7-8-33-16)24-13-22(31)20-11-17(26(32)28(2)3)10-18(25(20)35-24)15-29-6-9-34-23-5-4-19(27)12-21(23)29/h4-5,10-13,16H,6-9,14-15H2,1-3H3/t16-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant PI3Kalpha using PIP2 as substrate preincubated for 20 mins followed by substrate addition measured after 80 mins by l... |
Bioorg Med Chem Lett 26: 2318-23 (2016)
Article DOI: 10.1016/j.bmcl.2016.03.034
BindingDB Entry DOI: 10.7270/Q2W098ZZ |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit beta isoform
(Homo sapiens (Human)) | BDBM50500915

(CHEMBL3797750)Show SMILES CN(C)C(=O)c1cc(CN2CCOc3c(F)cc(F)cc23)c2oc(cc(=O)c2c1)N1CCOCC1 Show InChI InChI=1S/C25H25F2N3O5/c1-28(2)25(32)15-9-16(14-30-5-8-34-24-19(27)11-17(26)12-20(24)30)23-18(10-15)21(31)13-22(35-23)29-3-6-33-7-4-29/h9-13H,3-8,14H2,1-2H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kbeta in PTEN deficient human MDA-MB-468 cells assessed as inhibition of AKT phosphorylation at Ser-473 residue after 2 hrs by plate... |
Bioorg Med Chem Lett 26: 2318-23 (2016)
Article DOI: 10.1016/j.bmcl.2016.03.034
BindingDB Entry DOI: 10.7270/Q2W098ZZ |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit beta isoform
(Homo sapiens (Human)) | BDBM50500921

(CHEMBL3799187)Show SMILES CN(C)C(=O)c1cc(CN2CCOc3ccc(F)cc23)c2oc(cc(=O)c2c1)N1CCOCC1 Show InChI InChI=1S/C25H26FN3O5/c1-27(2)25(31)16-11-17(15-29-7-10-33-22-4-3-18(26)13-20(22)29)24-19(12-16)21(30)14-23(34-24)28-5-8-32-9-6-28/h3-4,11-14H,5-10,15H2,1-2H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant PI3Kbeta using PIP2 as substrate preincubated for 20 mins followed by substrate addition measured after 80 mins by lu... |
Bioorg Med Chem Lett 26: 2318-23 (2016)
Article DOI: 10.1016/j.bmcl.2016.03.034
BindingDB Entry DOI: 10.7270/Q2W098ZZ |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50500917

(CHEMBL3797698)Show SMILES C[C@@H]1CN(CCO1)c1cc(=O)c2cc(cc(CN(C)c3cc(F)cc(F)c3)c2o1)C(=O)N(C)C |r| Show InChI InChI=1S/C25H27F2N3O4/c1-15-13-30(5-6-33-15)23-12-22(31)21-8-16(25(32)28(2)3)7-17(24(21)34-23)14-29(4)20-10-18(26)9-19(27)11-20/h7-12,15H,5-6,13-14H2,1-4H3/t15-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Tight binding affinity to human recombinant PI3Kalpha using PIP2 as substrate preincubated for 20 mins followed by substrate addition measured after ... |
Bioorg Med Chem Lett 26: 2318-23 (2016)
Article DOI: 10.1016/j.bmcl.2016.03.034
BindingDB Entry DOI: 10.7270/Q2W098ZZ |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit beta isoform
(Homo sapiens (Human)) | BDBM50070301

(CHEMBL3408273)Show SMILES CN(C)C(=O)c1cc(CN(C)c2cc(F)cc(F)c2)c2oc(cc(=O)c2c1)N1CCOCC1 Show InChI InChI=1S/C24H25F2N3O4/c1-27(2)24(31)15-8-16(14-28(3)19-11-17(25)10-18(26)12-19)23-20(9-15)21(30)13-22(33-23)29-4-6-32-7-5-29/h8-13H,4-7,14H2,1-3H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Tight binding affinity to human recombinant PI3Kbeta using PIP2 as substrate preincubated for 20 mins followed by substrate addition measured after 8... |
Bioorg Med Chem Lett 26: 2318-23 (2016)
Article DOI: 10.1016/j.bmcl.2016.03.034
BindingDB Entry DOI: 10.7270/Q2W098ZZ |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50500918

(CHEMBL3799511)Show SMILES C[C@@H]1CN(CCO1)c1cc(=O)c2cc(cc(CN3CCOc4ccc(F)cc34)c2o1)C(=O)N(C)C |r| Show InChI InChI=1S/C26H28FN3O5/c1-16-14-30(7-8-33-16)24-13-22(31)20-11-17(26(32)28(2)3)10-18(25(20)35-24)15-29-6-9-34-23-5-4-19(27)12-21(23)29/h4-5,10-13,16H,6-9,14-15H2,1-3H3/t16-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Tight binding affinity to human recombinant PI3Kalpha using PIP2 as substrate preincubated for 20 mins followed by substrate addition measured after ... |
Bioorg Med Chem Lett 26: 2318-23 (2016)
Article DOI: 10.1016/j.bmcl.2016.03.034
BindingDB Entry DOI: 10.7270/Q2W098ZZ |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50500920

(CHEMBL3798914)Show SMILES C[C@@H]1CN(CCO1)c1cc(=O)c2cc(cc(CN3CCOc4c(F)cccc34)c2o1)C(=O)N(C)C |r| Show InChI InChI=1S/C26H28FN3O5/c1-16-14-30(8-9-33-16)23-13-22(31)19-12-17(26(32)28(2)3)11-18(24(19)35-23)15-29-7-10-34-25-20(27)5-4-6-21(25)29/h4-6,11-13,16H,7-10,14-15H2,1-3H3/t16-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Tight binding affinity to human recombinant PI3Kalpha using PIP2 as substrate preincubated for 20 mins followed by substrate addition measured after ... |
Bioorg Med Chem Lett 26: 2318-23 (2016)
Article DOI: 10.1016/j.bmcl.2016.03.034
BindingDB Entry DOI: 10.7270/Q2W098ZZ |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit beta isoform
(Homo sapiens (Human)) | BDBM50500920

(CHEMBL3798914)Show SMILES C[C@@H]1CN(CCO1)c1cc(=O)c2cc(cc(CN3CCOc4c(F)cccc34)c2o1)C(=O)N(C)C |r| Show InChI InChI=1S/C26H28FN3O5/c1-16-14-30(8-9-33-16)23-13-22(31)19-12-17(26(32)28(2)3)11-18(24(19)35-23)15-29-7-10-34-25-20(27)5-4-6-21(25)29/h4-6,11-13,16H,7-10,14-15H2,1-3H3/t16-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Tight binding affinity to human recombinant PI3Kbeta using PIP2 as substrate preincubated for 20 mins followed by substrate addition measured after 8... |
Bioorg Med Chem Lett 26: 2318-23 (2016)
Article DOI: 10.1016/j.bmcl.2016.03.034
BindingDB Entry DOI: 10.7270/Q2W098ZZ |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit beta isoform
(Homo sapiens (Human)) | BDBM50500915

(CHEMBL3797750)Show SMILES CN(C)C(=O)c1cc(CN2CCOc3c(F)cc(F)cc23)c2oc(cc(=O)c2c1)N1CCOCC1 Show InChI InChI=1S/C25H25F2N3O5/c1-28(2)25(32)15-9-16(14-30-5-8-34-24-19(27)11-17(26)12-20(24)30)23-18(10-15)21(31)13-22(35-23)29-3-6-33-7-4-29/h9-13H,3-8,14H2,1-2H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Tight binding affinity to human recombinant PI3Kbeta using PIP2 as substrate preincubated for 20 mins followed by substrate addition measured after 8... |
Bioorg Med Chem Lett 26: 2318-23 (2016)
Article DOI: 10.1016/j.bmcl.2016.03.034
BindingDB Entry DOI: 10.7270/Q2W098ZZ |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50500921

(CHEMBL3799187)Show SMILES CN(C)C(=O)c1cc(CN2CCOc3ccc(F)cc23)c2oc(cc(=O)c2c1)N1CCOCC1 Show InChI InChI=1S/C25H26FN3O5/c1-27(2)25(31)16-11-17(15-29-7-10-33-22-4-3-18(26)13-20(22)29)24-19(12-16)21(30)14-23(34-24)28-5-8-32-9-6-28/h3-4,11-14H,5-10,15H2,1-2H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Tight binding affinity to human recombinant PI3Kalpha using PIP2 as substrate preincubated for 20 mins followed by substrate addition measured after ... |
Bioorg Med Chem Lett 26: 2318-23 (2016)
Article DOI: 10.1016/j.bmcl.2016.03.034
BindingDB Entry DOI: 10.7270/Q2W098ZZ |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit beta isoform
(Homo sapiens (Human)) | BDBM50500917

(CHEMBL3797698)Show SMILES C[C@@H]1CN(CCO1)c1cc(=O)c2cc(cc(CN(C)c3cc(F)cc(F)c3)c2o1)C(=O)N(C)C |r| Show InChI InChI=1S/C25H27F2N3O4/c1-15-13-30(5-6-33-15)23-12-22(31)21-8-16(25(32)28(2)3)7-17(24(21)34-23)14-29(4)20-10-18(26)9-19(27)11-20/h7-12,15H,5-6,13-14H2,1-4H3/t15-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Tight binding affinity to human recombinant PI3Kbeta using PIP2 as substrate preincubated for 20 mins followed by substrate addition measured after 8... |
Bioorg Med Chem Lett 26: 2318-23 (2016)
Article DOI: 10.1016/j.bmcl.2016.03.034
BindingDB Entry DOI: 10.7270/Q2W098ZZ |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50500915

(CHEMBL3797750)Show SMILES CN(C)C(=O)c1cc(CN2CCOc3c(F)cc(F)cc23)c2oc(cc(=O)c2c1)N1CCOCC1 Show InChI InChI=1S/C25H25F2N3O5/c1-28(2)25(32)15-9-16(14-30-5-8-34-24-19(27)11-17(26)12-20(24)30)23-18(10-15)21(31)13-22(35-23)29-3-6-33-7-4-29/h9-13H,3-8,14H2,1-2H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Tight binding affinity to human recombinant PI3Kalpha using PIP2 as substrate preincubated for 20 mins followed by substrate addition measured after ... |
Bioorg Med Chem Lett 26: 2318-23 (2016)
Article DOI: 10.1016/j.bmcl.2016.03.034
BindingDB Entry DOI: 10.7270/Q2W098ZZ |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50070301

(CHEMBL3408273)Show SMILES CN(C)C(=O)c1cc(CN(C)c2cc(F)cc(F)c2)c2oc(cc(=O)c2c1)N1CCOCC1 Show InChI InChI=1S/C24H25F2N3O4/c1-27(2)24(31)15-8-16(14-28(3)19-11-17(25)10-18(26)12-19)23-20(9-15)21(30)13-22(33-23)29-4-6-32-7-5-29/h8-13H,4-7,14H2,1-3H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Tight binding affinity to human recombinant PI3Kalpha using PIP2 as substrate preincubated for 20 mins followed by substrate addition measured after ... |
Bioorg Med Chem Lett 26: 2318-23 (2016)
Article DOI: 10.1016/j.bmcl.2016.03.034
BindingDB Entry DOI: 10.7270/Q2W098ZZ |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM50500920

(CHEMBL3798914)Show SMILES C[C@@H]1CN(CCO1)c1cc(=O)c2cc(cc(CN3CCOc4c(F)cccc34)c2o1)C(=O)N(C)C |r| Show InChI InChI=1S/C26H28FN3O5/c1-16-14-30(8-9-33-16)23-13-22(31)19-12-17(26(32)28(2)3)11-18(24(19)35-23)15-29-7-10-34-25-20(27)5-4-6-21(25)29/h4-6,11-13,16H,7-10,14-15H2,1-3H3/t16-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant PI3Kdelta using PIP2 as substrate preincubated for 20 mins followed by substrate addition measured after 80 mins by l... |
Bioorg Med Chem Lett 26: 2318-23 (2016)
Article DOI: 10.1016/j.bmcl.2016.03.034
BindingDB Entry DOI: 10.7270/Q2W098ZZ |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit beta isoform
(Homo sapiens (Human)) | BDBM50500922

(CHEMBL3797812)Show SMILES C[C@@H]1CN(CCO1)c1cc(=O)c2cc(cc(CN3CCOc4c(F)cc(F)cc34)c2o1)C(=O)N(C)C |r| Show InChI InChI=1S/C26H27F2N3O5/c1-15-13-31(5-6-34-15)23-12-22(32)19-9-16(26(33)29(2)3)8-17(24(19)36-23)14-30-4-7-35-25-20(28)10-18(27)11-21(25)30/h8-12,15H,4-7,13-14H2,1-3H3/t15-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Tight binding affinity to human recombinant PI3Kbeta using PIP2 as substrate preincubated for 20 mins followed by substrate addition measured after 8... |
Bioorg Med Chem Lett 26: 2318-23 (2016)
Article DOI: 10.1016/j.bmcl.2016.03.034
BindingDB Entry DOI: 10.7270/Q2W098ZZ |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50500914

(CHEMBL3799831)Show SMILES CN(C)C(=O)c1cc(CN2CCOc3c(F)cccc23)c2oc(cc(=O)c2c1)N1CCOCC1 Show InChI InChI=1S/C25H26FN3O5/c1-27(2)25(31)16-12-17(15-29-8-11-33-24-19(26)4-3-5-20(24)29)23-18(13-16)21(30)14-22(34-23)28-6-9-32-10-7-28/h3-5,12-14H,6-11,15H2,1-2H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Tight binding affinity to human recombinant PI3Kalpha using PIP2 as substrate preincubated for 20 mins followed by substrate addition measured after ... |
Bioorg Med Chem Lett 26: 2318-23 (2016)
Article DOI: 10.1016/j.bmcl.2016.03.034
BindingDB Entry DOI: 10.7270/Q2W098ZZ |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit beta isoform
(Homo sapiens (Human)) | BDBM50500914

(CHEMBL3799831)Show SMILES CN(C)C(=O)c1cc(CN2CCOc3c(F)cccc23)c2oc(cc(=O)c2c1)N1CCOCC1 Show InChI InChI=1S/C25H26FN3O5/c1-27(2)25(31)16-12-17(15-29-8-11-33-24-19(26)4-3-5-20(24)29)23-18(13-16)21(30)14-22(34-23)28-6-9-32-10-7-28/h3-5,12-14H,6-11,15H2,1-2H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Tight binding affinity to human recombinant PI3Kbeta using PIP2 as substrate preincubated for 20 mins followed by substrate addition measured after 8... |
Bioorg Med Chem Lett 26: 2318-23 (2016)
Article DOI: 10.1016/j.bmcl.2016.03.034
BindingDB Entry DOI: 10.7270/Q2W098ZZ |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit beta isoform
(Homo sapiens (Human)) | BDBM50500921

(CHEMBL3799187)Show SMILES CN(C)C(=O)c1cc(CN2CCOc3ccc(F)cc23)c2oc(cc(=O)c2c1)N1CCOCC1 Show InChI InChI=1S/C25H26FN3O5/c1-27(2)25(31)16-11-17(15-29-7-10-33-22-4-3-18(26)13-20(22)29)24-19(12-16)21(30)14-23(34-24)28-5-8-32-9-6-28/h3-4,11-14H,5-10,15H2,1-2H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Tight binding affinity to human recombinant PI3Kbeta using PIP2 as substrate preincubated for 20 mins followed by substrate addition measured after 8... |
Bioorg Med Chem Lett 26: 2318-23 (2016)
Article DOI: 10.1016/j.bmcl.2016.03.034
BindingDB Entry DOI: 10.7270/Q2W098ZZ |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit beta isoform
(Homo sapiens (Human)) | BDBM50500918

(CHEMBL3799511)Show SMILES C[C@@H]1CN(CCO1)c1cc(=O)c2cc(cc(CN3CCOc4ccc(F)cc34)c2o1)C(=O)N(C)C |r| Show InChI InChI=1S/C26H28FN3O5/c1-16-14-30(7-8-33-16)24-13-22(31)20-11-17(26(32)28(2)3)10-18(25(20)35-24)15-29-6-9-34-23-5-4-19(27)12-21(23)29/h4-5,10-13,16H,6-9,14-15H2,1-3H3/t16-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Tight binding affinity to human recombinant PI3Kbeta using PIP2 as substrate preincubated for 20 mins followed by substrate addition measured after 8... |
Bioorg Med Chem Lett 26: 2318-23 (2016)
Article DOI: 10.1016/j.bmcl.2016.03.034
BindingDB Entry DOI: 10.7270/Q2W098ZZ |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50500922

(CHEMBL3797812)Show SMILES C[C@@H]1CN(CCO1)c1cc(=O)c2cc(cc(CN3CCOc4c(F)cc(F)cc34)c2o1)C(=O)N(C)C |r| Show InChI InChI=1S/C26H27F2N3O5/c1-15-13-31(5-6-34-15)23-12-22(32)19-9-16(26(33)29(2)3)8-17(24(19)36-23)14-30-4-7-35-25-20(28)10-18(27)11-21(25)30/h8-12,15H,4-7,13-14H2,1-3H3/t15-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Tight binding affinity to human recombinant PI3Kalpha using PIP2 as substrate preincubated for 20 mins followed by substrate addition measured after ... |
Bioorg Med Chem Lett 26: 2318-23 (2016)
Article DOI: 10.1016/j.bmcl.2016.03.034
BindingDB Entry DOI: 10.7270/Q2W098ZZ |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM50500914

(CHEMBL3799831)Show SMILES CN(C)C(=O)c1cc(CN2CCOc3c(F)cccc23)c2oc(cc(=O)c2c1)N1CCOCC1 Show InChI InChI=1S/C25H26FN3O5/c1-27(2)25(31)16-12-17(15-29-8-11-33-24-19(26)4-3-5-20(24)29)23-18(13-16)21(30)14-22(34-23)28-6-9-32-10-7-28/h3-5,12-14H,6-11,15H2,1-2H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant PI3Kdelta using PIP2 as substrate preincubated for 20 mins followed by substrate addition measured after 80 mins by l... |
Bioorg Med Chem Lett 26: 2318-23 (2016)
Article DOI: 10.1016/j.bmcl.2016.03.034
BindingDB Entry DOI: 10.7270/Q2W098ZZ |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM50500918

(CHEMBL3799511)Show SMILES C[C@@H]1CN(CCO1)c1cc(=O)c2cc(cc(CN3CCOc4ccc(F)cc34)c2o1)C(=O)N(C)C |r| Show InChI InChI=1S/C26H28FN3O5/c1-16-14-30(7-8-33-16)24-13-22(31)20-11-17(26(32)28(2)3)10-18(25(20)35-24)15-29-6-9-34-23-5-4-19(27)12-21(23)29/h4-5,10-13,16H,6-9,14-15H2,1-3H3/t16-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant PI3Kdelta using PIP2 as substrate preincubated for 20 mins followed by substrate addition measured after 80 mins by l... |
Bioorg Med Chem Lett 26: 2318-23 (2016)
Article DOI: 10.1016/j.bmcl.2016.03.034
BindingDB Entry DOI: 10.7270/Q2W098ZZ |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit beta isoform
(Homo sapiens (Human)) | BDBM50500914

(CHEMBL3799831)Show SMILES CN(C)C(=O)c1cc(CN2CCOc3c(F)cccc23)c2oc(cc(=O)c2c1)N1CCOCC1 Show InChI InChI=1S/C25H26FN3O5/c1-27(2)25(31)16-12-17(15-29-8-11-33-24-19(26)4-3-5-20(24)29)23-18(13-16)21(30)14-22(34-23)28-6-9-32-10-7-28/h3-5,12-14H,6-11,15H2,1-2H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kbeta in PTEN deficient human MDA-MB-468 cells assessed as inhibition of AKT phosphorylation at Ser-473 residue after 2 hrs by plate... |
Bioorg Med Chem Lett 26: 2318-23 (2016)
Article DOI: 10.1016/j.bmcl.2016.03.034
BindingDB Entry DOI: 10.7270/Q2W098ZZ |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit beta isoform
(Homo sapiens (Human)) | BDBM50500927
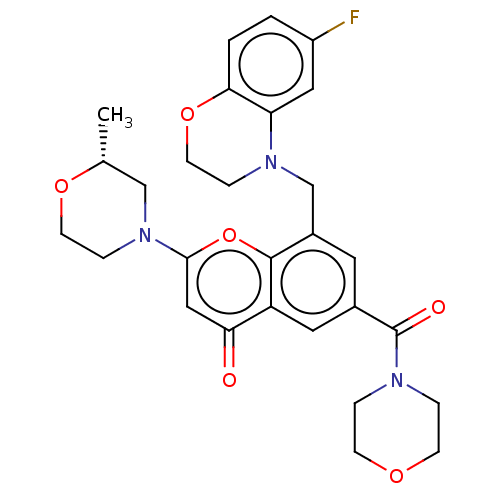
(CHEMBL3799504)Show SMILES C[C@@H]1CN(CCO1)c1cc(=O)c2cc(cc(CN3CCOc4ccc(F)cc34)c2o1)C(=O)N1CCOCC1 |r| Show InChI InChI=1S/C28H30FN3O6/c1-18-16-32(7-10-36-18)26-15-24(33)22-13-19(28(34)30-4-8-35-9-5-30)12-20(27(22)38-26)17-31-6-11-37-25-3-2-21(29)14-23(25)31/h2-3,12-15,18H,4-11,16-17H2,1H3/t18-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kbeta in PTEN deficient human MDA-MB-468 cells assessed as inhibition of AKT phosphorylation at Ser-473 residue after 2 hrs by plate... |
Bioorg Med Chem Lett 26: 2318-23 (2016)
Article DOI: 10.1016/j.bmcl.2016.03.034
BindingDB Entry DOI: 10.7270/Q2W098ZZ |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM50500921

(CHEMBL3799187)Show SMILES CN(C)C(=O)c1cc(CN2CCOc3ccc(F)cc23)c2oc(cc(=O)c2c1)N1CCOCC1 Show InChI InChI=1S/C25H26FN3O5/c1-27(2)25(31)16-11-17(15-29-7-10-33-22-4-3-18(26)13-20(22)29)24-19(12-16)21(30)14-23(34-24)28-5-8-32-9-6-28/h3-4,11-14H,5-10,15H2,1-2H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant PI3Kdelta using PIP2 as substrate preincubated for 20 mins followed by substrate addition measured after 80 mins by l... |
Bioorg Med Chem Lett 26: 2318-23 (2016)
Article DOI: 10.1016/j.bmcl.2016.03.034
BindingDB Entry DOI: 10.7270/Q2W098ZZ |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM50500915

(CHEMBL3797750)Show SMILES CN(C)C(=O)c1cc(CN2CCOc3c(F)cc(F)cc23)c2oc(cc(=O)c2c1)N1CCOCC1 Show InChI InChI=1S/C25H25F2N3O5/c1-28(2)25(32)15-9-16(14-30-5-8-34-24-19(27)11-17(26)12-20(24)30)23-18(10-15)21(31)13-22(35-23)29-3-6-33-7-4-29/h9-13H,3-8,14H2,1-2H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant PI3Kdelta using PIP2 as substrate preincubated for 20 mins followed by substrate addition measured after 80 mins by l... |
Bioorg Med Chem Lett 26: 2318-23 (2016)
Article DOI: 10.1016/j.bmcl.2016.03.034
BindingDB Entry DOI: 10.7270/Q2W098ZZ |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM50500914

(CHEMBL3799831)Show SMILES CN(C)C(=O)c1cc(CN2CCOc3c(F)cccc23)c2oc(cc(=O)c2c1)N1CCOCC1 Show InChI InChI=1S/C25H26FN3O5/c1-27(2)25(31)16-12-17(15-29-8-11-33-24-19(26)4-3-5-20(24)29)23-18(13-16)21(30)14-22(34-23)28-6-9-32-10-7-28/h3-5,12-14H,6-11,15H2,1-2H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Tight binding affinity to human recombinant PI3Kdelta using PIP2 as substrate preincubated for 20 mins followed by substrate addition measured after ... |
Bioorg Med Chem Lett 26: 2318-23 (2016)
Article DOI: 10.1016/j.bmcl.2016.03.034
BindingDB Entry DOI: 10.7270/Q2W098ZZ |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50500914

(CHEMBL3799831)Show SMILES CN(C)C(=O)c1cc(CN2CCOc3c(F)cccc23)c2oc(cc(=O)c2c1)N1CCOCC1 Show InChI InChI=1S/C25H26FN3O5/c1-27(2)25(31)16-12-17(15-29-8-11-33-24-19(26)4-3-5-20(24)29)23-18(13-16)21(30)14-22(34-23)28-6-9-32-10-7-28/h3-5,12-14H,6-11,15H2,1-2H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant PI3Kalpha using PIP2 as substrate preincubated for 20 mins followed by substrate addition measured after 80 mins by l... |
Bioorg Med Chem Lett 26: 2318-23 (2016)
Article DOI: 10.1016/j.bmcl.2016.03.034
BindingDB Entry DOI: 10.7270/Q2W098ZZ |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50500915

(CHEMBL3797750)Show SMILES CN(C)C(=O)c1cc(CN2CCOc3c(F)cc(F)cc23)c2oc(cc(=O)c2c1)N1CCOCC1 Show InChI InChI=1S/C25H25F2N3O5/c1-28(2)25(32)15-9-16(14-30-5-8-34-24-19(27)11-17(26)12-20(24)30)23-18(10-15)21(31)13-22(35-23)29-3-6-33-7-4-29/h9-13H,3-8,14H2,1-2H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant PI3Kalpha using PIP2 as substrate preincubated for 20 mins followed by substrate addition measured after 80 mins by l... |
Bioorg Med Chem Lett 26: 2318-23 (2016)
Article DOI: 10.1016/j.bmcl.2016.03.034
BindingDB Entry DOI: 10.7270/Q2W098ZZ |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit beta isoform
(Homo sapiens (Human)) | BDBM50500916
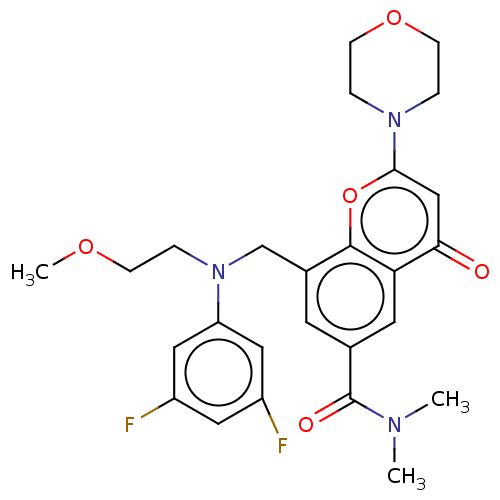
(CHEMBL3800314)Show SMILES COCCN(Cc1cc(cc2c1oc(cc2=O)N1CCOCC1)C(=O)N(C)C)c1cc(F)cc(F)c1 Show InChI InChI=1S/C26H29F2N3O5/c1-29(2)26(33)17-10-18(16-31(4-7-34-3)21-13-19(27)12-20(28)14-21)25-22(11-17)23(32)15-24(36-25)30-5-8-35-9-6-30/h10-15H,4-9,16H2,1-3H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 17 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kbeta in PTEN deficient human MDA-MB-468 cells assessed as inhibition of AKT phosphorylation at Ser-473 residue after 2 hrs by plate... |
Bioorg Med Chem Lett 26: 2318-23 (2016)
Article DOI: 10.1016/j.bmcl.2016.03.034
BindingDB Entry DOI: 10.7270/Q2W098ZZ |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50070301

(CHEMBL3408273)Show SMILES CN(C)C(=O)c1cc(CN(C)c2cc(F)cc(F)c2)c2oc(cc(=O)c2c1)N1CCOCC1 Show InChI InChI=1S/C24H25F2N3O4/c1-27(2)24(31)15-8-16(14-28(3)19-11-17(25)10-18(26)12-19)23-20(9-15)21(30)13-22(33-23)29-4-6-32-7-5-29/h8-13H,4-7,14H2,1-3H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 25 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant PI3Kalpha using PIP2 as substrate preincubated for 20 mins followed by substrate addition measured after 80 mins by l... |
Bioorg Med Chem Lett 26: 2318-23 (2016)
Article DOI: 10.1016/j.bmcl.2016.03.034
BindingDB Entry DOI: 10.7270/Q2W098ZZ |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM50070301

(CHEMBL3408273)Show SMILES CN(C)C(=O)c1cc(CN(C)c2cc(F)cc(F)c2)c2oc(cc(=O)c2c1)N1CCOCC1 Show InChI InChI=1S/C24H25F2N3O4/c1-27(2)24(31)15-8-16(14-28(3)19-11-17(25)10-18(26)12-19)23-20(9-15)21(30)13-22(33-23)29-4-6-32-7-5-29/h8-13H,4-7,14H2,1-3H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 27 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant PI3Kdelta using PIP2 as substrate preincubated for 20 mins followed by substrate addition measured after 80 mins by l... |
Bioorg Med Chem Lett 26: 2318-23 (2016)
Article DOI: 10.1016/j.bmcl.2016.03.034
BindingDB Entry DOI: 10.7270/Q2W098ZZ |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50500921

(CHEMBL3799187)Show SMILES CN(C)C(=O)c1cc(CN2CCOc3ccc(F)cc23)c2oc(cc(=O)c2c1)N1CCOCC1 Show InChI InChI=1S/C25H26FN3O5/c1-27(2)25(31)16-11-17(15-29-7-10-33-22-4-3-18(26)13-20(22)29)24-19(12-16)21(30)14-23(34-24)28-5-8-32-9-6-28/h3-4,11-14H,5-10,15H2,1-2H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 27 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant PI3Kalpha using PIP2 as substrate preincubated for 20 mins followed by substrate addition measured after 80 mins by l... |
Bioorg Med Chem Lett 26: 2318-23 (2016)
Article DOI: 10.1016/j.bmcl.2016.03.034
BindingDB Entry DOI: 10.7270/Q2W098ZZ |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit beta isoform
(Homo sapiens (Human)) | BDBM50070301

(CHEMBL3408273)Show SMILES CN(C)C(=O)c1cc(CN(C)c2cc(F)cc(F)c2)c2oc(cc(=O)c2c1)N1CCOCC1 Show InChI InChI=1S/C24H25F2N3O4/c1-27(2)24(31)15-8-16(14-28(3)19-11-17(25)10-18(26)12-19)23-20(9-15)21(30)13-22(33-23)29-4-6-32-7-5-29/h8-13H,4-7,14H2,1-3H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 35 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kbeta in PTEN deficient human MDA-MB-468 cells assessed as inhibition of AKT phosphorylation at Ser-473 residue after 2 hrs by plate... |
Bioorg Med Chem Lett 26: 2318-23 (2016)
Article DOI: 10.1016/j.bmcl.2016.03.034
BindingDB Entry DOI: 10.7270/Q2W098ZZ |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit beta isoform
(Homo sapiens (Human)) | BDBM50500921

(CHEMBL3799187)Show SMILES CN(C)C(=O)c1cc(CN2CCOc3ccc(F)cc23)c2oc(cc(=O)c2c1)N1CCOCC1 Show InChI InChI=1S/C25H26FN3O5/c1-27(2)25(31)16-11-17(15-29-7-10-33-22-4-3-18(26)13-20(22)29)24-19(12-16)21(30)14-23(34-24)28-5-8-32-9-6-28/h3-4,11-14H,5-10,15H2,1-2H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 37 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kbeta in PTEN deficient human MDA-MB-468 cells assessed as inhibition of AKT phosphorylation at Ser-473 residue after 2 hrs by plate... |
Bioorg Med Chem Lett 26: 2318-23 (2016)
Article DOI: 10.1016/j.bmcl.2016.03.034
BindingDB Entry DOI: 10.7270/Q2W098ZZ |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit beta isoform
(Homo sapiens (Human)) | BDBM50500928

(CHEMBL3797528)Show SMILES CC(N1CCOc2c(F)cc(F)cc12)c1cc(cc2c1oc(cc2=O)N1CCOCC1)C(=O)N(C)C Show InChI InChI=1S/C26H27F2N3O5/c1-15(31-6-9-35-25-20(28)12-17(27)13-21(25)31)18-10-16(26(33)29(2)3)11-19-22(32)14-23(36-24(18)19)30-4-7-34-8-5-30/h10-15H,4-9H2,1-3H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 45 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kbeta in PTEN deficient human MDA-MB-468 cells assessed as inhibition of AKT phosphorylation at Ser-473 residue after 2 hrs by plate... |
Bioorg Med Chem Lett 26: 2318-23 (2016)
Article DOI: 10.1016/j.bmcl.2016.03.034
BindingDB Entry DOI: 10.7270/Q2W098ZZ |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM50500914

(CHEMBL3799831)Show SMILES CN(C)C(=O)c1cc(CN2CCOc3c(F)cccc23)c2oc(cc(=O)c2c1)N1CCOCC1 Show InChI InChI=1S/C25H26FN3O5/c1-27(2)25(31)16-12-17(15-29-8-11-33-24-19(26)4-3-5-20(24)29)23-18(13-16)21(30)14-22(34-23)28-6-9-32-10-7-28/h3-5,12-14H,6-11,15H2,1-2H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 47 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kdelta in human JeKo1 cells assessed as inhibition of AKT phosphorylation at Ser-473 residue after 1 hr |
Bioorg Med Chem Lett 26: 2318-23 (2016)
Article DOI: 10.1016/j.bmcl.2016.03.034
BindingDB Entry DOI: 10.7270/Q2W098ZZ |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit beta isoform
(Homo sapiens (Human)) | BDBM50500919

(CHEMBL3797821)Show SMILES CN(C)C(=O)c1cc(CN(CCC#N)c2cc(F)cc(F)c2)c2oc(cc(=O)c2c1)N1CCOCC1 Show InChI InChI=1S/C26H26F2N4O4/c1-30(2)26(34)17-10-18(16-32(5-3-4-29)21-13-19(27)12-20(28)14-21)25-22(11-17)23(33)15-24(36-25)31-6-8-35-9-7-31/h10-15H,3,5-9,16H2,1-2H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 107 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kbeta in PTEN deficient human MDA-MB-468 cells assessed as inhibition of AKT phosphorylation at Ser-473 residue after 2 hrs by plate... |
Bioorg Med Chem Lett 26: 2318-23 (2016)
Article DOI: 10.1016/j.bmcl.2016.03.034
BindingDB Entry DOI: 10.7270/Q2W098ZZ |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit beta isoform
(Homo sapiens (Human)) | BDBM50500923

(CHEMBL3798108)Show SMILES CN(C)C(=O)c1cc(CN2CCOc3ccccc23)c2oc(cc(=O)c2c1)N1CCOCC1 Show InChI InChI=1S/C25H27N3O5/c1-26(2)25(30)17-13-18(16-28-9-12-32-22-6-4-3-5-20(22)28)24-19(14-17)21(29)15-23(33-24)27-7-10-31-11-8-27/h3-6,13-15H,7-12,16H2,1-2H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 120 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kbeta in PTEN deficient human MDA-MB-468 cells assessed as inhibition of AKT phosphorylation at Ser-473 residue after 2 hrs by plate... |
Bioorg Med Chem Lett 26: 2318-23 (2016)
Article DOI: 10.1016/j.bmcl.2016.03.034
BindingDB Entry DOI: 10.7270/Q2W098ZZ |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform
(Homo sapiens (Human)) | BDBM50500920

(CHEMBL3798914)Show SMILES C[C@@H]1CN(CCO1)c1cc(=O)c2cc(cc(CN3CCOc4c(F)cccc34)c2o1)C(=O)N(C)C |r| Show InChI InChI=1S/C26H28FN3O5/c1-16-14-30(8-9-33-16)23-13-22(31)19-12-17(26(32)28(2)3)11-18(24(19)35-23)15-29-7-10-34-25-20(27)5-4-6-21(25)29/h4-6,11-13,16H,7-10,14-15H2,1-3H3/t16-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 120 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant PI3Kgamma using PIP2 as substrate preincubated for 20 mins followed by substrate addition measured after 80 mins by l... |
Bioorg Med Chem Lett 26: 2318-23 (2016)
Article DOI: 10.1016/j.bmcl.2016.03.034
BindingDB Entry DOI: 10.7270/Q2W098ZZ |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform
(Homo sapiens (Human)) | BDBM50500922

(CHEMBL3797812)Show SMILES C[C@@H]1CN(CCO1)c1cc(=O)c2cc(cc(CN3CCOc4c(F)cc(F)cc34)c2o1)C(=O)N(C)C |r| Show InChI InChI=1S/C26H27F2N3O5/c1-15-13-31(5-6-34-15)23-12-22(32)19-9-16(26(33)29(2)3)8-17(24(19)36-23)14-30-4-7-35-25-20(28)10-18(27)11-21(25)30/h8-12,15H,4-7,13-14H2,1-3H3/t15-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 140 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant PI3Kgamma using PIP2 as substrate preincubated for 20 mins followed by substrate addition measured after 80 mins by l... |
Bioorg Med Chem Lett 26: 2318-23 (2016)
Article DOI: 10.1016/j.bmcl.2016.03.034
BindingDB Entry DOI: 10.7270/Q2W098ZZ |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit beta isoform
(Homo sapiens (Human)) | BDBM50500926
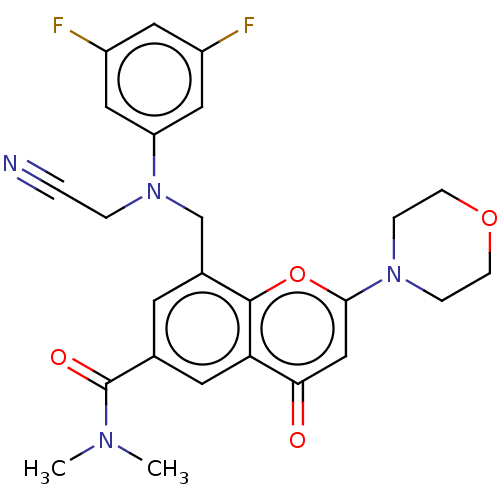
(CHEMBL3799515)Show SMILES CN(C)C(=O)c1cc(CN(CC#N)c2cc(F)cc(F)c2)c2oc(cc(=O)c2c1)N1CCOCC1 Show InChI InChI=1S/C25H24F2N4O4/c1-29(2)25(33)16-9-17(15-31(4-3-28)20-12-18(26)11-19(27)13-20)24-21(10-16)22(32)14-23(35-24)30-5-7-34-8-6-30/h9-14H,4-8,15H2,1-2H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 197 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kbeta in PTEN deficient human MDA-MB-468 cells assessed as inhibition of AKT phosphorylation at Ser-473 residue after 2 hrs by plate... |
Bioorg Med Chem Lett 26: 2318-23 (2016)
Article DOI: 10.1016/j.bmcl.2016.03.034
BindingDB Entry DOI: 10.7270/Q2W098ZZ |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform
(Homo sapiens (Human)) | BDBM50500917

(CHEMBL3797698)Show SMILES C[C@@H]1CN(CCO1)c1cc(=O)c2cc(cc(CN(C)c3cc(F)cc(F)c3)c2o1)C(=O)N(C)C |r| Show InChI InChI=1S/C25H27F2N3O4/c1-15-13-30(5-6-33-15)23-12-22(31)21-8-16(25(32)28(2)3)7-17(24(21)34-23)14-29(4)20-10-18(26)9-19(27)11-20/h7-12,15H,5-6,13-14H2,1-4H3/t15-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 240 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant PI3Kgamma using PIP2 as substrate preincubated for 20 mins followed by substrate addition measured after 80 mins by l... |
Bioorg Med Chem Lett 26: 2318-23 (2016)
Article DOI: 10.1016/j.bmcl.2016.03.034
BindingDB Entry DOI: 10.7270/Q2W098ZZ |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform
(Homo sapiens (Human)) | BDBM50500918

(CHEMBL3799511)Show SMILES C[C@@H]1CN(CCO1)c1cc(=O)c2cc(cc(CN3CCOc4ccc(F)cc34)c2o1)C(=O)N(C)C |r| Show InChI InChI=1S/C26H28FN3O5/c1-16-14-30(7-8-33-16)24-13-22(31)20-11-17(26(32)28(2)3)10-18(25(20)35-24)15-29-6-9-34-23-5-4-19(27)12-21(23)29/h4-5,10-13,16H,6-9,14-15H2,1-3H3/t16-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 440 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant PI3Kgamma using PIP2 as substrate preincubated for 20 mins followed by substrate addition measured after 80 mins by l... |
Bioorg Med Chem Lett 26: 2318-23 (2016)
Article DOI: 10.1016/j.bmcl.2016.03.034
BindingDB Entry DOI: 10.7270/Q2W098ZZ |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform
(Homo sapiens (Human)) | BDBM50500914

(CHEMBL3799831)Show SMILES CN(C)C(=O)c1cc(CN2CCOc3c(F)cccc23)c2oc(cc(=O)c2c1)N1CCOCC1 Show InChI InChI=1S/C25H26FN3O5/c1-27(2)25(31)16-12-17(15-29-8-11-33-24-19(26)4-3-5-20(24)29)23-18(13-16)21(30)14-22(34-23)28-6-9-32-10-7-28/h3-5,12-14H,6-11,15H2,1-2H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 570 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant PI3Kgamma using PIP2 as substrate preincubated for 20 mins followed by substrate addition measured after 80 mins by l... |
Bioorg Med Chem Lett 26: 2318-23 (2016)
Article DOI: 10.1016/j.bmcl.2016.03.034
BindingDB Entry DOI: 10.7270/Q2W098ZZ |
More data for this
Ligand-Target Pair | |
DNA-dependent protein kinase catalytic subunit
(Homo sapiens (Human)) | BDBM50500914

(CHEMBL3799831)Show SMILES CN(C)C(=O)c1cc(CN2CCOc3c(F)cccc23)c2oc(cc(=O)c2c1)N1CCOCC1 Show InChI InChI=1S/C25H26FN3O5/c1-27(2)25(31)16-12-17(15-29-8-11-33-24-19(26)4-3-5-20(24)29)23-18(13-16)21(30)14-22(34-23)28-6-9-32-10-7-28/h3-5,12-14H,6-11,15H2,1-2H3 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 690 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of DNAPK (unknown origin) |
Bioorg Med Chem Lett 26: 2318-23 (2016)
Article DOI: 10.1016/j.bmcl.2016.03.034
BindingDB Entry DOI: 10.7270/Q2W098ZZ |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform
(Homo sapiens (Human)) | BDBM50500915

(CHEMBL3797750)Show SMILES CN(C)C(=O)c1cc(CN2CCOc3c(F)cc(F)cc23)c2oc(cc(=O)c2c1)N1CCOCC1 Show InChI InChI=1S/C25H25F2N3O5/c1-28(2)25(32)15-9-16(14-30-5-8-34-24-19(27)11-17(26)12-20(24)30)23-18(10-15)21(31)13-22(35-23)29-3-6-33-7-4-29/h9-13H,3-8,14H2,1-2H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 720 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant PI3Kgamma using PIP2 as substrate preincubated for 20 mins followed by substrate addition measured after 80 mins by l... |
Bioorg Med Chem Lett 26: 2318-23 (2016)
Article DOI: 10.1016/j.bmcl.2016.03.034
BindingDB Entry DOI: 10.7270/Q2W098ZZ |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform
(Homo sapiens (Human)) | BDBM50070301

(CHEMBL3408273)Show SMILES CN(C)C(=O)c1cc(CN(C)c2cc(F)cc(F)c2)c2oc(cc(=O)c2c1)N1CCOCC1 Show InChI InChI=1S/C24H25F2N3O4/c1-27(2)24(31)15-8-16(14-28(3)19-11-17(25)10-18(26)12-19)23-20(9-15)21(30)13-22(33-23)29-4-6-32-7-5-29/h8-13H,4-7,14H2,1-3H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant PI3Kgamma using PIP2 as substrate preincubated for 20 mins followed by substrate addition measured after 80 mins by l... |
Bioorg Med Chem Lett 26: 2318-23 (2016)
Article DOI: 10.1016/j.bmcl.2016.03.034
BindingDB Entry DOI: 10.7270/Q2W098ZZ |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform
(Homo sapiens (Human)) | BDBM50500921

(CHEMBL3799187)Show SMILES CN(C)C(=O)c1cc(CN2CCOc3ccc(F)cc23)c2oc(cc(=O)c2c1)N1CCOCC1 Show InChI InChI=1S/C25H26FN3O5/c1-27(2)25(31)16-11-17(15-29-7-10-33-22-4-3-18(26)13-20(22)29)24-19(12-16)21(30)14-23(34-24)28-5-8-32-9-6-28/h3-4,11-14H,5-10,15H2,1-2H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant PI3Kgamma using PIP2 as substrate preincubated for 20 mins followed by substrate addition measured after 80 mins by l... |
Bioorg Med Chem Lett 26: 2318-23 (2016)
Article DOI: 10.1016/j.bmcl.2016.03.034
BindingDB Entry DOI: 10.7270/Q2W098ZZ |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C9
(Homo sapiens (Human)) | BDBM50500914

(CHEMBL3799831)Show SMILES CN(C)C(=O)c1cc(CN2CCOc3c(F)cccc23)c2oc(cc(=O)c2c1)N1CCOCC1 Show InChI InChI=1S/C25H26FN3O5/c1-27(2)25(31)16-12-17(15-29-8-11-33-24-19(26)4-3-5-20(24)29)23-18(13-16)21(30)14-22(34-23)28-6-9-32-10-7-28/h3-5,12-14H,6-11,15H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of CYP2C9 (unknown origin) |
Bioorg Med Chem Lett 26: 2318-23 (2016)
Article DOI: 10.1016/j.bmcl.2016.03.034
BindingDB Entry DOI: 10.7270/Q2W098ZZ |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2D6
(Homo sapiens (Human)) | BDBM50500914

(CHEMBL3799831)Show SMILES CN(C)C(=O)c1cc(CN2CCOc3c(F)cccc23)c2oc(cc(=O)c2c1)N1CCOCC1 Show InChI InChI=1S/C25H26FN3O5/c1-27(2)25(31)16-12-17(15-29-8-11-33-24-19(26)4-3-5-20(24)29)23-18(13-16)21(30)14-22(34-23)28-6-9-32-10-7-28/h3-5,12-14H,6-11,15H2,1-2H3 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of CYP2D6 (unknown origin) |
Bioorg Med Chem Lett 26: 2318-23 (2016)
Article DOI: 10.1016/j.bmcl.2016.03.034
BindingDB Entry DOI: 10.7270/Q2W098ZZ |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50500914

(CHEMBL3799831)Show SMILES CN(C)C(=O)c1cc(CN2CCOc3c(F)cccc23)c2oc(cc(=O)c2c1)N1CCOCC1 Show InChI InChI=1S/C25H26FN3O5/c1-27(2)25(31)16-12-17(15-29-8-11-33-24-19(26)4-3-5-20(24)29)23-18(13-16)21(30)14-22(34-23)28-6-9-32-10-7-28/h3-5,12-14H,6-11,15H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of CYP3A4 (unknown origin) |
Bioorg Med Chem Lett 26: 2318-23 (2016)
Article DOI: 10.1016/j.bmcl.2016.03.034
BindingDB Entry DOI: 10.7270/Q2W098ZZ |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C19
(Homo sapiens (Human)) | BDBM50500914

(CHEMBL3799831)Show SMILES CN(C)C(=O)c1cc(CN2CCOc3c(F)cccc23)c2oc(cc(=O)c2c1)N1CCOCC1 Show InChI InChI=1S/C25H26FN3O5/c1-27(2)25(31)16-12-17(15-29-8-11-33-24-19(26)4-3-5-20(24)29)23-18(13-16)21(30)14-22(34-23)28-6-9-32-10-7-28/h3-5,12-14H,6-11,15H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of CYP2C19 (unknown origin) |
Bioorg Med Chem Lett 26: 2318-23 (2016)
Article DOI: 10.1016/j.bmcl.2016.03.034
BindingDB Entry DOI: 10.7270/Q2W098ZZ |
More data for this
Ligand-Target Pair | |
Cytochrome P450 1A2
(Homo sapiens (Human)) | BDBM50500914

(CHEMBL3799831)Show SMILES CN(C)C(=O)c1cc(CN2CCOc3c(F)cccc23)c2oc(cc(=O)c2c1)N1CCOCC1 Show InChI InChI=1S/C25H26FN3O5/c1-27(2)25(31)16-12-17(15-29-8-11-33-24-19(26)4-3-5-20(24)29)23-18(13-16)21(30)14-22(34-23)28-6-9-32-10-7-28/h3-5,12-14H,6-11,15H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of CYP1A2 (unknown origin) |
Bioorg Med Chem Lett 26: 2318-23 (2016)
Article DOI: 10.1016/j.bmcl.2016.03.034
BindingDB Entry DOI: 10.7270/Q2W098ZZ |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase mTOR
(Homo sapiens (Human)) | BDBM50500914

(CHEMBL3799831)Show SMILES CN(C)C(=O)c1cc(CN2CCOc3c(F)cccc23)c2oc(cc(=O)c2c1)N1CCOCC1 Show InChI InChI=1S/C25H26FN3O5/c1-27(2)25(31)16-12-17(15-29-8-11-33-24-19(26)4-3-5-20(24)29)23-18(13-16)21(30)14-22(34-23)28-6-9-32-10-7-28/h3-5,12-14H,6-11,15H2,1-2H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of mTOR (unknown origin) |
Bioorg Med Chem Lett 26: 2318-23 (2016)
Article DOI: 10.1016/j.bmcl.2016.03.034
BindingDB Entry DOI: 10.7270/Q2W098ZZ |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50500914

(CHEMBL3799831)Show SMILES CN(C)C(=O)c1cc(CN2CCOc3c(F)cccc23)c2oc(cc(=O)c2c1)N1CCOCC1 Show InChI InChI=1S/C25H26FN3O5/c1-27(2)25(31)16-12-17(15-29-8-11-33-24-19(26)4-3-5-20(24)29)23-18(13-16)21(30)14-22(34-23)28-6-9-32-10-7-28/h3-5,12-14H,6-11,15H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 9.40E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of human ERG |
Bioorg Med Chem Lett 26: 2318-23 (2016)
Article DOI: 10.1016/j.bmcl.2016.03.034
BindingDB Entry DOI: 10.7270/Q2W098ZZ |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data