

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Catechol O-methyltransferase (Rattus norvegicus (Rat)) | BDBM50017865 (2,5-Bis-(3,4-dihydroxy-5-nitro-benzylidene)-cyclop...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Orion Corporation Ltd. Curated by ChEMBL | Assay Description In vitro inhibitory activity against catechol O-methyltransferase of rat brain using 3,4-dihydroxybenzoic acid as the substrate. | J Med Chem 32: 841-6 (1989) BindingDB Entry DOI: 10.7270/Q2GF0SHP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Catechol O-methyltransferase (Rattus norvegicus (Rat)) | BDBM50017845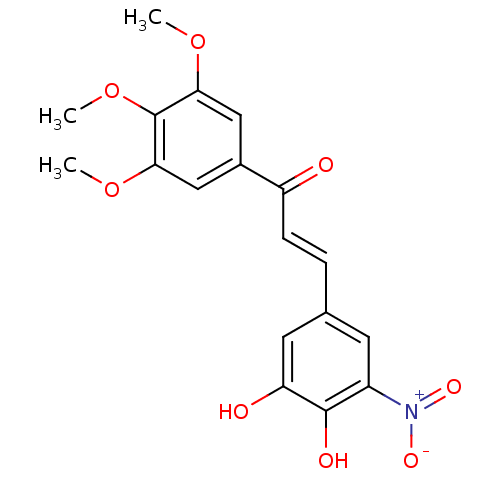 (3-(3,4-Dihydroxy-5-nitro-phenyl)-1-(3,4,5-trimetho...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Orion Corporation Ltd. Curated by ChEMBL | Assay Description In vitro inhibitory activity against catechol O-methyltransferase of rat brain using 3,4-dihydroxybenzoic acid as the substrate. | J Med Chem 32: 841-6 (1989) BindingDB Entry DOI: 10.7270/Q2GF0SHP | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| Catechol O-methyltransferase (Rattus norvegicus (Rat)) | BDBM50017857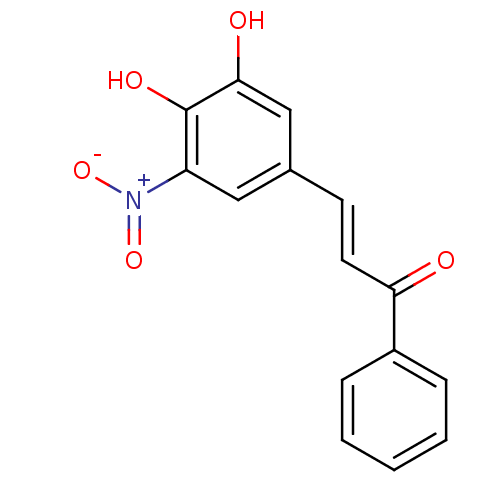 (3-(3,4-Dihydroxy-5-nitro-phenyl)-1-phenyl-propenon...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Orion Corporation Ltd. Curated by ChEMBL | Assay Description In vitro inhibitory activity against catechol O-methyltransferase of rat brain using 3,4-dihydroxybenzoic acid as the substrate. | J Med Chem 32: 841-6 (1989) BindingDB Entry DOI: 10.7270/Q2GF0SHP | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| Catechol O-methyltransferase (Rattus norvegicus (Rat)) | BDBM50017846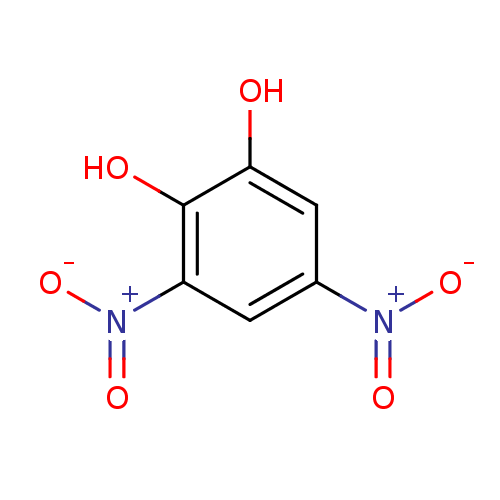 (3,5-DINITROCATECHOL | 3,5-Dinitro-benzene-1,2-diol...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | PDB PubMed | n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
Orion Corporation Ltd. Curated by ChEMBL | Assay Description In vitro inhibitory activity against catechol O-methyltransferase of rat brain using 3,4-dihydroxybenzoic acid as the substrate. | J Med Chem 32: 841-6 (1989) BindingDB Entry DOI: 10.7270/Q2GF0SHP | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Catechol O-methyltransferase (Rattus norvegicus (Rat)) | BDBM50017852 (4-(3,4-Dihydroxy-5-nitro-phenyl)-3-methyl-but-3-en...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
Orion Corporation Ltd. Curated by ChEMBL | Assay Description In vitro inhibitory activity against catechol O-methyltransferase of rat brain using 3,4-dihydroxybenzoic acid as the substrate. | J Med Chem 32: 841-6 (1989) BindingDB Entry DOI: 10.7270/Q2GF0SHP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Catechol O-methyltransferase (Rattus norvegicus (Rat)) | BDBM50017844 (1-(3,4-Dihydroxy-5-nitro-phenyl)-ethanone | CHEMBL...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
Orion Corporation Ltd. Curated by ChEMBL | Assay Description In vitro inhibitory activity against catechol O-methyltransferase of rat brain using 3,4-dihydroxybenzoic acid as the substrate. | J Med Chem 32: 841-6 (1989) BindingDB Entry DOI: 10.7270/Q2GF0SHP | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| Catechol O-methyltransferase (Rattus norvegicus (Rat)) | BDBM50017861 (2-Cyano-3-(3,4-dihydroxy-5-nitro-phenyl)-N,N-dimet...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 18 | n/a | n/a | n/a | n/a | n/a | n/a |
Orion Corporation Ltd. Curated by ChEMBL | Assay Description In vitro inhibitory activity against catechol O-methyltransferase of rat brain using 3,4-dihydroxybenzoic acid as the substrate. | J Med Chem 32: 841-6 (1989) BindingDB Entry DOI: 10.7270/Q2GF0SHP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Catechol O-methyltransferase (Rattus norvegicus (Rat)) | BDBM50017848 (3-(3,4-Dihydroxy-5-nitro-benzylidene)-pentane-2,4-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | PDB PubMed | n/a | n/a | 18 | n/a | n/a | n/a | n/a | n/a | n/a |
Orion Corporation Ltd. Curated by ChEMBL | Assay Description In vitro inhibitory activity against catechol O-methyltransferase of rat brain using 3,4-dihydroxybenzoic acid as the substrate. | J Med Chem 32: 841-6 (1989) BindingDB Entry DOI: 10.7270/Q2GF0SHP | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Catechol O-methyltransferase (Rattus norvegicus (Rat)) | BDBM50017866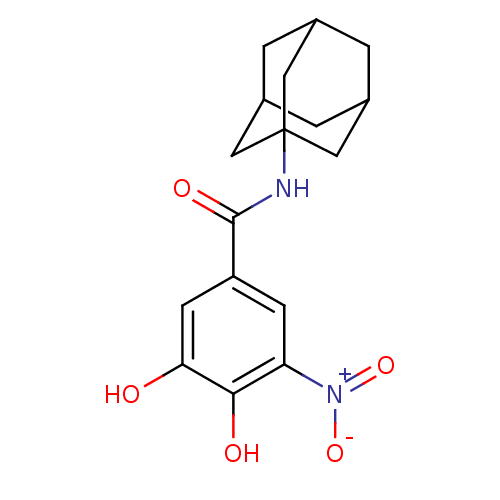 (CHEMBL350927 | N-Adamantan-1-yl-3,4-dihydroxy-5-ni...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | PubMed | n/a | n/a | 19 | n/a | n/a | n/a | n/a | n/a | n/a |
Orion Corporation Ltd. Curated by ChEMBL | Assay Description In vitro inhibitory activity against catechol O-methyltransferase of rat brain using 3,4-dihydroxybenzoic acid as the substrate. | J Med Chem 32: 841-6 (1989) BindingDB Entry DOI: 10.7270/Q2GF0SHP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Catechol O-methyltransferase (Rattus norvegicus (Rat)) | BDBM50017871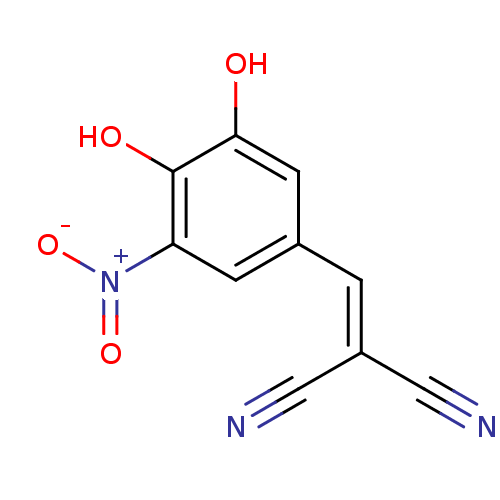 (2-(3,4-Dihydroxy-5-nitro-benzylidene)-malononitril...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Orion Corporation Ltd. Curated by ChEMBL | Assay Description In vitro inhibitory activity against catechol O-methyltransferase of rat brain using 3,4-dihydroxybenzoic acid as the substrate. | J Med Chem 32: 841-6 (1989) BindingDB Entry DOI: 10.7270/Q2GF0SHP | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| Catechol O-methyltransferase (Rattus norvegicus (Rat)) | BDBM50004043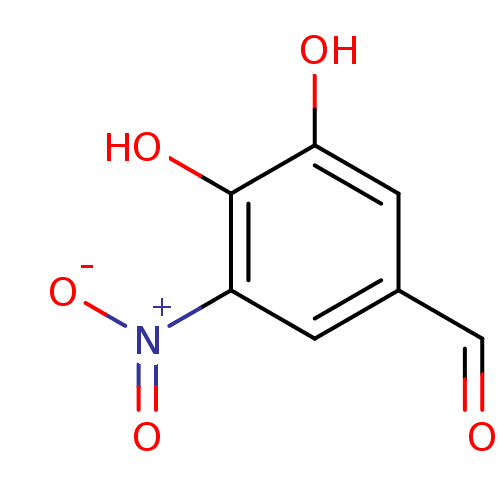 (3,4-Dihydroxy-5-Nitrobenzaldehyde (Rat) | 3,4-Dihy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 24 | n/a | n/a | n/a | n/a | n/a | n/a |
Orion Corporation Ltd. Curated by ChEMBL | Assay Description In vitro inhibitory activity against catechol O-methyltransferase of rat brain using 3,4-dihydroxybenzoic acid as the substrate. | J Med Chem 32: 841-6 (1989) BindingDB Entry DOI: 10.7270/Q2GF0SHP | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| Catechol O-methyltransferase (Rattus norvegicus (Rat)) | BDBM50017867 (5-Chloro-3-nitro-benzene-1,2-diol | CHEMBL355017) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | PubMed | n/a | n/a | 25 | n/a | n/a | n/a | n/a | n/a | n/a |
Orion Corporation Ltd. Curated by ChEMBL | Assay Description In vitro inhibitory activity against catechol O-methyltransferase of rat brain using 3,4-dihydroxybenzoic acid as the substrate. | J Med Chem 32: 841-6 (1989) BindingDB Entry DOI: 10.7270/Q2GF0SHP | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| Catechol O-methyltransferase (Rattus norvegicus (Rat)) | BDBM50017856 (3,4-Dihydroxy-5-nitro-benzonitrile | CHEMBL169891) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents | PubMed | n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
Orion Corporation Ltd. Curated by ChEMBL | Assay Description In vitro inhibitory activity against catechol O-methyltransferase of rat brain using 3,4-dihydroxybenzoic acid as the substrate. | J Med Chem 32: 841-6 (1989) BindingDB Entry DOI: 10.7270/Q2GF0SHP | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| Catechol O-methyltransferase (Rattus norvegicus (Rat)) | BDBM50017870 (2-Cyano-3-(3,4-dihydroxy-5-nitro-phenyl)-acrylic a...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
Orion Corporation Ltd. Curated by ChEMBL | Assay Description In vitro inhibitory activity against catechol O-methyltransferase of rat brain using 3,4-dihydroxybenzoic acid as the substrate. | J Med Chem 32: 841-6 (1989) BindingDB Entry DOI: 10.7270/Q2GF0SHP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Catechol O-methyltransferase (Rattus norvegicus (Rat)) | BDBM50017863 (CHEMBL167172 | N-Adamantan-1-yl-3-cyano-4,5-dihydr...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | PubMed | n/a | n/a | 90 | n/a | n/a | n/a | n/a | n/a | n/a |
Orion Corporation Ltd. Curated by ChEMBL | Assay Description In vitro inhibitory activity against catechol O-methyltransferase of rat brain using 3,4-dihydroxybenzoic acid as the substrate. | J Med Chem 32: 841-6 (1989) BindingDB Entry DOI: 10.7270/Q2GF0SHP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Catechol O-methyltransferase (Rattus norvegicus (Rat)) | BDBM50017853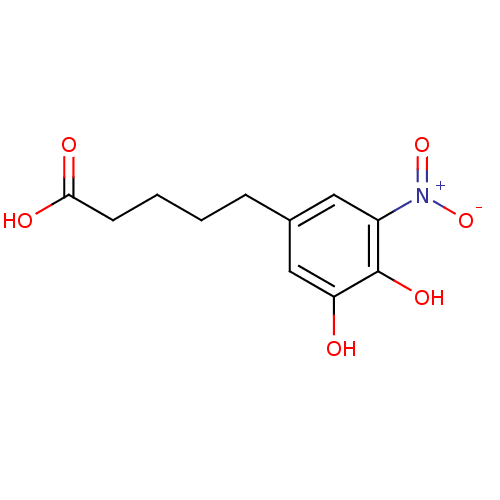 (5-(3,4-Dihydroxy-5-nitro-phenyl)-pentanoic acid | ...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | PubMed | n/a | n/a | 90 | n/a | n/a | n/a | n/a | n/a | n/a |
Orion Corporation Ltd. Curated by ChEMBL | Assay Description In vitro inhibitory activity against catechol O-methyltransferase of rat brain using 3,4-dihydroxybenzoic acid as the substrate. | J Med Chem 32: 841-6 (1989) BindingDB Entry DOI: 10.7270/Q2GF0SHP | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| Catechol O-methyltransferase (Rattus norvegicus (Rat)) | BDBM50017862 (5-Formyl-2,3-dihydroxy-benzonitrile | CHEMBL169465) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents | PubMed | n/a | n/a | 160 | n/a | n/a | n/a | n/a | n/a | n/a |
Orion Corporation Ltd. Curated by ChEMBL | Assay Description In vitro inhibitory activity against catechol O-methyltransferase of rat brain using 3,4-dihydroxybenzoic acid as the substrate. | J Med Chem 32: 841-6 (1989) BindingDB Entry DOI: 10.7270/Q2GF0SHP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Catechol O-methyltransferase (Rattus norvegicus (Rat)) | BDBM50017860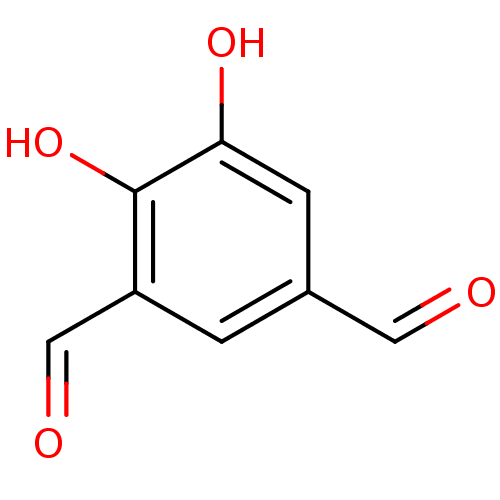 (4,5-Dihydroxy-benzene-1,3-dicarbaldehyde | CHEMBL3...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 160 | n/a | n/a | n/a | n/a | n/a | n/a |
Orion Corporation Ltd. Curated by ChEMBL | Assay Description In vitro inhibitory activity against catechol O-methyltransferase of rat brain using 3,4-dihydroxybenzoic acid as the substrate. | J Med Chem 32: 841-6 (1989) BindingDB Entry DOI: 10.7270/Q2GF0SHP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Catechol O-methyltransferase (Rattus norvegicus (Rat)) | BDBM50017869 (5-Hydroxymethyl-3-nitro-benzene-1,2-diol | CHEMBL3...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 180 | n/a | n/a | n/a | n/a | n/a | n/a |
Orion Corporation Ltd. Curated by ChEMBL | Assay Description In vitro inhibitory activity against catechol O-methyltransferase of rat brain using 3,4-dihydroxybenzoic acid as the substrate. | J Med Chem 32: 841-6 (1989) BindingDB Entry DOI: 10.7270/Q2GF0SHP | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| Catechol O-methyltransferase (Rattus norvegicus (Rat)) | BDBM50017868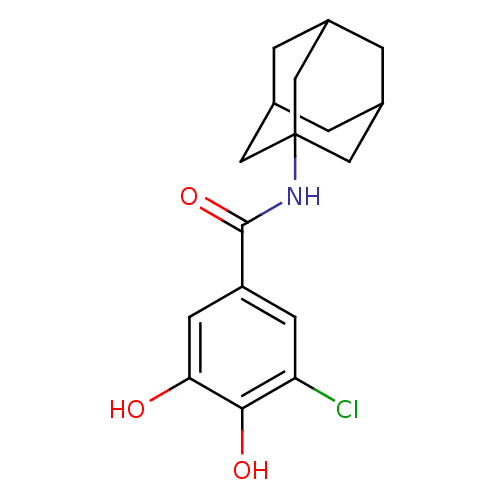 (CHEMBL168451 | N-Adamantan-1-yl-3-chloro-4,5-dihyd...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 400 | n/a | n/a | n/a | n/a | n/a | n/a |
Orion Corporation Ltd. Curated by ChEMBL | Assay Description In vitro inhibitory activity against catechol O-methyltransferase of rat brain using 3,4-dihydroxybenzoic acid as the substrate. | J Med Chem 32: 841-6 (1989) BindingDB Entry DOI: 10.7270/Q2GF0SHP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Catechol O-methyltransferase (Rattus norvegicus (Rat)) | BDBM50017858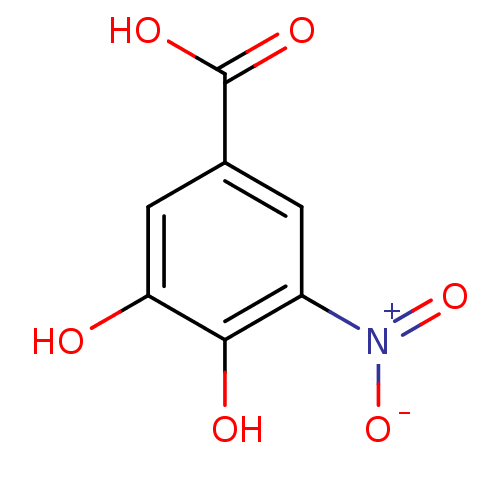 (3,4-Dihydroxy-5-nitro-benzoic acid | CHEMBL170549) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 620 | n/a | n/a | n/a | n/a | n/a | n/a |
Orion Corporation Ltd. Curated by ChEMBL | Assay Description In vitro inhibitory activity against catechol O-methyltransferase of rat brain using 3,4-dihydroxybenzoic acid as the substrate. | J Med Chem 32: 841-6 (1989) BindingDB Entry DOI: 10.7270/Q2GF0SHP | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| Catechol O-methyltransferase (Rattus norvegicus (Rat)) | BDBM50017859 (CHEMBL352270 | N-Adamantan-1-yl-4,5-dihydroxy-2-ni...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | PubMed | n/a | n/a | 1.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Orion Corporation Ltd. Curated by ChEMBL | Assay Description In vitro inhibitory activity against catechol O-methyltransferase of rat brain using 3,4-dihydroxybenzoic acid as the substrate. | J Med Chem 32: 841-6 (1989) BindingDB Entry DOI: 10.7270/Q2GF0SHP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Catechol O-methyltransferase (Rattus norvegicus (Rat)) | BDBM50017851 (3-(3,4-Dihydroxy-5-trifluoromethyl-benzylidene)-pe...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | PubMed | n/a | n/a | 1.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Orion Corporation Ltd. Curated by ChEMBL | Assay Description In vitro inhibitory activity against catechol O-methyltransferase of rat brain using 3,4-dihydroxybenzoic acid as the substrate. | J Med Chem 32: 841-6 (1989) BindingDB Entry DOI: 10.7270/Q2GF0SHP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Catechol O-methyltransferase (Rattus norvegicus (Rat)) | BDBM50017847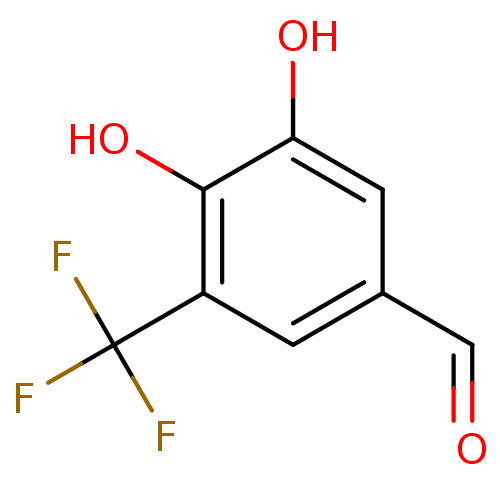 (3,4-Dihydroxy-5-trifluoromethyl-benzaldehyde | CHE...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | PubMed | n/a | n/a | 2.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Orion Corporation Ltd. Curated by ChEMBL | Assay Description In vitro inhibitory activity against catechol O-methyltransferase of rat brain using 3,4-dihydroxybenzoic acid as the substrate. | J Med Chem 32: 841-6 (1989) BindingDB Entry DOI: 10.7270/Q2GF0SHP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Catechol O-methyltransferase (Rattus norvegicus (Rat)) | BDBM50017849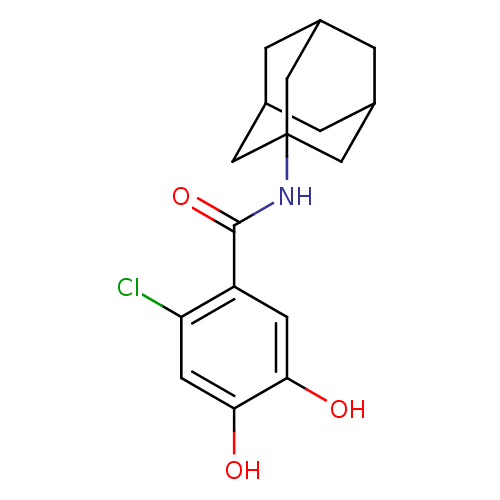 (CHEMBL169679 | N-Adamantan-1-yl-2-chloro-4,5-dihyd...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 2.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Orion Corporation Ltd. Curated by ChEMBL | Assay Description In vitro inhibitory activity against catechol O-methyltransferase of rat brain using 3,4-dihydroxybenzoic acid as the substrate. | J Med Chem 32: 841-6 (1989) BindingDB Entry DOI: 10.7270/Q2GF0SHP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Catechol O-methyltransferase (Rattus norvegicus (Rat)) | BDBM50017850 (1-(3,4-Dihydroxy-phenyl)-2-methyl-propan-1-one | C...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid UniChem Patents | PubMed | n/a | n/a | 6.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Orion Corporation Ltd. Curated by ChEMBL | Assay Description In vitro inhibitory activity against catechol O-methyltransferase of rat brain using 3,4-dihydroxybenzoic acid as the substrate. | J Med Chem 32: 841-6 (1989) BindingDB Entry DOI: 10.7270/Q2GF0SHP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Catechol O-methyltransferase (Rattus norvegicus (Rat)) | BDBM50017855 (5-(3-Chloro-4,5-dihydroxy-phenyl)-pentanoic acid |...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | PubMed | n/a | n/a | 8.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Orion Corporation Ltd. Curated by ChEMBL | Assay Description In vitro inhibitory activity against catechol O-methyltransferase of rat brain using 3,4-dihydroxybenzoic acid as the substrate. | J Med Chem 32: 841-6 (1989) BindingDB Entry DOI: 10.7270/Q2GF0SHP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Catechol O-methyltransferase (Rattus norvegicus (Rat)) | BDBM50017854 (CHEMBL169208 | N-Adamantan-1-yl-2-chloro-3,4-dihyd...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Orion Corporation Ltd. Curated by ChEMBL | Assay Description In vitro inhibitory activity against catechol O-methyltransferase of rat brain using 3,4-dihydroxybenzoic acid as the substrate. | J Med Chem 32: 841-6 (1989) BindingDB Entry DOI: 10.7270/Q2GF0SHP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Catechol O-methyltransferase (Rattus norvegicus (Rat)) | BDBM50017864 (3,4-Dihydroxy-5-methanesulfonyl-benzaldehyde | CHE...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | PubMed | n/a | n/a | 2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Orion Corporation Ltd. Curated by ChEMBL | Assay Description In vitro inhibitory activity against catechol O-methyltransferase of rat brain using 3,4-dihydroxybenzoic acid as the substrate. | J Med Chem 32: 841-6 (1989) BindingDB Entry DOI: 10.7270/Q2GF0SHP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine 3-monooxygenase (Bos taurus) | BDBM50017845 (3-(3,4-Dihydroxy-5-nitro-phenyl)-1-(3,4,5-trimetho...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 2.10E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Orion Corporation Ltd. Curated by ChEMBL | Assay Description Inhibitory activity against the tyrosine hydroxylase | J Med Chem 32: 841-6 (1989) BindingDB Entry DOI: 10.7270/Q2GF0SHP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine 3-monooxygenase (Bos taurus) | BDBM50017850 (1-(3,4-Dihydroxy-phenyl)-2-methyl-propan-1-one | C...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid UniChem Patents | PubMed | n/a | n/a | 2.40E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Orion Corporation Ltd. Curated by ChEMBL | Assay Description Inhibitory activity against the tyrosine hydroxylase | J Med Chem 32: 841-6 (1989) BindingDB Entry DOI: 10.7270/Q2GF0SHP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] B (Rattus norvegicus (rat)) | BDBM50017845 (3-(3,4-Dihydroxy-5-nitro-phenyl)-1-(3,4,5-trimetho...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Orion Corporation Ltd. Curated by ChEMBL | Assay Description Inhibitory activity against the Monoamine oxidase B | J Med Chem 32: 841-6 (1989) BindingDB Entry DOI: 10.7270/Q2GF0SHP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] B (Rattus norvegicus (rat)) | BDBM50017857 (3-(3,4-Dihydroxy-5-nitro-phenyl)-1-phenyl-propenon...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Orion Corporation Ltd. Curated by ChEMBL | Assay Description Inhibitory activity against the Monoamine oxidase B | J Med Chem 32: 841-6 (1989) BindingDB Entry DOI: 10.7270/Q2GF0SHP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatic-L-amino-acid decarboxylase (Sus scrofa) | BDBM50017850 (1-(3,4-Dihydroxy-phenyl)-2-methyl-propan-1-one | C...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid UniChem Patents | PubMed | n/a | n/a | 5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Orion Corporation Ltd. Curated by ChEMBL | Assay Description Inhibitory activity against the dopamine decarboxylase | J Med Chem 32: 841-6 (1989) BindingDB Entry DOI: 10.7270/Q2GF0SHP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dopamine beta-hydroxylase (Homo sapiens (Human)) | BDBM50017857 (3-(3,4-Dihydroxy-5-nitro-phenyl)-1-phenyl-propenon...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Orion Corporation Ltd. Curated by ChEMBL | Assay Description Inhibitory activity against the dopamine beta hydroxylase | J Med Chem 32: 841-6 (1989) BindingDB Entry DOI: 10.7270/Q2GF0SHP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatic-L-amino-acid decarboxylase (Sus scrofa) | BDBM50017857 (3-(3,4-Dihydroxy-5-nitro-phenyl)-1-phenyl-propenon...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Orion Corporation Ltd. Curated by ChEMBL | Assay Description Inhibitory activity against the dopamine decarboxylase | J Med Chem 32: 841-6 (1989) BindingDB Entry DOI: 10.7270/Q2GF0SHP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] A (Mus musculus) | BDBM50017845 (3-(3,4-Dihydroxy-5-nitro-phenyl)-1-(3,4,5-trimetho...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Orion Corporation Ltd. Curated by ChEMBL | Assay Description Inhibitory activity against the Monoamine oxidase A | J Med Chem 32: 841-6 (1989) BindingDB Entry DOI: 10.7270/Q2GF0SHP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] B (Rattus norvegicus (rat)) | BDBM50017850 (1-(3,4-Dihydroxy-phenyl)-2-methyl-propan-1-one | C...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid UniChem Patents | PubMed | n/a | n/a | 5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Orion Corporation Ltd. Curated by ChEMBL | Assay Description Inhibitory activity against the Monoamine oxidase B | J Med Chem 32: 841-6 (1989) BindingDB Entry DOI: 10.7270/Q2GF0SHP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dopamine beta-hydroxylase (Homo sapiens (Human)) | BDBM50017845 (3-(3,4-Dihydroxy-5-nitro-phenyl)-1-(3,4,5-trimetho...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Orion Corporation Ltd. Curated by ChEMBL | Assay Description Inhibitory activity against the dopamine beta hydroxylase | J Med Chem 32: 841-6 (1989) BindingDB Entry DOI: 10.7270/Q2GF0SHP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dopamine beta-hydroxylase (Homo sapiens (Human)) | BDBM50017850 (1-(3,4-Dihydroxy-phenyl)-2-methyl-propan-1-one | C...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid UniChem Patents | PubMed | n/a | n/a | 5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Orion Corporation Ltd. Curated by ChEMBL | Assay Description Inhibitory activity against the dopamine beta hydroxylase | J Med Chem 32: 841-6 (1989) BindingDB Entry DOI: 10.7270/Q2GF0SHP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] A (Mus musculus) | BDBM50017850 (1-(3,4-Dihydroxy-phenyl)-2-methyl-propan-1-one | C...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid UniChem Patents | PubMed | n/a | n/a | 5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Orion Corporation Ltd. Curated by ChEMBL | Assay Description Inhibitory activity against the Monoamine oxidase A | J Med Chem 32: 841-6 (1989) BindingDB Entry DOI: 10.7270/Q2GF0SHP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] A (Mus musculus) | BDBM50017857 (3-(3,4-Dihydroxy-5-nitro-phenyl)-1-phenyl-propenon...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Orion Corporation Ltd. Curated by ChEMBL | Assay Description Inhibitory activity against the Monoamine oxidase A | J Med Chem 32: 841-6 (1989) BindingDB Entry DOI: 10.7270/Q2GF0SHP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatic-L-amino-acid decarboxylase (Sus scrofa) | BDBM50017845 (3-(3,4-Dihydroxy-5-nitro-phenyl)-1-(3,4,5-trimetho...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Orion Corporation Ltd. Curated by ChEMBL | Assay Description Inhibitory activity against the dopamine decarboxylase | J Med Chem 32: 841-6 (1989) BindingDB Entry DOI: 10.7270/Q2GF0SHP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine 3-monooxygenase (Bos taurus) | BDBM50017857 (3-(3,4-Dihydroxy-5-nitro-phenyl)-1-phenyl-propenon...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Orion Corporation Ltd. Curated by ChEMBL | Assay Description Inhibitory activity against the tyrosine hydroxylase | J Med Chem 32: 841-6 (1989) BindingDB Entry DOI: 10.7270/Q2GF0SHP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||