

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cholinesterase (Equus caballus (Horse)) | BDBM8961 (1,2,3,4-tetrahydro-9-acridinamine | 1,2,3,4-tetrah...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 5.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Wuhan University of Technology Curated by ChEMBL | Assay Description Inhibition of horse serum BChE using butrylthiocholine iodide as substrate preincubated for 5 mins followed by substrate addition measured for 10 min... | Bioorg Med Chem 24: 2352-9 (2016) Article DOI: 10.1016/j.bmc.2016.04.015 BindingDB Entry DOI: 10.7270/Q2S46TVJ | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50161803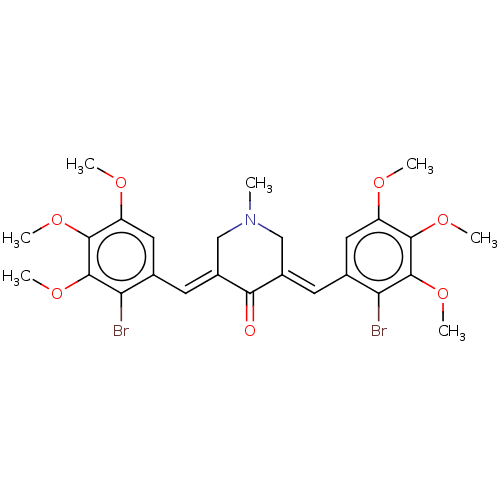 (CHEMBL3785375) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 37 | n/a | n/a | n/a | n/a | n/a | n/a |
Wuhan University of Technology Curated by ChEMBL | Assay Description Inhibition of electric eel AChE using acetylthiocholine iodide as substrate preincubated for 5 mins followed by substrate addition measured for 10 mi... | Bioorg Med Chem 24: 2352-9 (2016) Article DOI: 10.1016/j.bmc.2016.04.015 BindingDB Entry DOI: 10.7270/Q2S46TVJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM8961 (1,2,3,4-tetrahydro-9-acridinamine | 1,2,3,4-tetrah...) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 38 | n/a | n/a | n/a | n/a | n/a | n/a |
Wuhan University of Technology Curated by ChEMBL | Assay Description Inhibition of electric eel AChE using acetylthiocholine iodide as substrate preincubated for 5 mins followed by substrate addition measured for 10 mi... | Bioorg Med Chem 24: 2352-9 (2016) Article DOI: 10.1016/j.bmc.2016.04.015 BindingDB Entry DOI: 10.7270/Q2S46TVJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM8960 ((+/-)-2-[(1-benzylpiperidin-4-yl)methyl]-5,6-dimet...) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 57 | n/a | n/a | n/a | n/a | n/a | n/a |
Wuhan University of Technology Curated by ChEMBL | Assay Description Inhibition of electric eel AChE using acetylthiocholine iodide as substrate preincubated for 5 mins followed by substrate addition measured for 10 mi... | Bioorg Med Chem 24: 2352-9 (2016) Article DOI: 10.1016/j.bmc.2016.04.015 BindingDB Entry DOI: 10.7270/Q2S46TVJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50161797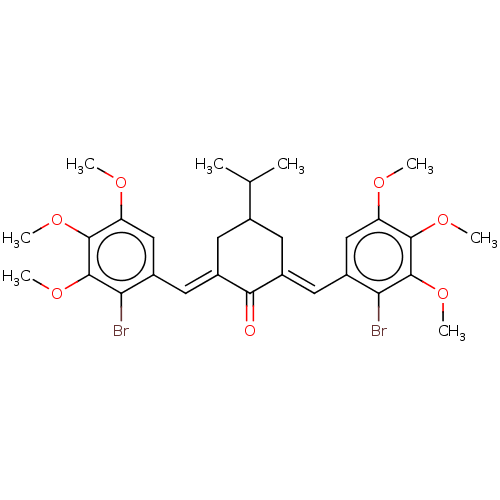 (CHEMBL3785402) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 510 | n/a | n/a | n/a | n/a | n/a | n/a |
Wuhan University of Technology Curated by ChEMBL | Assay Description Inhibition of electric eel AChE using acetylthiocholine iodide as substrate preincubated for 5 mins followed by substrate addition measured for 10 mi... | Bioorg Med Chem 24: 2352-9 (2016) Article DOI: 10.1016/j.bmc.2016.04.015 BindingDB Entry DOI: 10.7270/Q2S46TVJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50161802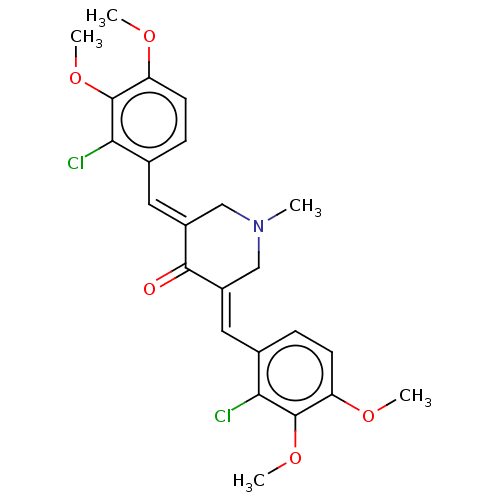 (CHEMBL3787299) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 630 | n/a | n/a | n/a | n/a | n/a | n/a |
Wuhan University of Technology Curated by ChEMBL | Assay Description Inhibition of electric eel AChE using acetylthiocholine iodide as substrate preincubated for 5 mins followed by substrate addition measured for 10 mi... | Bioorg Med Chem 24: 2352-9 (2016) Article DOI: 10.1016/j.bmc.2016.04.015 BindingDB Entry DOI: 10.7270/Q2S46TVJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50161796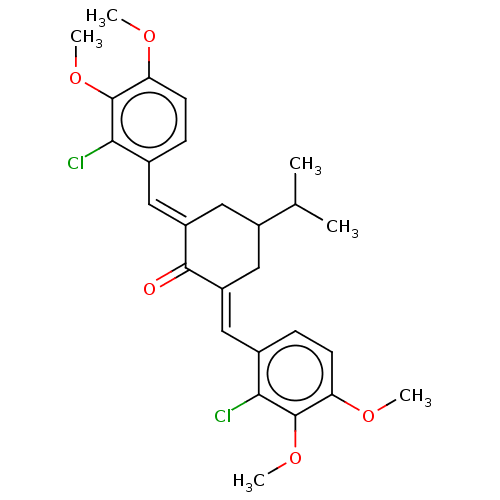 (CHEMBL3787250) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 920 | n/a | n/a | n/a | n/a | n/a | n/a |
Wuhan University of Technology Curated by ChEMBL | Assay Description Inhibition of electric eel AChE using acetylthiocholine iodide as substrate preincubated for 5 mins followed by substrate addition measured for 10 mi... | Bioorg Med Chem 24: 2352-9 (2016) Article DOI: 10.1016/j.bmc.2016.04.015 BindingDB Entry DOI: 10.7270/Q2S46TVJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50161801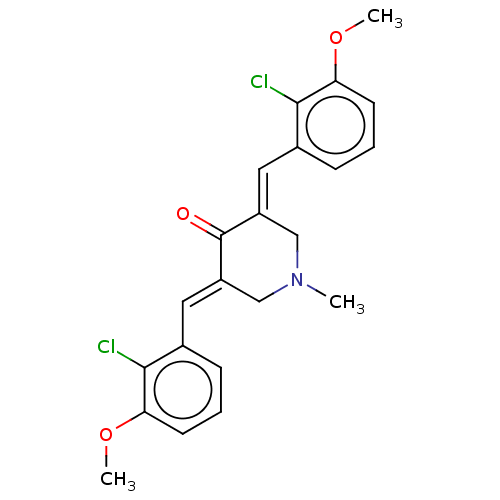 (CHEMBL3787510) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Wuhan University of Technology Curated by ChEMBL | Assay Description Inhibition of electric eel AChE using acetylthiocholine iodide as substrate preincubated for 5 mins followed by substrate addition measured for 10 mi... | Bioorg Med Chem 24: 2352-9 (2016) Article DOI: 10.1016/j.bmc.2016.04.015 BindingDB Entry DOI: 10.7270/Q2S46TVJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50161795 (CHEMBL3787274) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Wuhan University of Technology Curated by ChEMBL | Assay Description Inhibition of electric eel AChE using acetylthiocholine iodide as substrate preincubated for 5 mins followed by substrate addition measured for 10 mi... | Bioorg Med Chem 24: 2352-9 (2016) Article DOI: 10.1016/j.bmc.2016.04.015 BindingDB Entry DOI: 10.7270/Q2S46TVJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50161809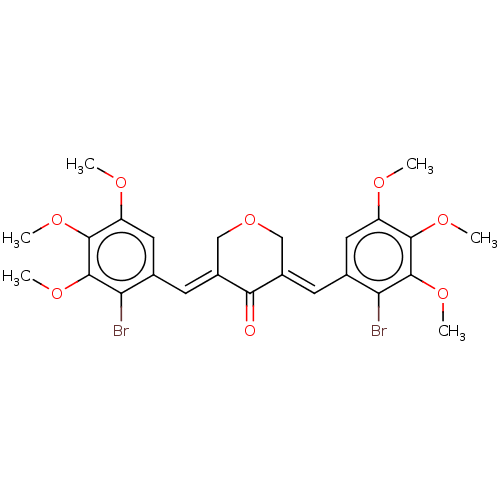 (CHEMBL3787119) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Wuhan University of Technology Curated by ChEMBL | Assay Description Inhibition of electric eel AChE using acetylthiocholine iodide as substrate preincubated for 5 mins followed by substrate addition measured for 10 mi... | Bioorg Med Chem 24: 2352-9 (2016) Article DOI: 10.1016/j.bmc.2016.04.015 BindingDB Entry DOI: 10.7270/Q2S46TVJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50161794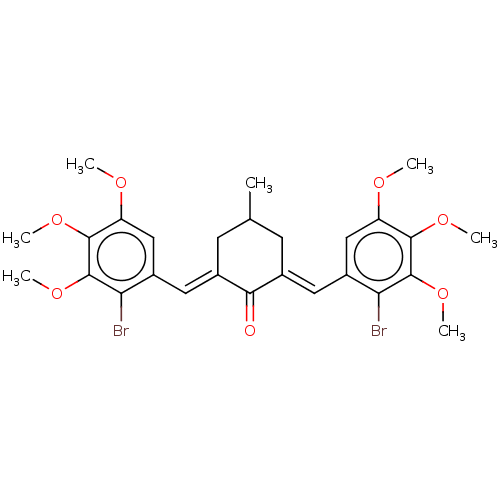 (CHEMBL3786129) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Wuhan University of Technology Curated by ChEMBL | Assay Description Inhibition of electric eel AChE using acetylthiocholine iodide as substrate preincubated for 5 mins followed by substrate addition measured for 10 mi... | Bioorg Med Chem 24: 2352-9 (2016) Article DOI: 10.1016/j.bmc.2016.04.015 BindingDB Entry DOI: 10.7270/Q2S46TVJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50161800 (CHEMBL3786742) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Wuhan University of Technology Curated by ChEMBL | Assay Description Inhibition of electric eel AChE using acetylthiocholine iodide as substrate preincubated for 5 mins followed by substrate addition measured for 10 mi... | Bioorg Med Chem 24: 2352-9 (2016) Article DOI: 10.1016/j.bmc.2016.04.015 BindingDB Entry DOI: 10.7270/Q2S46TVJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50161793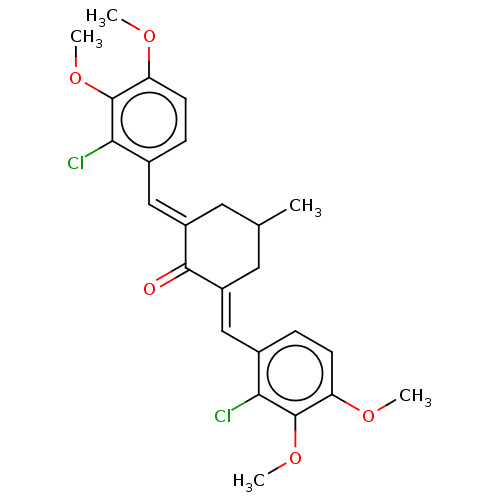 (CHEMBL3785504) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Wuhan University of Technology Curated by ChEMBL | Assay Description Inhibition of electric eel AChE using acetylthiocholine iodide as substrate preincubated for 5 mins followed by substrate addition measured for 10 mi... | Bioorg Med Chem 24: 2352-9 (2016) Article DOI: 10.1016/j.bmc.2016.04.015 BindingDB Entry DOI: 10.7270/Q2S46TVJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50161802 (CHEMBL3787299) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Wuhan University of Technology Curated by ChEMBL | Assay Description Inhibition of horse serum BChE using butrylthiocholine iodide as substrate preincubated for 5 mins followed by substrate addition measured for 10 min... | Bioorg Med Chem 24: 2352-9 (2016) Article DOI: 10.1016/j.bmc.2016.04.015 BindingDB Entry DOI: 10.7270/Q2S46TVJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50161799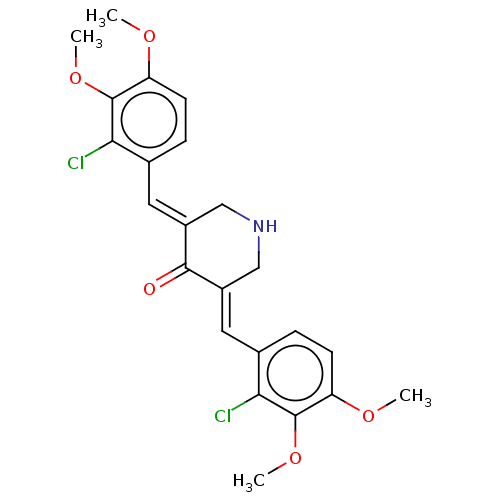 (CHEMBL3787626) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Wuhan University of Technology Curated by ChEMBL | Assay Description Inhibition of electric eel AChE using acetylthiocholine iodide as substrate preincubated for 5 mins followed by substrate addition measured for 10 mi... | Bioorg Med Chem 24: 2352-9 (2016) Article DOI: 10.1016/j.bmc.2016.04.015 BindingDB Entry DOI: 10.7270/Q2S46TVJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50161798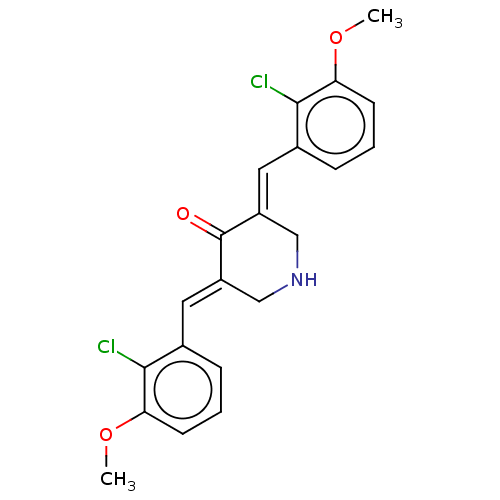 (CHEMBL3785522) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Wuhan University of Technology Curated by ChEMBL | Assay Description Inhibition of electric eel AChE using acetylthiocholine iodide as substrate preincubated for 5 mins followed by substrate addition measured for 10 mi... | Bioorg Med Chem 24: 2352-9 (2016) Article DOI: 10.1016/j.bmc.2016.04.015 BindingDB Entry DOI: 10.7270/Q2S46TVJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50161792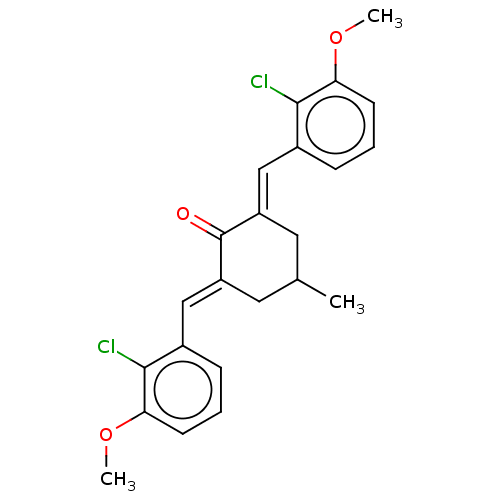 (CHEMBL3786205) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Wuhan University of Technology Curated by ChEMBL | Assay Description Inhibition of electric eel AChE using acetylthiocholine iodide as substrate preincubated for 5 mins followed by substrate addition measured for 10 mi... | Bioorg Med Chem 24: 2352-9 (2016) Article DOI: 10.1016/j.bmc.2016.04.015 BindingDB Entry DOI: 10.7270/Q2S46TVJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM8960 ((+/-)-2-[(1-benzylpiperidin-4-yl)methyl]-5,6-dimet...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 6.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Wuhan University of Technology Curated by ChEMBL | Assay Description Inhibition of horse serum BChE using butrylthiocholine iodide as substrate preincubated for 5 mins followed by substrate addition measured for 10 min... | Bioorg Med Chem 24: 2352-9 (2016) Article DOI: 10.1016/j.bmc.2016.04.015 BindingDB Entry DOI: 10.7270/Q2S46TVJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50161808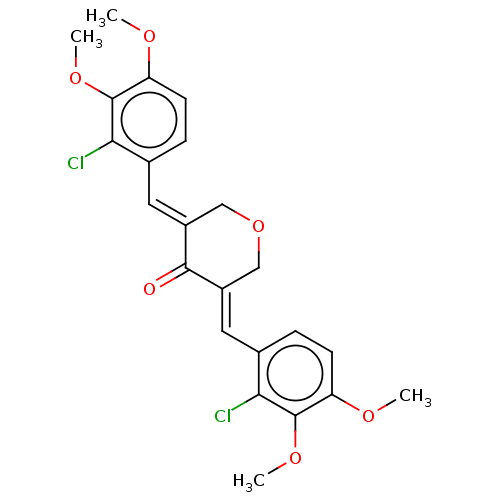 (CHEMBL3785460) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 7.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Wuhan University of Technology Curated by ChEMBL | Assay Description Inhibition of electric eel AChE using acetylthiocholine iodide as substrate preincubated for 5 mins followed by substrate addition measured for 10 mi... | Bioorg Med Chem 24: 2352-9 (2016) Article DOI: 10.1016/j.bmc.2016.04.015 BindingDB Entry DOI: 10.7270/Q2S46TVJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50161807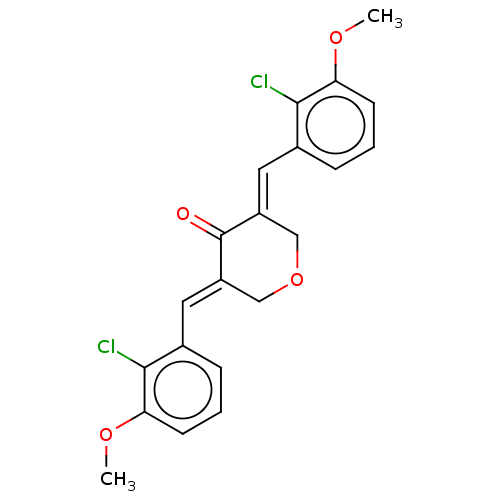 (CHEMBL3786918) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 9.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Wuhan University of Technology Curated by ChEMBL | Assay Description Inhibition of electric eel AChE using acetylthiocholine iodide as substrate preincubated for 5 mins followed by substrate addition measured for 10 mi... | Bioorg Med Chem 24: 2352-9 (2016) Article DOI: 10.1016/j.bmc.2016.04.015 BindingDB Entry DOI: 10.7270/Q2S46TVJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50161803 (CHEMBL3785375) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.05E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Wuhan University of Technology Curated by ChEMBL | Assay Description Inhibition of horse serum BChE using butrylthiocholine iodide as substrate preincubated for 5 mins followed by substrate addition measured for 10 min... | Bioorg Med Chem 24: 2352-9 (2016) Article DOI: 10.1016/j.bmc.2016.04.015 BindingDB Entry DOI: 10.7270/Q2S46TVJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50161793 (CHEMBL3785504) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.12E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Wuhan University of Technology Curated by ChEMBL | Assay Description Inhibition of horse serum BChE using butrylthiocholine iodide as substrate preincubated for 5 mins followed by substrate addition measured for 10 min... | Bioorg Med Chem 24: 2352-9 (2016) Article DOI: 10.1016/j.bmc.2016.04.015 BindingDB Entry DOI: 10.7270/Q2S46TVJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50161774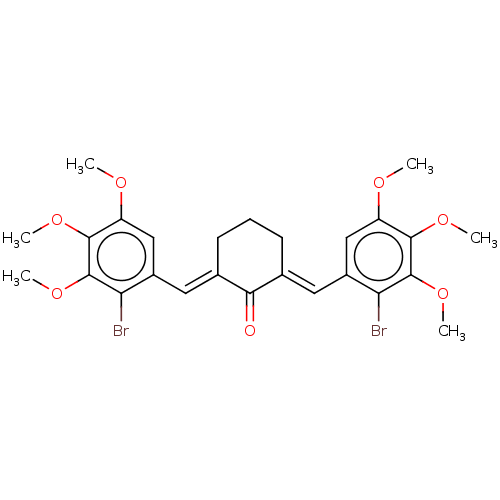 (CHEMBL3785268) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.26E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Wuhan University of Technology Curated by ChEMBL | Assay Description Inhibition of electric eel AChE using acetylthiocholine iodide as substrate preincubated for 5 mins followed by substrate addition measured for 10 mi... | Bioorg Med Chem 24: 2352-9 (2016) Article DOI: 10.1016/j.bmc.2016.04.015 BindingDB Entry DOI: 10.7270/Q2S46TVJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50161799 (CHEMBL3787626) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.67E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Wuhan University of Technology Curated by ChEMBL | Assay Description Inhibition of horse serum BChE using butrylthiocholine iodide as substrate preincubated for 5 mins followed by substrate addition measured for 10 min... | Bioorg Med Chem 24: 2352-9 (2016) Article DOI: 10.1016/j.bmc.2016.04.015 BindingDB Entry DOI: 10.7270/Q2S46TVJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50161773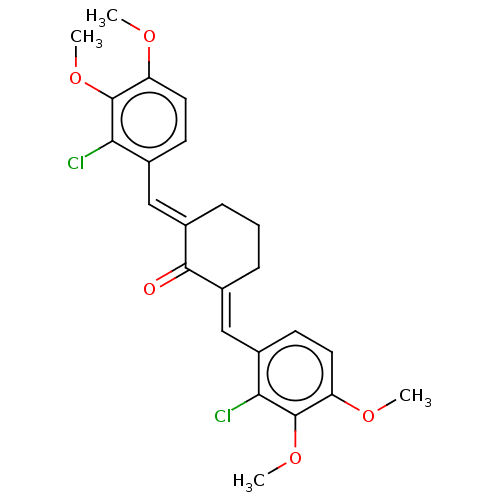 (CHEMBL3786643) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.75E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Wuhan University of Technology Curated by ChEMBL | Assay Description Inhibition of electric eel AChE using acetylthiocholine iodide as substrate preincubated for 5 mins followed by substrate addition measured for 10 mi... | Bioorg Med Chem 24: 2352-9 (2016) Article DOI: 10.1016/j.bmc.2016.04.015 BindingDB Entry DOI: 10.7270/Q2S46TVJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50161794 (CHEMBL3786129) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.95E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Wuhan University of Technology Curated by ChEMBL | Assay Description Inhibition of horse serum BChE using butrylthiocholine iodide as substrate preincubated for 5 mins followed by substrate addition measured for 10 min... | Bioorg Med Chem 24: 2352-9 (2016) Article DOI: 10.1016/j.bmc.2016.04.015 BindingDB Entry DOI: 10.7270/Q2S46TVJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50161772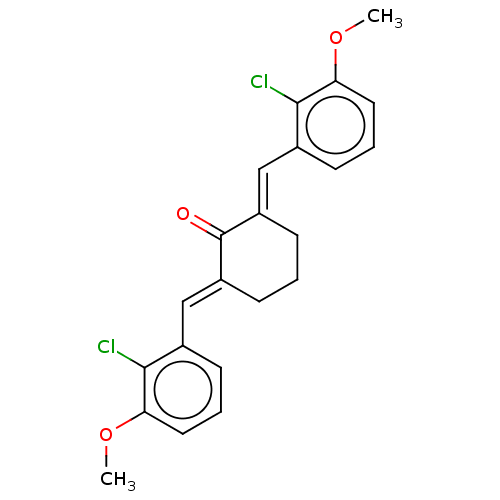 (CHEMBL3786095) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.24E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Wuhan University of Technology Curated by ChEMBL | Assay Description Inhibition of electric eel AChE using acetylthiocholine iodide as substrate preincubated for 5 mins followed by substrate addition measured for 10 mi... | Bioorg Med Chem 24: 2352-9 (2016) Article DOI: 10.1016/j.bmc.2016.04.015 BindingDB Entry DOI: 10.7270/Q2S46TVJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50161792 (CHEMBL3786205) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.97E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Wuhan University of Technology Curated by ChEMBL | Assay Description Inhibition of horse serum BChE using butrylthiocholine iodide as substrate preincubated for 5 mins followed by substrate addition measured for 10 min... | Bioorg Med Chem 24: 2352-9 (2016) Article DOI: 10.1016/j.bmc.2016.04.015 BindingDB Entry DOI: 10.7270/Q2S46TVJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50161800 (CHEMBL3786742) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6.88E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Wuhan University of Technology Curated by ChEMBL | Assay Description Inhibition of horse serum BChE using butrylthiocholine iodide as substrate preincubated for 5 mins followed by substrate addition measured for 10 min... | Bioorg Med Chem 24: 2352-9 (2016) Article DOI: 10.1016/j.bmc.2016.04.015 BindingDB Entry DOI: 10.7270/Q2S46TVJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50161806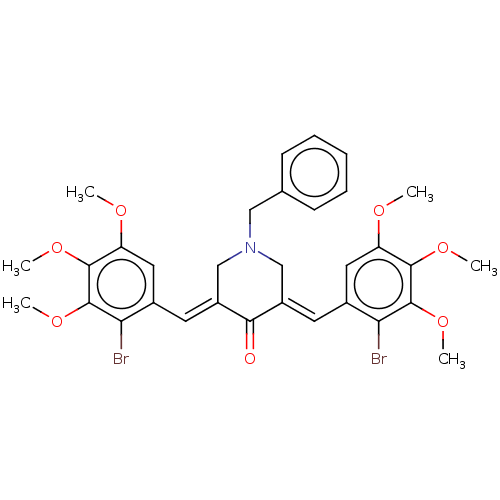 (CHEMBL3786242) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Wuhan University of Technology Curated by ChEMBL | Assay Description Inhibition of electric eel AChE using acetylthiocholine iodide as substrate preincubated for 5 mins followed by substrate addition measured for 10 mi... | Bioorg Med Chem 24: 2352-9 (2016) Article DOI: 10.1016/j.bmc.2016.04.015 BindingDB Entry DOI: 10.7270/Q2S46TVJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50161808 (CHEMBL3785460) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Wuhan University of Technology Curated by ChEMBL | Assay Description Inhibition of horse serum BChE using butrylthiocholine iodide as substrate preincubated for 5 mins followed by substrate addition measured for 10 min... | Bioorg Med Chem 24: 2352-9 (2016) Article DOI: 10.1016/j.bmc.2016.04.015 BindingDB Entry DOI: 10.7270/Q2S46TVJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50161806 (CHEMBL3786242) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Wuhan University of Technology Curated by ChEMBL | Assay Description Inhibition of horse serum BChE using butrylthiocholine iodide as substrate preincubated for 5 mins followed by substrate addition measured for 10 min... | Bioorg Med Chem 24: 2352-9 (2016) Article DOI: 10.1016/j.bmc.2016.04.015 BindingDB Entry DOI: 10.7270/Q2S46TVJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50161805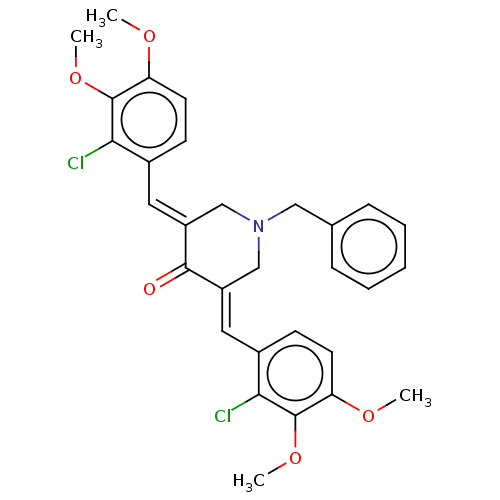 (CHEMBL3787471) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Wuhan University of Technology Curated by ChEMBL | Assay Description Inhibition of electric eel AChE using acetylthiocholine iodide as substrate preincubated for 5 mins followed by substrate addition measured for 10 mi... | Bioorg Med Chem 24: 2352-9 (2016) Article DOI: 10.1016/j.bmc.2016.04.015 BindingDB Entry DOI: 10.7270/Q2S46TVJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50161796 (CHEMBL3787250) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Wuhan University of Technology Curated by ChEMBL | Assay Description Inhibition of horse serum BChE using butrylthiocholine iodide as substrate preincubated for 5 mins followed by substrate addition measured for 10 min... | Bioorg Med Chem 24: 2352-9 (2016) Article DOI: 10.1016/j.bmc.2016.04.015 BindingDB Entry DOI: 10.7270/Q2S46TVJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50161805 (CHEMBL3787471) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Wuhan University of Technology Curated by ChEMBL | Assay Description Inhibition of horse serum BChE using butrylthiocholine iodide as substrate preincubated for 5 mins followed by substrate addition measured for 10 min... | Bioorg Med Chem 24: 2352-9 (2016) Article DOI: 10.1016/j.bmc.2016.04.015 BindingDB Entry DOI: 10.7270/Q2S46TVJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50161774 (CHEMBL3785268) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Wuhan University of Technology Curated by ChEMBL | Assay Description Inhibition of horse serum BChE using butrylthiocholine iodide as substrate preincubated for 5 mins followed by substrate addition measured for 10 min... | Bioorg Med Chem 24: 2352-9 (2016) Article DOI: 10.1016/j.bmc.2016.04.015 BindingDB Entry DOI: 10.7270/Q2S46TVJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50161804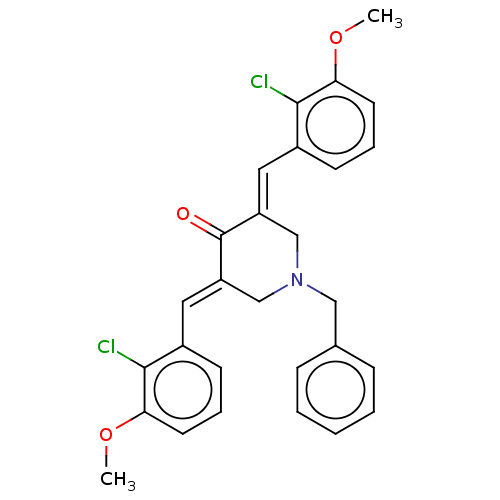 (CHEMBL3786212) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Wuhan University of Technology Curated by ChEMBL | Assay Description Inhibition of horse serum BChE using butrylthiocholine iodide as substrate preincubated for 5 mins followed by substrate addition measured for 10 min... | Bioorg Med Chem 24: 2352-9 (2016) Article DOI: 10.1016/j.bmc.2016.04.015 BindingDB Entry DOI: 10.7270/Q2S46TVJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50161797 (CHEMBL3785402) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Wuhan University of Technology Curated by ChEMBL | Assay Description Inhibition of horse serum BChE using butrylthiocholine iodide as substrate preincubated for 5 mins followed by substrate addition measured for 10 min... | Bioorg Med Chem 24: 2352-9 (2016) Article DOI: 10.1016/j.bmc.2016.04.015 BindingDB Entry DOI: 10.7270/Q2S46TVJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50161773 (CHEMBL3786643) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Wuhan University of Technology Curated by ChEMBL | Assay Description Inhibition of horse serum BChE using butrylthiocholine iodide as substrate preincubated for 5 mins followed by substrate addition measured for 10 min... | Bioorg Med Chem 24: 2352-9 (2016) Article DOI: 10.1016/j.bmc.2016.04.015 BindingDB Entry DOI: 10.7270/Q2S46TVJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50161801 (CHEMBL3787510) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Wuhan University of Technology Curated by ChEMBL | Assay Description Inhibition of horse serum BChE using butrylthiocholine iodide as substrate preincubated for 5 mins followed by substrate addition measured for 10 min... | Bioorg Med Chem 24: 2352-9 (2016) Article DOI: 10.1016/j.bmc.2016.04.015 BindingDB Entry DOI: 10.7270/Q2S46TVJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50161809 (CHEMBL3787119) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Wuhan University of Technology Curated by ChEMBL | Assay Description Inhibition of horse serum BChE using butrylthiocholine iodide as substrate preincubated for 5 mins followed by substrate addition measured for 10 min... | Bioorg Med Chem 24: 2352-9 (2016) Article DOI: 10.1016/j.bmc.2016.04.015 BindingDB Entry DOI: 10.7270/Q2S46TVJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50161807 (CHEMBL3786918) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Wuhan University of Technology Curated by ChEMBL | Assay Description Inhibition of horse serum BChE using butrylthiocholine iodide as substrate preincubated for 5 mins followed by substrate addition measured for 10 min... | Bioorg Med Chem 24: 2352-9 (2016) Article DOI: 10.1016/j.bmc.2016.04.015 BindingDB Entry DOI: 10.7270/Q2S46TVJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50161795 (CHEMBL3787274) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Wuhan University of Technology Curated by ChEMBL | Assay Description Inhibition of horse serum BChE using butrylthiocholine iodide as substrate preincubated for 5 mins followed by substrate addition measured for 10 min... | Bioorg Med Chem 24: 2352-9 (2016) Article DOI: 10.1016/j.bmc.2016.04.015 BindingDB Entry DOI: 10.7270/Q2S46TVJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50161772 (CHEMBL3786095) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Wuhan University of Technology Curated by ChEMBL | Assay Description Inhibition of horse serum BChE using butrylthiocholine iodide as substrate preincubated for 5 mins followed by substrate addition measured for 10 min... | Bioorg Med Chem 24: 2352-9 (2016) Article DOI: 10.1016/j.bmc.2016.04.015 BindingDB Entry DOI: 10.7270/Q2S46TVJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50161798 (CHEMBL3785522) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Wuhan University of Technology Curated by ChEMBL | Assay Description Inhibition of horse serum BChE using butrylthiocholine iodide as substrate preincubated for 5 mins followed by substrate addition measured for 10 min... | Bioorg Med Chem 24: 2352-9 (2016) Article DOI: 10.1016/j.bmc.2016.04.015 BindingDB Entry DOI: 10.7270/Q2S46TVJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50161804 (CHEMBL3786212) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Wuhan University of Technology Curated by ChEMBL | Assay Description Inhibition of electric eel AChE using acetylthiocholine iodide as substrate preincubated for 5 mins followed by substrate addition measured for 10 mi... | Bioorg Med Chem 24: 2352-9 (2016) Article DOI: 10.1016/j.bmc.2016.04.015 BindingDB Entry DOI: 10.7270/Q2S46TVJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||