

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Branched-chain-amino-acid aminotransferase, mitochondrial (Homo sapiens (Human)) | BDBM50173927 (CHEMBL3808902) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of human BCATm (28 to 392 residues) using L-Leucine and alpha-ketogluterate as substrate assessed as L-glutamate production after 10 mins ... | ACS Med Chem Lett 7: 379-84 (2016) Article DOI: 10.1021/acsmedchemlett.5b00389 BindingDB Entry DOI: 10.7270/Q2W097V6 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Branched-chain-amino-acid aminotransferase, mitochondrial (Homo sapiens (Human)) | BDBM50173927 (CHEMBL3808902) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | 50.1 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of human BCATm (28 to 392 residues) using L-Leucine and alpha-ketogluterate as substrate assessed as L-glutamate production after 10 mins ... | ACS Med Chem Lett 7: 379-84 (2016) Article DOI: 10.1021/acsmedchemlett.5b00389 BindingDB Entry DOI: 10.7270/Q2W097V6 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Branched-chain-amino-acid aminotransferase, mitochondrial (Homo sapiens (Human)) | BDBM50173926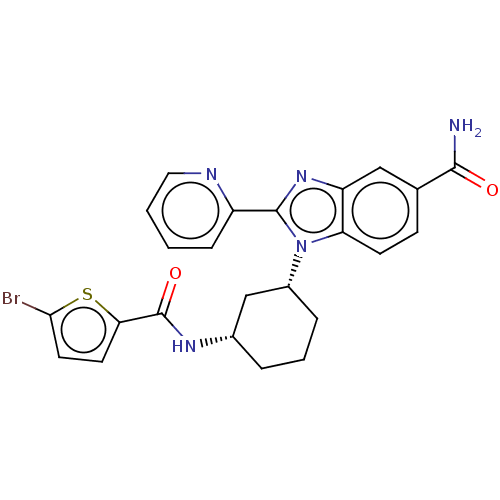 (CHEMBL3808971) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 63 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of human BCATm (28 to 392 residues) using L-Leucine and alpha-ketogluterate as substrate assessed as L-glutamate production after 10 mins ... | ACS Med Chem Lett 7: 379-84 (2016) Article DOI: 10.1021/acsmedchemlett.5b00389 BindingDB Entry DOI: 10.7270/Q2W097V6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Branched-chain-amino-acid aminotransferase, mitochondrial (Homo sapiens (Human)) | BDBM50173928 (CHEMBL3809839) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 63 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of human BCATm (28 to 392 residues) using L-Leucine and alpha-ketogluterate as substrate assessed as L-glutamate production after 10 mins ... | ACS Med Chem Lett 7: 379-84 (2016) Article DOI: 10.1021/acsmedchemlett.5b00389 BindingDB Entry DOI: 10.7270/Q2W097V6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Branched-chain-amino-acid aminotransferase, mitochondrial (Homo sapiens (Human)) | BDBM50173926 (CHEMBL3808971) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 63.1 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of human BCATm (28 to 392 residues) using L-Leucine and alpha-ketogluterate as substrate assessed as L-glutamate production after 10 mins ... | ACS Med Chem Lett 7: 379-84 (2016) Article DOI: 10.1021/acsmedchemlett.5b00389 BindingDB Entry DOI: 10.7270/Q2W097V6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Branched-chain-amino-acid aminotransferase, mitochondrial (Homo sapiens (Human)) | BDBM50173928 (CHEMBL3809839) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 63.1 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of human BCATm (28 to 392 residues) using L-Leucine and alpha-ketogluterate as substrate assessed as L-glutamate production after 10 mins ... | ACS Med Chem Lett 7: 379-84 (2016) Article DOI: 10.1021/acsmedchemlett.5b00389 BindingDB Entry DOI: 10.7270/Q2W097V6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Branched-chain-amino-acid aminotransferase, mitochondrial (Homo sapiens (Human)) | BDBM50173923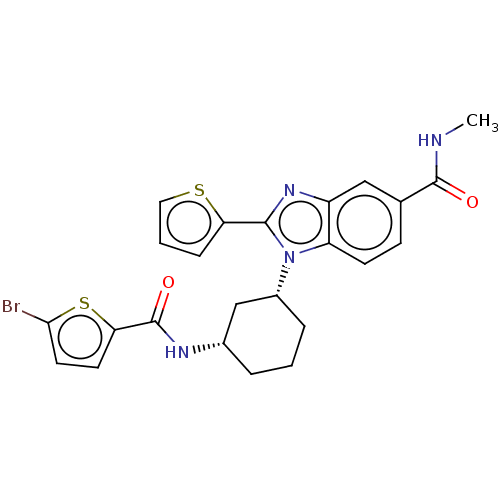 (CHEMBL3810317) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 79 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of human BCATm (28 to 392 residues) using L-Leucine and alpha-ketogluterate as substrate assessed as L-glutamate production after 10 mins ... | ACS Med Chem Lett 7: 379-84 (2016) Article DOI: 10.1021/acsmedchemlett.5b00389 BindingDB Entry DOI: 10.7270/Q2W097V6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Branched-chain-amino-acid aminotransferase, mitochondrial (Homo sapiens (Human)) | BDBM50173922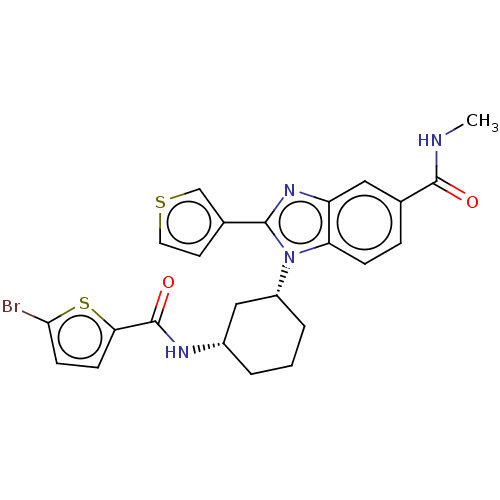 (CHEMBL3809282) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of human BCATm (28 to 392 residues) using L-Leucine and alpha-ketogluterate as substrate assessed as L-glutamate production after 10 mins ... | ACS Med Chem Lett 7: 379-84 (2016) Article DOI: 10.1021/acsmedchemlett.5b00389 BindingDB Entry DOI: 10.7270/Q2W097V6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Branched-chain-amino-acid aminotransferase, mitochondrial (Homo sapiens (Human)) | BDBM50173923 (CHEMBL3810317) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of BCATm in differentiated primary human adipocytes using L-Serine and L-Leucine as substrate after overnight incubation by reverse-phase ... | ACS Med Chem Lett 7: 379-84 (2016) Article DOI: 10.1021/acsmedchemlett.5b00389 BindingDB Entry DOI: 10.7270/Q2W097V6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Branched-chain-amino-acid aminotransferase, mitochondrial (Homo sapiens (Human)) | BDBM50173922 (CHEMBL3809282) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of BCATm in differentiated primary human adipocytes using L-Serine and L-Leucine as substrate after overnight incubation by reverse-phase ... | ACS Med Chem Lett 7: 379-84 (2016) Article DOI: 10.1021/acsmedchemlett.5b00389 BindingDB Entry DOI: 10.7270/Q2W097V6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Branched-chain-amino-acid aminotransferase, mitochondrial (Homo sapiens (Human)) | BDBM50173939 (CHEMBL3809237) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 158 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of BCATm in differentiated primary human adipocytes using L-Serine and L-Leucine as substrate after overnight incubation by reverse-phase ... | ACS Med Chem Lett 7: 379-84 (2016) Article DOI: 10.1021/acsmedchemlett.5b00389 BindingDB Entry DOI: 10.7270/Q2W097V6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Branched-chain-amino-acid aminotransferase, mitochondrial (Homo sapiens (Human)) | BDBM50173918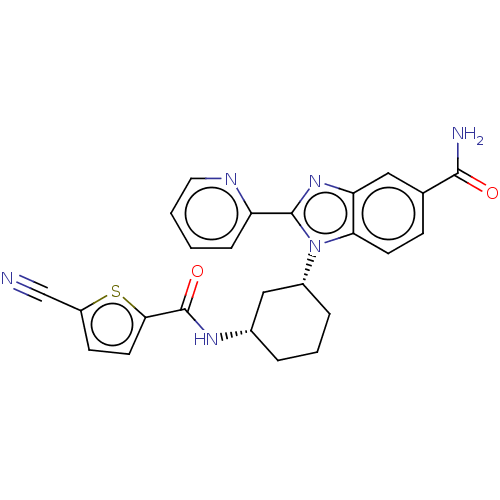 (CHEMBL3809916) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 251 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of human BCATm (28 to 392 residues) using L-Leucine and alpha-ketogluterate as substrate assessed as L-glutamate production after 10 mins ... | ACS Med Chem Lett 7: 379-84 (2016) Article DOI: 10.1021/acsmedchemlett.5b00389 BindingDB Entry DOI: 10.7270/Q2W097V6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Branched-chain-amino-acid aminotransferase, mitochondrial (Homo sapiens (Human)) | BDBM50173916 (CHEMBL3809321) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 251 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of human BCATm (28 to 392 residues) using L-Leucine and alpha-ketogluterate as substrate assessed as L-glutamate production after 10 mins ... | ACS Med Chem Lett 7: 379-84 (2016) Article DOI: 10.1021/acsmedchemlett.5b00389 BindingDB Entry DOI: 10.7270/Q2W097V6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Branched-chain-amino-acid aminotransferase, mitochondrial (Homo sapiens (Human)) | BDBM50173939 (CHEMBL3809237) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 251 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of human BCATm (28 to 392 residues) using L-Leucine and alpha-ketogluterate as substrate assessed as L-glutamate production after 10 mins ... | ACS Med Chem Lett 7: 379-84 (2016) Article DOI: 10.1021/acsmedchemlett.5b00389 BindingDB Entry DOI: 10.7270/Q2W097V6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Branched-chain-amino-acid aminotransferase, mitochondrial (Homo sapiens (Human)) | BDBM50173933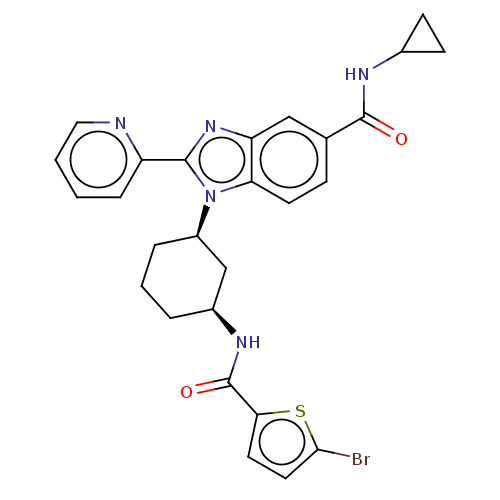 (CHEMBL3808894) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 251 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of human BCATm (28 to 392 residues) using L-Leucine and alpha-ketogluterate as substrate assessed as L-glutamate production after 10 mins ... | ACS Med Chem Lett 7: 379-84 (2016) Article DOI: 10.1021/acsmedchemlett.5b00389 BindingDB Entry DOI: 10.7270/Q2W097V6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Branched-chain-amino-acid aminotransferase, mitochondrial (Homo sapiens (Human)) | BDBM50173925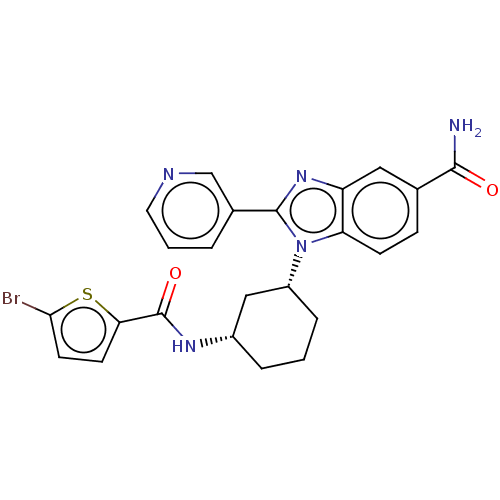 (CHEMBL3808495) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 501 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of human BCATm (28 to 392 residues) using L-Leucine and alpha-ketogluterate as substrate assessed as L-glutamate production after 10 mins ... | ACS Med Chem Lett 7: 379-84 (2016) Article DOI: 10.1021/acsmedchemlett.5b00389 BindingDB Entry DOI: 10.7270/Q2W097V6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Branched-chain-amino-acid aminotransferase, mitochondrial (Homo sapiens (Human)) | BDBM50173931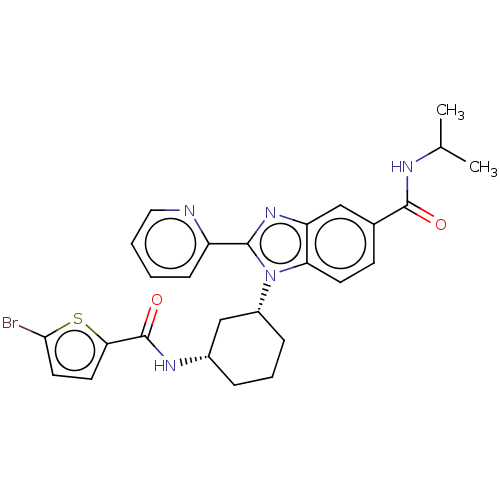 (CHEMBL3808975) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 631 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of human BCATm (28 to 392 residues) using L-Leucine and alpha-ketogluterate as substrate assessed as L-glutamate production after 10 mins ... | ACS Med Chem Lett 7: 379-84 (2016) Article DOI: 10.1021/acsmedchemlett.5b00389 BindingDB Entry DOI: 10.7270/Q2W097V6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Branched-chain-amino-acid aminotransferase, mitochondrial (Homo sapiens (Human)) | BDBM50173921 (CHEMBL3809574) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 631 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of human BCATm (28 to 392 residues) using L-Leucine and alpha-ketogluterate as substrate assessed as L-glutamate production after 10 mins ... | ACS Med Chem Lett 7: 379-84 (2016) Article DOI: 10.1021/acsmedchemlett.5b00389 BindingDB Entry DOI: 10.7270/Q2W097V6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Branched-chain-amino-acid aminotransferase, mitochondrial (Homo sapiens (Human)) | BDBM50173920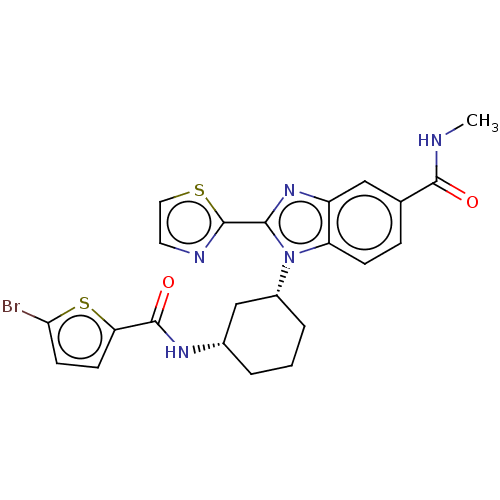 (CHEMBL3808530) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 794 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of human BCATm (28 to 392 residues) using L-Leucine and alpha-ketogluterate as substrate assessed as L-glutamate production after 10 mins ... | ACS Med Chem Lett 7: 379-84 (2016) Article DOI: 10.1021/acsmedchemlett.5b00389 BindingDB Entry DOI: 10.7270/Q2W097V6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Branched-chain-amino-acid aminotransferase, mitochondrial (Homo sapiens (Human)) | BDBM50173937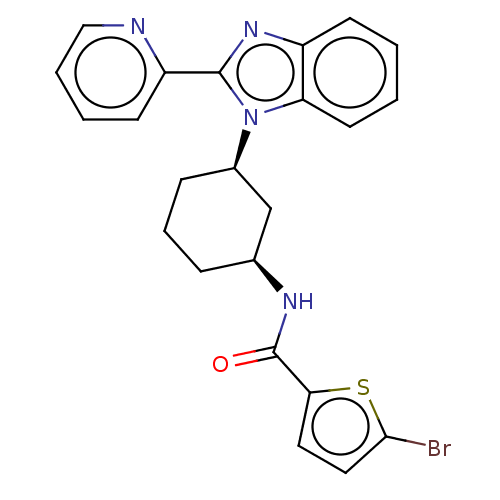 (CHEMBL3809820) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of human BCATm (28 to 392 residues) using L-Leucine and alpha-ketogluterate as substrate assessed as L-glutamate production after 10 mins ... | ACS Med Chem Lett 7: 379-84 (2016) Article DOI: 10.1021/acsmedchemlett.5b00389 BindingDB Entry DOI: 10.7270/Q2W097V6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Branched-chain-amino-acid aminotransferase, mitochondrial (Homo sapiens (Human)) | BDBM50173918 (CHEMBL3809916) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.26E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of BCATm in differentiated primary human adipocytes using L-Serine and L-Leucine as substrate after overnight incubation by reverse-phase ... | ACS Med Chem Lett 7: 379-84 (2016) Article DOI: 10.1021/acsmedchemlett.5b00389 BindingDB Entry DOI: 10.7270/Q2W097V6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Branched-chain-amino-acid aminotransferase, cytosolic (Homo sapiens (Human)) | BDBM50173939 (CHEMBL3809237) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.26E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of BCATc (unknown origin) | ACS Med Chem Lett 7: 379-84 (2016) Article DOI: 10.1021/acsmedchemlett.5b00389 BindingDB Entry DOI: 10.7270/Q2W097V6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Branched-chain-amino-acid aminotransferase, mitochondrial (Homo sapiens (Human)) | BDBM50173915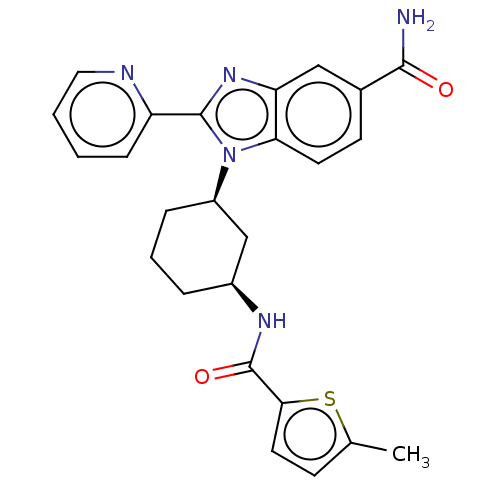 (CHEMBL3809792) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.26E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of human BCATm (28 to 392 residues) using L-Leucine and alpha-ketogluterate as substrate assessed as L-glutamate production after 10 mins ... | ACS Med Chem Lett 7: 379-84 (2016) Article DOI: 10.1021/acsmedchemlett.5b00389 BindingDB Entry DOI: 10.7270/Q2W097V6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Branched-chain-amino-acid aminotransferase, mitochondrial (Homo sapiens (Human)) | BDBM50173924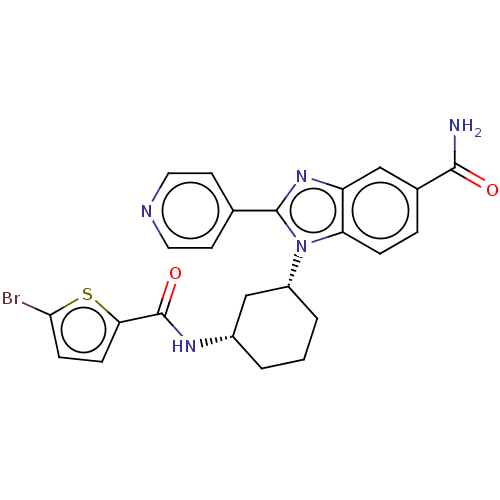 (CHEMBL3809704) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.59E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of human BCATm (28 to 392 residues) using L-Leucine and alpha-ketogluterate as substrate assessed as L-glutamate production after 10 mins ... | ACS Med Chem Lett 7: 379-84 (2016) Article DOI: 10.1021/acsmedchemlett.5b00389 BindingDB Entry DOI: 10.7270/Q2W097V6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Branched-chain-amino-acid aminotransferase, mitochondrial (Homo sapiens (Human)) | BDBM50173940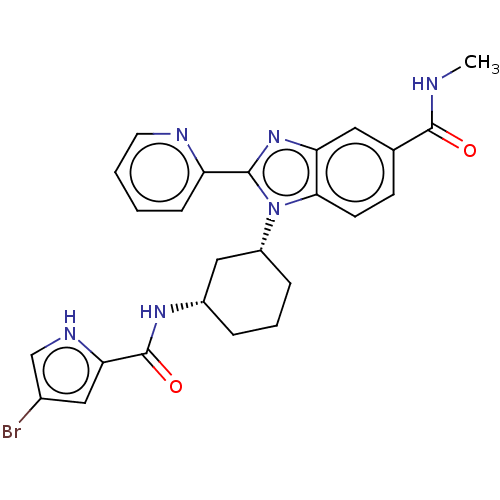 (CHEMBL3809515) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.59E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of human BCATm (28 to 392 residues) using L-Leucine and alpha-ketogluterate as substrate assessed as L-glutamate production after 10 mins ... | ACS Med Chem Lett 7: 379-84 (2016) Article DOI: 10.1021/acsmedchemlett.5b00389 BindingDB Entry DOI: 10.7270/Q2W097V6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Branched-chain-amino-acid aminotransferase, mitochondrial (Homo sapiens (Human)) | BDBM50173930 (CHEMBL3808610) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of human BCATm (28 to 392 residues) using L-Leucine and alpha-ketogluterate as substrate assessed as L-glutamate production after 10 mins ... | ACS Med Chem Lett 7: 379-84 (2016) Article DOI: 10.1021/acsmedchemlett.5b00389 BindingDB Entry DOI: 10.7270/Q2W097V6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Branched-chain-amino-acid aminotransferase, mitochondrial (Homo sapiens (Human)) | BDBM50173938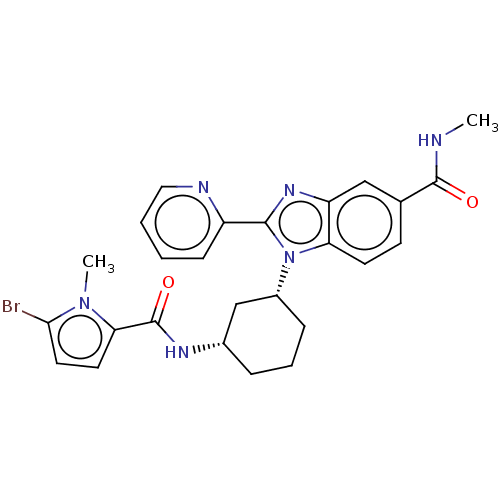 (CHEMBL3808493) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.51E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of human BCATm (28 to 392 residues) using L-Leucine and alpha-ketogluterate as substrate assessed as L-glutamate production after 10 mins ... | ACS Med Chem Lett 7: 379-84 (2016) Article DOI: 10.1021/acsmedchemlett.5b00389 BindingDB Entry DOI: 10.7270/Q2W097V6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Branched-chain-amino-acid aminotransferase, mitochondrial (Homo sapiens (Human)) | BDBM50173919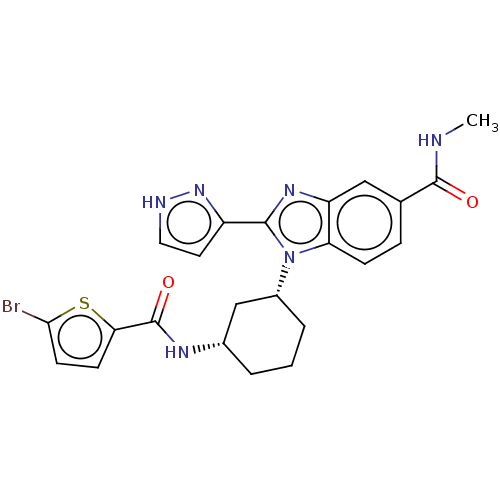 (CHEMBL3808453) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.51E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of human BCATm (28 to 392 residues) using L-Leucine and alpha-ketogluterate as substrate assessed as L-glutamate production after 10 mins ... | ACS Med Chem Lett 7: 379-84 (2016) Article DOI: 10.1021/acsmedchemlett.5b00389 BindingDB Entry DOI: 10.7270/Q2W097V6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Branched-chain-amino-acid aminotransferase, mitochondrial (Homo sapiens (Human)) | BDBM50173988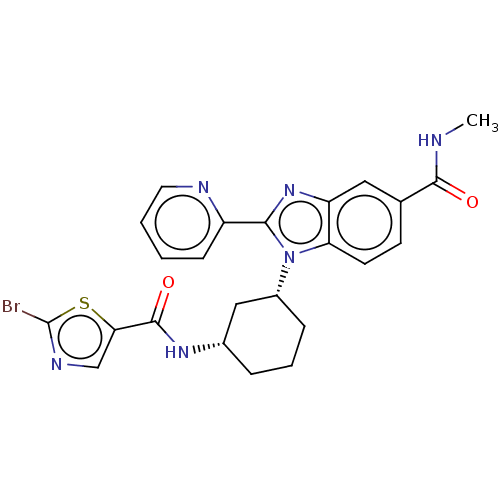 (CHEMBL3809941) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.16E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of human BCATm (28 to 392 residues) using L-Leucine and alpha-ketogluterate as substrate assessed as L-glutamate production after 10 mins ... | ACS Med Chem Lett 7: 379-84 (2016) Article DOI: 10.1021/acsmedchemlett.5b00389 BindingDB Entry DOI: 10.7270/Q2W097V6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Branched-chain-amino-acid aminotransferase, cytosolic (Homo sapiens (Human)) | BDBM50173927 (CHEMBL3808902) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | 3.98E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of BCATc (unknown origin) | ACS Med Chem Lett 7: 379-84 (2016) Article DOI: 10.1021/acsmedchemlett.5b00389 BindingDB Entry DOI: 10.7270/Q2W097V6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Branched-chain-amino-acid aminotransferase, mitochondrial (Homo sapiens (Human)) | BDBM50173934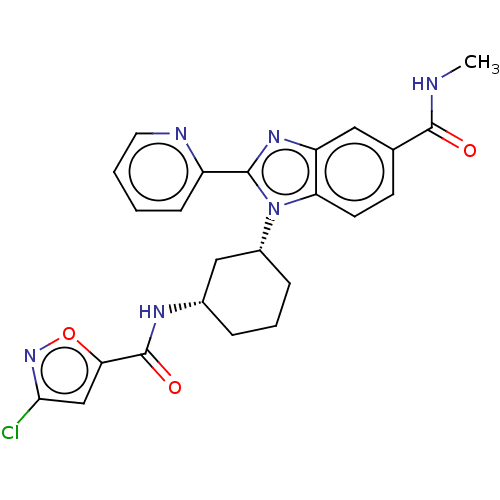 (CHEMBL3809651) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6.31E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of human BCATm (28 to 392 residues) using L-Leucine and alpha-ketogluterate as substrate assessed as L-glutamate production after 10 mins ... | ACS Med Chem Lett 7: 379-84 (2016) Article DOI: 10.1021/acsmedchemlett.5b00389 BindingDB Entry DOI: 10.7270/Q2W097V6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Branched-chain-amino-acid aminotransferase, mitochondrial (Homo sapiens (Human)) | BDBM50173932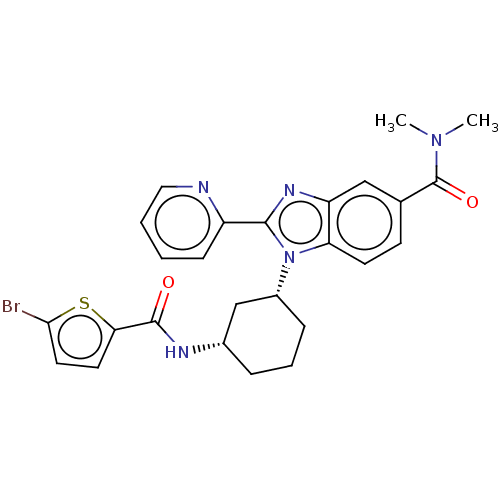 (CHEMBL3809727) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of human BCATm (28 to 392 residues) using L-Leucine and alpha-ketogluterate as substrate assessed as L-glutamate production after 10 mins ... | ACS Med Chem Lett 7: 379-84 (2016) Article DOI: 10.1021/acsmedchemlett.5b00389 BindingDB Entry DOI: 10.7270/Q2W097V6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Branched-chain-amino-acid aminotransferase, mitochondrial (Homo sapiens (Human)) | BDBM50173946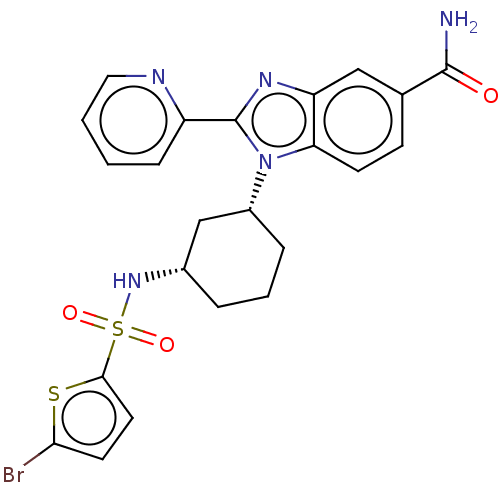 (CHEMBL3809828) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of human BCATm (28 to 392 residues) using L-Leucine and alpha-ketogluterate as substrate assessed as L-glutamate production after 10 mins ... | ACS Med Chem Lett 7: 379-84 (2016) Article DOI: 10.1021/acsmedchemlett.5b00389 BindingDB Entry DOI: 10.7270/Q2W097V6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Branched-chain-amino-acid aminotransferase, mitochondrial (Homo sapiens (Human)) | BDBM50173937 (CHEMBL3809820) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | <5.01E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of BCATm in differentiated primary human adipocytes using L-Serine and L-Leucine as substrate after overnight incubation by reverse-phase ... | ACS Med Chem Lett 7: 379-84 (2016) Article DOI: 10.1021/acsmedchemlett.5b00389 BindingDB Entry DOI: 10.7270/Q2W097V6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||